Investigation of ATG16L1 rs2241880 Polymorphism with Cancer Risk: A Meta-Analysis
Abstract
:1. Introduction
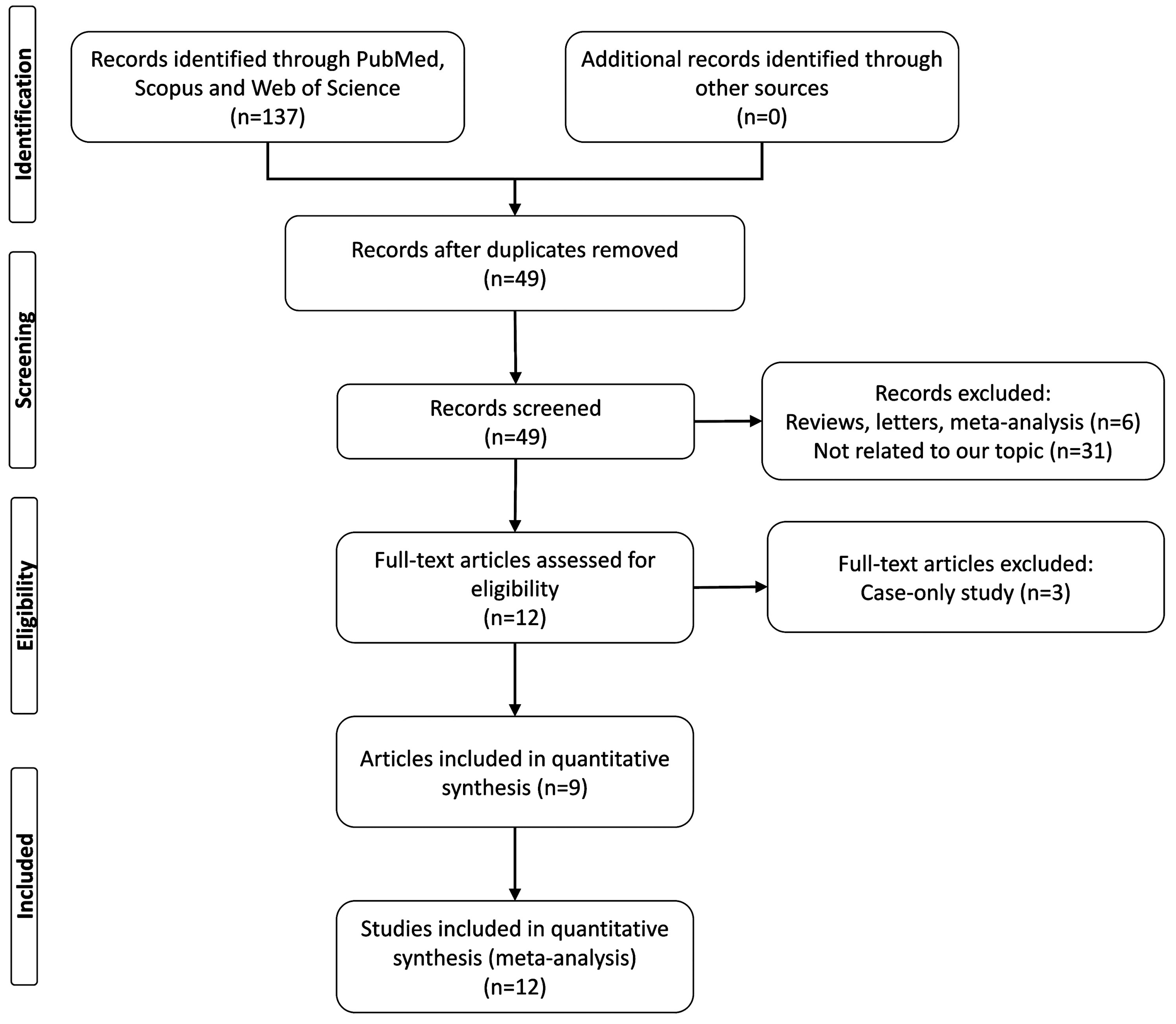
2. Methods
2.1. Literature Search
2.2. Data Extraction
2.3. Statistical Analysis
3. Results
3.1. Study Characteristics
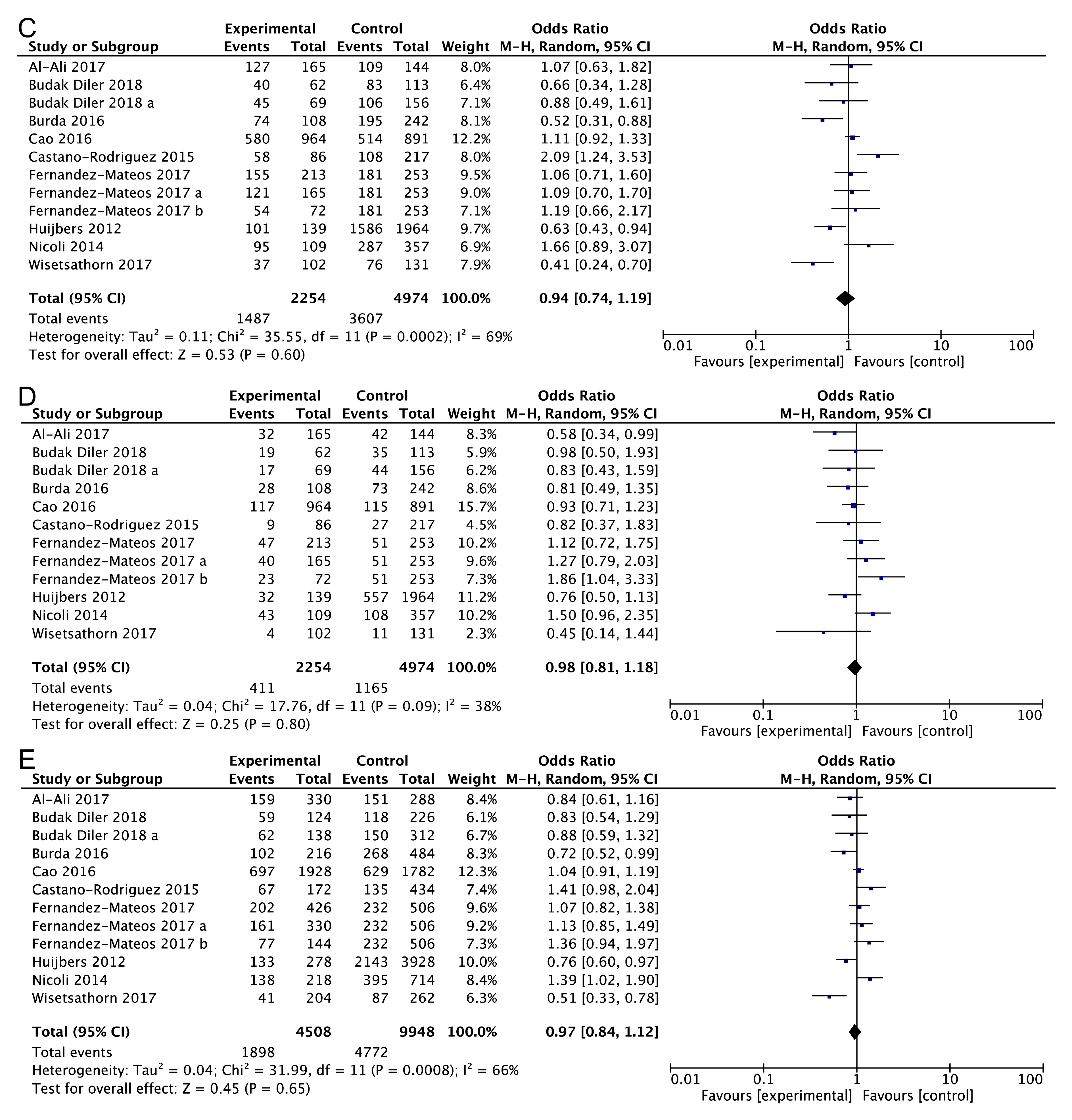
3.2. Main Analysis Results
3.3. Subgroup Analysis
3.4. Heterogeneity and Publication Bias
3.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Cancer; Fitzmaurice, C.; Akinyemiju, T.F.; Al Lami, F.H.; Alam, T.; Alizadeh-Navaei, R.; Allen, C.; Alsharif, U.; Alvis-Guzman, N.; Amini, E.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018, 4, 1553–1568. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, P.; Holm, N.V.; Verkasalo, P.K.; Iliadou, A.; Kaprio, J.; Koskenvuo, M.; Pukkala, E.; Skytthe, A.; Hemminki, K. Environmental and heritable factors in the causation of cancer—Analyses of cohorts of twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 2000, 343, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Moazeni-Roodi, A.; Ghavami, S.; Hashemi, M. Association Between miR-423 rs6505162 Polymorphism and Susceptibility to Cancer. Arch. Med. Res. 2019, 50, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Martini-Stoica, H.; Xu, Y.; Ballabio, A.; Zheng, H. The Autophagy-Lysosomal Pathway in Neurodegeneration: A TFEB Perspective. Trends Neurosci. 2016, 39, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, E.L.; Saftig, P. Autophagy: A lysosomal degradation pathway with a central role in health and disease. Biochim. Biophys. Acta 2009, 1793, 664–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.H.; Al-Aidaroos, A.Q.; Yuen, H.F.; Zhang, S.D.; Shen, H.M.; Rozycka, E.; McCrudden, C.M.; Tergaonkar, V.; Gupta, A.; Lin, Y.B.; et al. A role of autophagy in PTP4A3-driven cancer progression. Autophagy 2014, 10, 1787–1800. [Google Scholar] [Green Version]
- Hombach-Klonisch, S.; Mehrpour, M.; Shojaei, S.; Harlos, C.; Pitz, M.; Hamai, A.; Siemianowicz, K.; Likus, W.; Wiechec, E.; Toyota, B.D.; et al. Glioblastoma and chemoresistance to alkylating agents: Involvement of apoptosis, autophagy, and unfolded protein response. Pharmacol. Ther. 2018, 184, 13–41. [Google Scholar]
- Ghavami, S.; Gupta, S.; Ambrose, E.; Hnatowich, M.; Freed, D.H.; Dixon, I.M. Autophagy and heart disease: Implications for cardiac ischemia-reperfusion damage. Curr. Mol. Med. 2014, 14, 616–629. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef] [Green Version]
- Wasik, A.M.; Grabarek, J.; Pantovic, A.; Cieslar-Pobuda, A.; Asgari, H.R.; Bundgaard-Nielsen, C.; Rafat, M.; Dixon, I.M.; Ghavami, S.; Los, M.J. Reprogramming and carcinogenesis--parallels and distinctions. Int. Rev. Cell Mol. Biol. 2014, 308, 167–203. [Google Scholar] [PubMed]
- Ghavami, S.; Sharma, P.; Yeganeh, B.; Ojo, O.O.; Jha, A.; Mutawe, M.M.; Kashani, H.H.; Los, M.J.; Klonisch, T.; Unruh, H.; et al. Airway mesenchymal cell death by mevalonate cascade inhibition: Integration of autophagy, unfolded protein response and apoptosis focusing on Bcl2 family proteins. Biochim. Biophys. Acta 2014, 1843, 1259–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikseresht, M.; Shahverdi, M.; Dehghani, M.; Abidi, H.; Mahmoudi, R.; Ghalamfarsa, G.; Manzouri, L.; Ghavami, S. Association of single nucleotide autophagy-related protein 5 gene polymorphism rs2245214 with susceptibility to non-small cell lung cancer. J. Cell. Biochem. 2018, 120, 1924–1931. [Google Scholar] [CrossRef] [PubMed]
- Baehrecke, E.H. Autophagy: Dual roles in life and death? Nat. Rev. Mol. Cell Biol. 2005, 6, 505–510. [Google Scholar] [CrossRef]
- Choi, A.M.; Ryter, S.W.; Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 2013, 368, 1845–1846. [Google Scholar] [CrossRef] [PubMed]
- Mokarram, P.; Albokashy, M.; Zarghooni, M.; Moosavi, M.A.; Sepehri, Z.; Chen, Q.M.; Hudecki, A.; Sargazi, A.; Alizadeh, J.; Moghadam, A.R.; et al. New frontiers in the treatment of colorectal cancer: Autophagy and the unfolded protein response as promising targets. Autophagy 2017, 13, 781–819. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, S.; Shojaei, S.; Yeganeh, B.; Ande, S.R.; Jangamreddy, J.R.; Mehrpour, M.; Christoffersson, J.; Chaabane, W.; Moghadam, A.R.; Kashani, H.H.; et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol. 2014, 112, 24–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeganeh, B.; Ghavami, S.; Kroeker, A.L.; Mahood, T.H.; Stelmack, G.L.; Klonisch, T.; Coombs, K.M.; Halayko, A.J. Suppression of influenza A virus replication in human lung epithelial cells by noncytotoxic concentrations bafilomycin A1. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L270–L286. [Google Scholar] [CrossRef] [PubMed]
- Yeganeh, B.; Rezaei Moghadam, A.; Tran, A.T.; Rahim, M.N.; Ande, S.R.; Hashemi, M.; Coombs, K.M.; Ghavami, S. Asthma and influenza virus infection:focusing on cell death and stress pathways in influenza virus replication. Iran. J. Allergy Asthma Immunol. 2013, 12, 1–17. [Google Scholar]
- Ghavami, S.; Yeganeh, B.; Zeki, A.A.; Shojaei, S.; Kenyon, N.J.; Ott, S.; Samali, A.; Patterson, J.; Alizadeh, J.; Moghadam, A.R.; et al. Autophagy and the unfolded protein response promote profibrotic effects of TGF-beta1 in human lung fibroblasts. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L493–L504. [Google Scholar] [CrossRef]
- Alizadeh, J.; Glogowska, A.; Thliveris, J.; Kalantari, F.; Shojaei, S.; Hombach-Klonisch, S.; Klonisch, T.; Ghavami, S. Autophagy modulates transforming growth factor beta 1 induced epithelial to mesenchymal transition in non-small cell lung cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 749–768. [Google Scholar] [CrossRef]
- Kondo, Y.; Kanzawa, T.; Sawaya, R.; Kondo, S. The role of autophagy in cancer development and response to therapy. Nat. Rev. Cancer 2005, 5, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.; Karantza-Wadsworth, V.; White, E. Role of autophagy in cancer. Nat. Rev. Cancer 2007, 7, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Overholtzer, M.; Thompson, C.B. Autophagy in cellular metabolism and cancer. J. Clin. Investig. 2015, 125, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, J.; Shojaei, S.; Sepanjnia, A.; Hashemi, M.; Eftekharpour, E.; Ghavami, S. Simultaneous Detection of Autophagy and Epithelial to Mesenchymal Transition in the Non-small Cell Lung Cancer Cells. Methods Mol. Biol. 2019, 1854, 87–103. [Google Scholar]
- Moosavi, M.A.; Sharifi, M.; Ghafary, S.M.; Mohammadalipour, Z.; Khataee, A.; Rahmati, M.; Hajjaran, S.; Los, M.J.; Klonisch, T.; Ghavami, S. Photodynamic N-TiO2 Nanoparticle Treatment Induces Controlled ROS-mediated Autophagy and Terminal Differentiation of Leukemia Cells. Sci. Rep. 2016, 6, 34413. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Ji, C.; Li, J.; Jiang, H.; Ren, M.; Lu, Q.; Gu, S.; Mao, Y.; Xie, Y. Cloning and analysis of human Apg16L. DNA Seq. 2004, 15, 303–305. [Google Scholar] [CrossRef]
- Mizushima, N.; Kuma, A.; Kobayashi, Y.; Yamamoto, A.; Matsubae, M.; Takao, T.; Natsume, T.; Ohsumi, Y.; Yoshimori, T. Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the Apg12-Apg5 conjugate. J. Cell Sci. 2003, 116, 1679–1688. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, K.; Ulferts, R.; Jacquin, E.; Veith, T.; Gammoh, N.; Arasteh, J.M.; Mayer, U.; Carding, S.R.; Wileman, T.; Beale, R.; et al. The WD40 domain of ATG16L1 is required for its non-canonical role in lipidation of LC3 at single membranes. EMBO J. 2018, 37, e97840. [Google Scholar] [CrossRef]
- Li, Q.X.; Zhou, X.; Huang, T.T.; Tang, Y.; Liu, B.; Peng, P.; Sun, L.; Wang, Y.H.; Yuan, X.L. The Thr300Ala variant of ATG16L1 is associated with decreased risk of brain metastasis in patients with non-small cell lung cancer. Autophagy 2017, 13, 1053–1063. [Google Scholar] [CrossRef] [Green Version]
- Budak Diler, S.; Aybuga, F. Association of Autophagy Gene ATG16L1 Polymorphism with Human Prostate Cancer and Bladder Cancer in Turkish Population. Asian Pac. J. Cancer Prev. 2018, 19, 2625–2630. [Google Scholar]
- Burada, F.; Ciurea, M.E.; Nicoli, R.; Streata, I.; Vilcea, I.D.; Rogoveanu, I.; Ioana, M. ATG16L1 T300A Polymorphism is Correlated with Gastric Cancer Susceptibility. Pathol. Oncol. Res. 2016, 22, 317–322. [Google Scholar] [CrossRef]
- Castano-Rodriguez, N.; Kaakoush, N.O.; Goh, K.L.; Fock, K.M.; Mitchell, H.M. Autophagy in Helicobacter pylori Infection and Related Gastric Cancer. Helicobacter 2015, 20, 353–369. [Google Scholar] [CrossRef]
- Fernandez-Mateos, J.; Seijas-Tamayo, R.; Klain, J.C.A.; Borgonon, M.P.; Perez-Ruiz, E.; Mesia, R.; Del Barco, E.; Coloma, C.S.; Dominguez, A.R.; Daroqui, J.C.; et al. Analysis of autophagy gene polymorphisms in Spanish patients with head and neck squamous cell carcinoma. Sci. Rep. 2017, 7, 6887. [Google Scholar] [CrossRef]
- Huijbers, A.; Plantinga, T.S.; Joosten, L.A.; Aben, K.K.; Gudmundsson, J.; den Heijer, M.; Kiemeney, L.A.; Netea, M.G.; Hermus, A.R.; Netea-Maier, R.T. The effect of the ATG16L1 Thr300Ala polymorphism on susceptibility and outcome of patients with epithelial cell-derived thyroid carcinoma. Endocr. Relat. Cancer 2012, 19, L15–L18. [Google Scholar] [CrossRef]
- Nicoli, E.R.; Dumitrescu, T.; Uscatu, C.D.; Popescu, F.D.; Streata, I.; Serban Sosoi, S.; Ivanov, P.; Dumitrescu, A.; Barbalan, A.; Lungulescu, D.; et al. Determination of autophagy gene ATG16L1 polymorphism in human colorectal cancer. Rom. J. Morphol. Embryol. 2014, 55, 57–62. [Google Scholar]
- Wisetsathorn, S.; Tantithavorn, V.; Hirankarn, N.; Tangkijvanich, P.; Saethang, T.; Kimkong, I. Gene polymorphisms of autophagy machinery and the risk of hepatitis B virus-related hepatocellular carcinoma in a Thai population. Scienceasia 2017, 43, 362–368. [Google Scholar] [CrossRef]
- Cao, H.; Li, Z.L.; Zhou, D.; Wan, L.D.; Yu, D.; Zhang, J.; Xu, E.P.; Zhang, D.D.; Lai, M.D. ATG16L1 rs2241880 polymorphism predicts unfavorable clinical outcomes for colorectal cancer patients in the Chinese population. Int. J. Clin. Exp. Pathol. 2016, 9, 8586–8595. [Google Scholar]
- Al-Ali, R.; Fernandez-Mateos, J.; Gonzalez-Sarmiento, R. Association of autophagy gene polymorphisms with lung cancer. Gene Rep. 2017, 7, 74–77. [Google Scholar] [CrossRef]
- Cooney, R.; Baker, J.; Brain, O.; Danis, B.; Pichulik, T.; Allan, P.; Ferguson, D.J.; Campbell, B.J.; Jewell, D.; Simmons, A. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat. Med. 2010, 16, 90–97. [Google Scholar] [CrossRef]
- Plantinga, T.S.; Crisan, T.O.; Oosting, M.; van de Veerdonk, F.L.; de Jong, D.J.; Philpott, D.J.; van der Meer, J.W.; Girardin, S.E.; Joosten, L.A.; Netea, M.G. Crohn’s disease-associated ATG16L1 polymorphism modulates pro-inflammatory cytokine responses selectively upon activation of NOD2. Gut 2011, 60, 1229–1235. [Google Scholar] [CrossRef]
- Figlioli, G.; Elisei, R.; Romei, C.; Melaiu, O.; Cipollini, M.; Bambi, F.; Chen, B.; Kohler, A.; Cristaudo, A.; Hemminki, K.; et al. A Comprehensive Meta-analysis of Case-Control Association Studies to Evaluate Polymorphisms Associated with the Risk of Differentiated Thyroid Carcinoma. Cancer Epidemiol. Biomark. Prev. 2016, 25, 700–713. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, N.; Yin, L.; Zhu, H.; Zhang, L.; Zhou, L.; Yang, M. Clinical Implications of the Autophagy Core Gene Variations in Advanced Lung Adenocarcinoma Treated with Gefitinib. Sci. Rep. 2017, 7, 17814. [Google Scholar] [CrossRef]



| First Author | Year | Country | Ethnicity | Cancer Type | Source of Control | Genotyping Method | Case/Control | Cases | Controls | HWE (P) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | AG | GG | A | G | AA | AG | GG | A | G | |||||||||
| Al-Ali et al. [39] | 2017 | Spain | Caucasian | Lung cancer | PB | TaqMan | 165/144 | 38 | 95 | 32 | 171 | 159 | 35 | 67 | 42 | 137 | 151 | 0.420 |
| Budak Diler et al. [31] | 2018 | Turkey | Asian | Prostate cancer | PB | PCR-RFLP | 62/113 | 22 | 21 | 19 | 65 | 59 | 30 | 48 | 35 | 108 | 118 | 0.114 |
| Budak Diler et al. [31] | 2018 | Turkey | Asian | Bladder cancer | PB | PCR-RFLP | 69/156 | 24 | 28 | 17 | 76 | 62 | 50 | 62 | 44 | 162 | 150 | 0.011 |
| Burada et al. [32] | 2016 | Romania | Caucasian | Gastric cancer | HB | TaqMan | 108/242 | 34 | 46 | 28 | 114 | 102 | 47 | 122 | 73 | 216 | 268 | 0.755 |
| Cao et al. [38] | 2016 | China | Asian | Colorectal cancer | HB | Illumina | 964/891 | 384 | 463 | 117 | 1231 | 697 | 377 | 399 | 115 | 1153 | 629 | 0.558 |
| Castano-Rodriguez et al. [33] | 2015 | Singapore | Asian | Gastric cancer | HB | MassARRAY iPLEX | 86/217 | 28 | 49 | 9 | 105 | 67 | 109 | 81 | 27 | 299 | 135 | 0.057 |
| Fernandez-Mateos et al. [34] | 2017 | Spain | Caucasian | Larynx cancer | HB | TaqMan | 213/253 | 58 | 108 | 47 | 224 | 202 | 72 | 130 | 51 | 274 | 232 | 0.580 |
| Fernandez-Mateos et al. [34] | 2017 | Spain | Caucasian | Pharynx cancer | HB | TaqMan | 165/253 | 44 | 81 | 40 | 169 | 161 | 72 | 130 | 51 | 274 | 232 | 0.580 |
| Fernandez-Mateos et al. [34] | 2017 | Spain | Caucasian | Oral cavity cancer | HB | TaqMan | 72/253 | 18 | 31 | 23 | 67 | 77 | 72 | 130 | 51 | 274 | 232 | 0.580 |
| Huijbers et al. [35] | 2012 | Netherlands | Caucasian | Thyroid cancer | PB | - | 139/1964 | 38 | 69 | 32 | 145 | 133 | 378 | 1029 | 557 | 1785 | 2143 | 0.012 |
| Nicoli et al. [36] | 2014 | Romania | Caucasian | Colorectal cancer | HB | TaqMan | 109/357 | 14 | 52 | 43 | 80 | 138 | 70 | 179 | 108 | 319 | 395 | 0.787 |
| Wisetsathorn et al. [37] | 2017 | Thailand | Asian | HCC | HB | PCR-RFLP | 102/131 | 65 | 33 | 4 | 163 | 41 | 55 | 65 | 11 | 175 | 87 | 0.175 |
| Genetic Model | Association Test | Heterogeneity Test | Test of Publication Bias | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Z | p | χ2 | I2 (%) | p | Egger’s Test p | Begg’s Test p | |
| AG vs. AA | 0.94 (0.74–1.20) | 0.48 | 0.63 | 33.17 | 67 | 0.000 | 0.425 | 0.411 |
| GG vs. AA | 0.93 (0.72–1.20) | 0.55 | 0.58 | 22.30 | 51 | 0.022 | 0.726 | 0.891 |
| AG + GG vs. AA | 0.94 (0.74–1.19) | 0.53 | 0.60 | 35.55 | 69 | 0.000 | 0.523 | 0.891 |
| GG vs. AG + AA | 0.98 (0.81–1.18) | 0.25 | 0.80 | 17.76 | 38 | 0.087 | 0.677 | 0.493 |
| AG vs. GG + AA | 0.97 (0.80–1.17) | 0.36 | 0.72 | 27.55 | 60 | 0.004 | 0.321 | 0.411 |
| G vs. A | 0.97 (0.84–1.12) | 0.45 | 0.65 | 31.99 | 66 | 0.001 | 0.567 | 0.583 |
| Type of Cancer | N | AG vs. AA | GG vs. AA | AG + GG vs. AA | GG vs. AG + AA | AG vs. GG + AA | G vs. A | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ||
| Cancer type | |||||||||||||
| Digestive tract system | 4 | 1.19 (0.71–1.98) | 0.51 | 1.05 (0.65–1.70) | 0.85 | 1.17 (0.72–1.92) | 0.52 | 1.00 (0.81–1.22) | 0.98 | 1.12 (0.78–1.62) | 0.54 | 1.09 90.84–1.41) | 0.51 |
| Colorectal cancer | 2 | 1.16 (0.96–1.40) | 0.12 | 1.32 (0.68–2.55) | 0.42 | 1.21 (0.87–1.67) | 0.25 | 1.06 (0.84–1.34) | 0.62 | 1.10 (0.93–1.30) | 0.26 | 1.16 (0.88–1.54) | 0.30 |
| Gastric cancer | 2 | 1.11 (0.25–4.86) | 0.89 | 0.79 (0.33–1.88) | 0.59 | 1.05 (0.27–4.06) | 0.95 | 0.81 (0.53–1.25) | 0.35 | 1.27 (0.43–3.77) | 0.67 | 1.00 (0.52–1.94) | 0.99 |
| Head and neck squamous cell carcinoma | 3 | 1.01 (0.76–1.34) | 0.94 | 1.32 (0.94–1.85) | 0.11 | 1.10 (0.84–1.44) | 0.49 | 1.31 (0.99–1.74) | 0.06 | 0.89 (0.70–1.13) | 0.35 | 1.14 (0.97–1.35) | 0.12 |
| Ethnicity | |||||||||||||
| Caucasian | 7 | 0.92 (0.76–1.11) | 0.37 | 1.00 (0.68–1.47) | 0.98 | 0.95 (0.72–1.25) | 0.70 | 1.04 (0.78–1.39) | 0.77 | 0.94 (0.80–1.09) | 0.40 | 1.00 (0.83–1.21) | 0.99 |
| Asian | 5 | 0.94 (0.57–1.57) | 0.81 | 0.92 (0.72–1.17) | 0.47 | 0.91 (0.57–1.46) | 0.69 | 0.89 (0.71–1.11) | 0.30 | 1.00 (0.65–1.54) | 0.99 | 0.91 (0.69–1.20) | 0.50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moazeni-Roodi, A.; Tabasi, F.; Ghavami, S.; Hashemi, M. Investigation of ATG16L1 rs2241880 Polymorphism with Cancer Risk: A Meta-Analysis. Medicina 2019, 55, 425. https://doi.org/10.3390/medicina55080425
Moazeni-Roodi A, Tabasi F, Ghavami S, Hashemi M. Investigation of ATG16L1 rs2241880 Polymorphism with Cancer Risk: A Meta-Analysis. Medicina. 2019; 55(8):425. https://doi.org/10.3390/medicina55080425
Chicago/Turabian StyleMoazeni-Roodi, Abdolkarim, Farhad Tabasi, Saeid Ghavami, and Mohammad Hashemi. 2019. "Investigation of ATG16L1 rs2241880 Polymorphism with Cancer Risk: A Meta-Analysis" Medicina 55, no. 8: 425. https://doi.org/10.3390/medicina55080425





