Exhaled Breath Analysis in Obstructive Sleep Apnea Syndrome: A Review of the Literature
Abstract
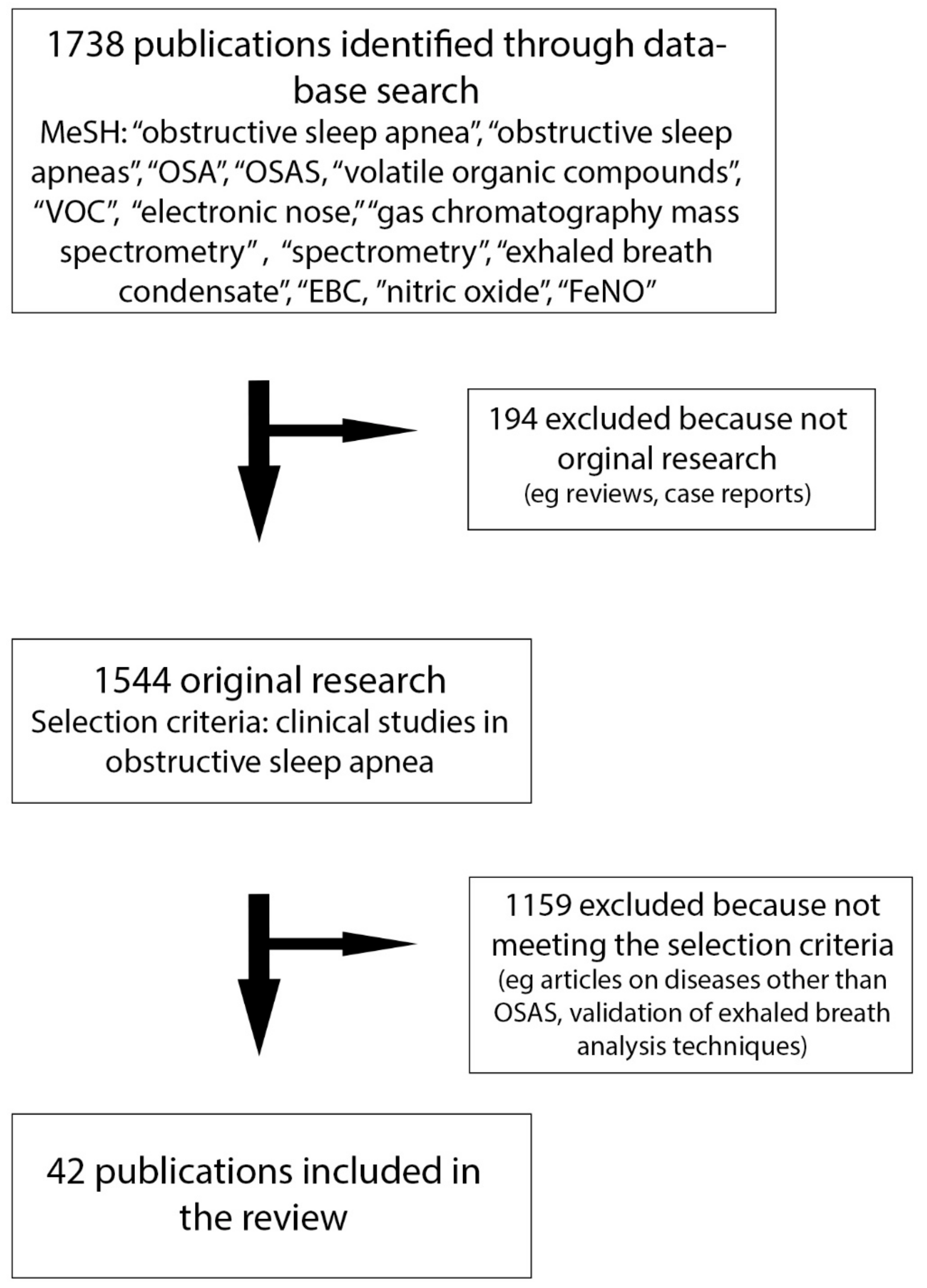
:1. Introduction
2. Materials and Methods
3. Results
3.1. FeNO and Exhaled Carbon Monoxide (eCO)
3.2. Exhaled Breath Condensate
3.2.1. EBC pH
3.2.2. EBC Cytokines
3.2.3. EBC Oxidative Stress
3.2.4. Other EBC Markers
3.3. Volatile Organic Compounds: Spectrometry and Electronic Nose
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mehra, R.; Benjamin, E.J.; Shahar, E.; Gottlieb, D.J.; Nawabit, R.; Kirchner, H.L. Association of nocturnal arrhythmias with sleep-disordered breathing: The sleep heart health study. Am. J. Respir. Crit. Care Med. 2006, 173, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Gami, A.S.; Olson, E.J.; Shen, W.K.; Wright, R.S.; Ballman, K.V.; Hodge, D.O.; Herges, R.M.; Howard, D.E.; Somers, V.K. Obstructive Sleep Apnea and the Risk of Sudden Cardiac Death: A Longitudinal Study of 10,701 Adults. J. Am. Coll. Cardiol. 2013, 62, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Peled, N.; Kassirer, M.; Shitrit, D.; Kogan, Y.; Shlomi, D.; Berliner, A.S.; Kramer, M.R. The association of OSA with insulin resistance, inflammation and metabolic syndrome. Respir. Med. 2007, 101, 1696–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, K.L.; Blackwell, T.L.; Ancoli-Israel, S.; Barrett-Connor, E.; Bauer, D.C.; Cauley, J.A.; Ensrud, K.E.; Hoffman, A.R.; Mehra, R.; Stefanick, M.L.; et al. Sleep Disordered Breathing and Risk of Stroke in Older Community-Dwelling Men. Sleep 2016, 39, 531–540. [Google Scholar] [Green Version]
- Redline, S. Obstructive Sleep Apnea–Hypopnea and Incident Stroke: The Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2010, 182, 1332–1333. [Google Scholar] [CrossRef]
- Mulgrew, A.T.; Nasvadi, G.; Butt, A.; Cheema, R.; Fox, N.; Fleetham, J.A.; Ryan, C.F.; Cooper, P.; Ayas, N.T. Risk and severity of motor vehicle crashes in patients with obstructive sleep apnoea/hypopnoea. Thorax 2008, 63, 536–541. [Google Scholar] [CrossRef] [Green Version]
- Schlaud, M.; Urschitz, M.S.; Urschitz-Duprat, P.M.; Poets, C.F.; Urschitz-Duprat, P.M. The German study on sleep-disordered breathing in primary school children: Epidemiological approach, representativeness of study sample, and preliminary screening results. Paediatr. Périnat. Epidemiol. 2004, 18, 431–440. [Google Scholar] [CrossRef]
- Mitchell, R.B.; Kelly, J. Behavior, neurocognition and quality-of-life in children with sleep-disordered breathing. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 395–406. [Google Scholar] [CrossRef]
- Sabato, R.; Guido, P.; Salerno, F.; Resta, O.; Spanevello, A.; Barbaro, M.F. Airway inflammation in patients affected by obstructive sleep apnea. Monaldi Arch. Chest Dis. 2006, 65, 102–105. [Google Scholar] [CrossRef]
- Holty, J.-E.C.; Owens, D.K.; Dallas, P.; Shekelle, P.; Qaseem, A.; Starkey, M. Management of Obstructive Sleep Apnea in Adults: A Clinical Practice Guideline from the American College of Physicians. Ann. Intern. Med. 2013, 159, 471–483. [Google Scholar]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Scarlata, S.; Pedone, C.; Curcio, G.; Cortese, L.; Chiurco, D.; Fontana, D.; Calabrese, M.; Fusiello, R.; Abbruzzese, G.; Santangelo, S.; et al. Pre-polysomnographic assessment using the Pittsburgh Sleep Quality Index questionnaire is not useful in identifying people at higher risk for obstructive sleep apnea. J. Med. Screen. 2013, 20, 220–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpagnano, G.E. Exhaled Breath Analysis and Sleep. J. Clin. Sleep Med. 2011, 7, S34–S37. [Google Scholar] [CrossRef] [PubMed]
- Bikov, A.; Hull, J.H.; Kunos, L.; Information, P.E.K.F.C. Exhaled breath analysis, a simple tool to study the pathophysiology of obstructive sleep apnoea. Sleep Med. Rev. 2016, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I.; Barnes, P.J.; Loukides, S.; Sterk, P.J.; Högman, M.; Olin, A.-C.; Amann, A.; Antus, B.; Baraldi, E.; Bikov, A.; et al. A European Respiratory Society technical standard: Exhaled biomarkers in lung disease. Eur. Respir. J. 2017, 49, 1600965. [Google Scholar] [CrossRef]
- Donnelly, L.E.; Barnes, P.J. Expression and Regulation of Inducible Nitric Oxide Synthase from Human Primary Airway Epithelial Cells. Am. J. Respir. Cell Mol. Boil. 2002, 26, 144–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alving, K.; Weitzberg, E.; Lundberg, J.M. Increased amount of nitric oxide in exhaled air of asthmatics. Eur. Respir. J. 1993, 6, 1368–1370. [Google Scholar]
- Kharitonov, S.; Yates, D.; Robbins, R.; Barnes, P.; Logan-Sinclair, R.; Shinebourne, E. Increased nitric oxide in exhaled air of asthmatic patients. Lancet 1994, 343, 133–135. [Google Scholar] [CrossRef]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.-C.; Plummer, A.L.; Taylor, D.R. An Official ATS Clinical Practice Guideline: Interpretation of Exhaled Nitric Oxide Levels (FeNO) for Clinical Applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef]
- Zhang, D.; Xiao, Y.; Luo, J.; Wang, X.; Qiao, Y.; Huang, R. Measurement of fractional exhaled nitric oxide and nasal nitric oxide in male patients with obstructive sleep apnea. Sleep Breath. 2018. [Google Scholar] [CrossRef]
- Przybyłowski, T.; Bielicki, P.; Kumor, M.; Hildebrand, K.; Maskey-Warzechowska, M.; Fangrat, A.; Górska, K.; Korczyński, P.; Chazan, R. Exhaled nitric oxide in patients with obstructive sleep apnea syndrome. Pneumonol. Alergol. Polska 2006, 74, 21–25. [Google Scholar]
- Petrosyan, M.; Perraki, E.; Simoes, D.; Koutsourelakis, I.; Vagiakis, E.; Roussos, C. Exhaled breath markers in patients with obstructive sleep apnoea. Sleep Breath. 2008, 12, 207–215. [Google Scholar] [CrossRef]
- Fortuna, A.; Miralda, R.; Calaf, N.; Gonzalez, M.; Casan, P.; Mayos, M. Airway and alveolar nitric oxide measurements in obstructive sleep apnea syndrome. Respir. Med. 2011, 105, 630–636. [Google Scholar] [CrossRef] [Green Version]
- Chua, A.-P.; Aboussouan, L.S.; Minai, O.A.; Paschke, K.; Laskowski, D.; Dweik, R.A. Long-Term Continuous Positive Airway Pressure Therapy Normalizes High Exhaled Nitric Oxide Levels in Obstructive Sleep Apnea. J. Clin. Sleep Med. 2013, 9, 529–535. [Google Scholar] [CrossRef]
- Duong-Quy, S.; Hua-Huy, T.; Tran-Mai-Thi, H.-T.; Le-Dong, N.-N.; Craig, T.J.; Dinh-Xuan, A.-T. Study of Exhaled Nitric Oxide in Subjects with Suspected Obstructive Sleep Apnea: A Pilot Study in Vietnam. Pulm. Med. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Duarte, R.L.M.; Rabahi, M.F.; Oliveira-E-Sá, T.S.; Magalhães-Da-Silveira, F.J.; Mello, F.C.Q.; Gozal, D. Fractional Exhaled Nitric Oxide Measurements and Screening of Obstructive Sleep Apnea in a Sleep-Laboratory Setting: A Cross-Sectional Study. Lung 2019, 197, 131–137. [Google Scholar] [CrossRef]
- Togores, B.; Agustí, A.G.; Barbé, F. Exhaled Nitric Oxide in Patients with Sleep Apnea. Sleep 1999, 22, 231–235. [Google Scholar]
- Barreto, M.; Montuschi, P.; Evangelisti, M.; Bonafoni, S.; Cecili, M.; Shohreh, R.; Santini, G.; Villa, M.P. Comparison of two exhaled biomarkers in children with and without sleep disordered breathing. Sleep Med. 2018, 45, 83–88. [Google Scholar] [CrossRef]
- Jalil Mirmohammadi, S.; Mehrparvar, A.H.; Safaei, S.; Samimi, E.; Jahromi, M.T. The association between exhaled nitric oxide and sleep apnea: The role of BMI. Respir. Med. 2014, 108, 1229–1233. [Google Scholar] [CrossRef] [Green Version]
- Foresi, A.; Leone, C.; Olivieri, D.; Cremona, G. Alveolar-Derived Exhaled Nitric Oxide Is Reduced in Obstructive Sleep Apnea Syndrome. Chest 2007, 132, 860–867. [Google Scholar] [CrossRef]
- Carpagnano, G.E.; Spanevello, A.; Sabato, R.; DePalo, A.; Turchiarelli, V.; Barbaro, M.P.F. Exhaled pH, exhaled nitric oxide, and induced sputum cellularity in obese patients with obstructive sleep apnea syndrome. Transl. Res. 2008, 151, 45–50. [Google Scholar] [CrossRef]
- Culla, B.; Guida, G.; Brussino, L.; Tribolo, A.; Cicolin, A.; Sciascia, S.; Badiu, I.; Mietta, S.; Bucca, C. Increased oral nitric oxide in obstructive sleep apnoea. Respir. Med. 2010, 104, 316–320. [Google Scholar] [CrossRef] [Green Version]
- DePalo, A.; Carpagnano, G.E.; Spanevello, A.; Sabato, R.; Cagnazzo, M.G.; Gramiccioni, C.; Foschino-Barbaro, M.P. Exhaled NO and iNOS expression in sputum cells of healthy, obese and OSA subjects. J. Int. Med. 2008, 263, 70–78. [Google Scholar] [CrossRef]
- Paulsen, F.P.; Steven, P.; Tsokos, M.; Jungmann, K.; Mueller, A.; Verse, T.; Pirsig, W. Upper Airway Epithelial Structural Changes in Obstructive Sleep-disordered Breathing. Am. J. Respir. Crit. Care Med. 2002, 166, 501–509. [Google Scholar] [CrossRef]
- Boyd, J.H.; Petrof, B.J.; Hamid, Q.; Fraser, R.; Kimoff, R.J. Upper Airway Muscle Inflammation and Denervation Changes in Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2004, 170, 541–546. [Google Scholar] [CrossRef]
- Paraskakis, E.; Vergadi, E.; Chatzimichael, A.; Bush, A. The Role of Flow-Independent Exhaled Nitric Oxide Parameters in the Assessment of Airway Diseases. Curr. Top. Med. Chem. 2016, 16, 1631–1642. [Google Scholar] [CrossRef]
- Hua-Huy, T.; Le-Dong, N.-N.; Duong-Quy, S.; Luchon, L.; Rouhani, S.; Dinh-Xuan, A.T. Increased alveolar nitric oxide concentration is related to nocturnal oxygen desaturation in obstructive sleep apnoea. Nitric Oxide 2015, 45, 27–34. [Google Scholar] [CrossRef]
- Ip, M.S.M.; Lam, B.; Chan, L.-Y.; Zheng, L.; Tsang, K.W.T.; Fung, P.C.W.; Lam, W.-K. Circulating Nitric Oxide Is Suppressed in Obstructive Sleep Apnea and Is Reversed by Nasal Continuous Positive Airway Pressure. Am. J. Respir. Crit. Care Med. 2000, 162, 2166–2171. [Google Scholar] [CrossRef]
- Olopade, C.O.; Christon, J.A.; Zakkar, M.; Swedler, W.I.; Rubinstein, I.; Hua, C.-W.; Scheff, P.A. Exhaled Pentane and Nitric Oxide Levels in Patients with Obstructive Sleep Apnea. Chest 1997, 111, 1500–1504. [Google Scholar] [CrossRef]
- Liu, J.; Li, Z.; Liu, Z.; Zhu, F.; Li, W.; Jiang, H. Exhaled nitric oxide from the central airway and alveoli in OSAHS patients: The potential correlations and clinical implications. Sleep Breath. 2016, 20, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Gut, G.; Tauman, R.; Greenfeld, M.; Armoni-Domany, K.; Sivan, Y. Nasal nitric oxide in sleep-disordered breathing in children. Sleep Breath. 2016, 20, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Kis, A.; Meszaros, M.; Tarnoki, D.L.; Tarnoki, A.D.; Lazar, Z.; Horvath, P.; Kunos, L.; Bikov, A. Exhaled carbon monoxide levels in obstructive sleep apnoea. J. Breath Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Greulich, T.; Hattesohl, A.; Grabisch, A.; Koepke, J.; Schmid, S.; Noeske, S. Detection of obstructive sleep apnoea by an electronic nose. Eur. Respir. J. 2013, 42, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Paget-Brown, A.O.; Ngamtrakulpanit, L.; Smith, A.; Bunyan, D.; Hom, S.; Nguyen, A.; Hunt, J.F. Normative Data for pH of Exhaled Breath Condensate. Chest 2006, 129, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Lloberes, P.; Sánchez-Vidaurre, S.; Ferré, Á.; Cruz, M.J.; Lorente, J.; Sampol, G.; Morell, F.; Muñoz, X. Effect of Continuous Positive Airway Pressure and Upper Airway Surgery on Exhaled Breath Condensate and Serum Biomarkers in Patients with Sleep Apnea. Arch. Bronconeumol. 2014, 50, 422–428. [Google Scholar] [CrossRef]
- Antonopoulou, S.; Loukides, S.; Papatheodorou, G.; Roussos, C.; Alchanatis, M. Airway inflammation in obstructive sleep apnea: Is leptin the missing link? Respir. Med. 2008, 102, 1399–1405. [Google Scholar] [CrossRef] [Green Version]
- Carpagnano, G.E.; Kharitonov, S.A.; Resta, O.; Foschino-Barbaro, M.P.; Gramiccioni, E.; Barnes, P.J. Increased 8-Isoprostane and Interleukin-6 in Breath Condensate of Obstructive Sleep Apnea Patients. Chest 2002, 122, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chongsuvivatwong, V.; Geater, A.; Liu, A. Exhaled breath condensate cytokine level as a diagnostic tool for obstructive sleep apnea syndrome. Sleep Med. 2009, 10, 95–103. [Google Scholar] [CrossRef]
- Carpagnano, G.E.; Spanevello, A.; Sabato, R.; DePalo, A.; Palladino, G.P.; Bergantino, L.; Barbaro, M.P.F. Systemic and airway inflammation in sleep apnea and obesity: The role of ICAM-1 and IL-8. Transl. Res. 2010, 155, 35–43. [Google Scholar] [CrossRef]
- Karamanlı, H.; Özol, D.; Ugur, K.S.; Yıldırım, Z.; Armutçu, F.; Bozkurt, B. Influence of CPAP treatment on airway and systemic inflammation in OSAS patients. Sleep Breath. 2014, 18, 251–256. [Google Scholar] [CrossRef]
- Li, Y.; Chongsuvivatwong, V.; Geater, A.; Liu, A. Are Biomarker Levels a Good Follow-Up Tool for Evaluating Obstructive Sleep Apnea Syndrome Treatments? Respir. Int. Rev. Thorac. Dis. 2008, 76, 317–323. [Google Scholar] [CrossRef]
- Carpagnano, G.E.; Kharitonov, S.A.; Resta, O.; Foschino-Barbaro, M.P.; Gramiccioni, E.; Barnes, P.J. 8-Isoprostane, a Marker of Oxidative Stress, Is Increased in Exhaled Breath Condensate of Patients With Obstructive Sleep Apnea After Night and Is Reduced by Continuous Positive Airway Pressure Therapy. Chest 2003, 124, 1386–1392. [Google Scholar] [CrossRef] [Green Version]
- Fernandez Alvarez, R.; Rubinos Cuadrado, G.; Alonso Arias, R.; Cascon Hernandez, J.A.; Palomo Antequera, B.; Iscar Urrutia, M. Snoring as a Determinant Factor of Oxidative Stress in the Airway of Patients with Obstructive Sleep Apnea. Lung 2016, 194, 469–473. [Google Scholar] [CrossRef]
- Malakasioti, G.; Alexopoulos, E.; Befani, C.; Tanou, K.; Varlami, V.; Ziogas, D. Oxidative stress and inflammatory markers in the exhaled breath condensate of children with OSA. Sleep Breath. 2012, 16, 703–708. [Google Scholar] [CrossRef]
- Vlasic, V.; Trifunovic, J.; Cepelak, I.; Nimac, P.; Topic, R.Z.; Dodig, S. Urates in exhaled breath condensate of children with obstructive sleep apnea. Biochem. Med. 2011, 21, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Goldbart, A.D.; Krishna, J.; Li, R.C.; Serpero, L.D.; Gozal, D. Inflammatory Mediators in Exhaled Breath Condensate of Children with Obstructive Sleep Apnea Syndrome. Chest 2006, 130, 143–148. [Google Scholar] [CrossRef]
- Carpagnano, G.E.; Resta, O.; De Pergola, G.; Sabato, R.; Barbaro, M.P.F. The role of obstructive sleep apnea syndrome and obesity in determining leptin in the exhaled breath condensate. J. Breath Res. 2010, 4, 36003. [Google Scholar] [CrossRef]
- Schulz, S.; Dickschat, J.S. Bacterial volatiles: The smell of small organisms. Nat. Prod. Rep. 2007, 24, 814–842. [Google Scholar] [CrossRef]
- Finamore, P.; Scarlata, S.; Incalzi, R.A. Breath analysis in respiratory diseases: State-of-the-art and future perspectives. Expert Rev. Mol. Diagn. 2019, 19, 47–61. [Google Scholar] [CrossRef]
- Greulich, T.; Fischer, H.; Lubbe, D.; Nell, C.; Baumbach, J.I.; Koehler, U.; Boeselt, T.; Vogelmeier, C.; Koczulla, A.R. Obstructive sleep apnea patients can be identified by ion mobility spectrometry-derived smell prints of different biological materials. J. Breath Res. 2018, 12, 026006. [Google Scholar] [CrossRef]
- Dragonieri, S.; Porcelli, F.; Longobardi, F.; Carratu, P.; Aliani, M.; Ventura, V.A.; Tutino, M.; Quaranta, V.N.; Resta, O.; De Gennaro, G. An electronic nose in the discrimination of obese patients with and without obstructive sleep apnoea. J. Breath Res. 2015, 9, 26005. [Google Scholar] [CrossRef]
- Aoki, T.; Nagaoka, T.; Kobayashi, N.; Kurahashi, M.; Tsuji, C.; Takiguchi, H. Editor’s highlight: Prospective analyses of volatile organic compounds in obstructive sleep apnea patients. Toxicol. Sci. Off. J. Soc. Toxicol. 2017, 156, 362–374. [Google Scholar] [CrossRef]
- Scarlata, S.; Pennazza, G.; Santonico, M.; Santangelo, S.; Bartoli, I.R.; Rivera, C.; Vernile, C.; De Vincentis, A.; Incalzi, R.A. Screening of Obstructive Sleep Apnea Syndrome by Electronic-Nose Analysis of Volatile Organic Compounds. Sci. Rep. 2017, 7, 11938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedek, P.; Lazar, Z.; Bikov, A.; Kunos, L.; Katona, G.; Horváth, I. Exhaled biomarker pattern is altered in children with obstructive sleep apnoea syndrome. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1244–1247. [Google Scholar] [CrossRef]
- Dragonieri, S.; Quaranta, V.N.; Carratu, P.; Ranieri, T.; Resta, O. Exhaled breath profiling in patients with COPD and OSA overlap syndrome: A pilot study. J. Breath Res. 2016, 10, 41001. [Google Scholar] [CrossRef]
- Kunos, L.; Bikov, A.; Lazar, Z.; Korosi, B.Z.; Benedek, P.; Losonczy, G. Evening and morning exhaled volatile compound patterns are different in obstructive sleep apnoea assessed with electronic nose. Sleep Breath. 2015, 19, 247–253. [Google Scholar] [CrossRef]
- Antonelli Incalzi, R.; Pennazza, G.; Scarlata, S.; Santonico, M.; Vernile, C.; Cortese, L. Comorbidity modulates non invasive ventilation-induced changes in breath print of obstructive sleep apnea syndrome patients. Sleep Breath. 2015, 19, 623–630. [Google Scholar] [CrossRef]
- Schwarz, E.I.; Martinez-Lozano Sinues, P.; Bregy, L.; Gaisl, T.; Garcia Gomez, D.; Gaugg, M.T. Effects of CPAP therapy withdrawal on exhaled breath pattern in obstructive sleep apnoea. Thorax 2016, 71, 110–117. [Google Scholar] [CrossRef]
- Scarlata, S.; Pennazza, G.; Santonico, M.; Pedone, C.; Incalzi, R.A. Exhaled breath analysis by electronic nose in respiratory diseases. Expert Rev. Mol. Diagn. 2015, 15, 1–24. [Google Scholar] [CrossRef]
- Pennazza, G.; Santonico, M.; Scarlata, S.; Santangelo, S.; Grasso, S.; Zompanti, A.; Incalzi, R.A. A Non Invasive Sensor System for the Screening of Obstructive Sleep Apnea Syndrome. Proceedings 2017, 1, 426. [Google Scholar] [CrossRef]
- Wang, J.; Yu, W.; Gao, M.; Zhang, F.; Gu, C.; Yu, Y.; Wei, Y. Impact of Obstructive Sleep Apnea Syndrome on Endothelial Function, Arterial Stiffening, and Serum Inflammatory Markers: An Updated Meta-analysis and Metaregression of 18 Studies. J. Am. Hear. Assoc. 2015, 4, e002454. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.R.; Chen, K.; Keaney, J.F. Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J. Biol. Chem. 2002, 277, 6017–6024. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nakamura, H.; Yodoi, J.; Bloom, E.T. Redox regulation of the signaling pathways leading to eNOS phosphorylation. Free Radic. Biol. Med. 2005, 38, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Bikov, A.; Gálffy, G.; Tamasi, L.; Lazar, Z.; Losonczy, G.; Horváth, I. Exhaled breath condensate pH is influenced by respiratory droplet dilution. J. Breath Res. 2012, 6, 46002. [Google Scholar] [CrossRef]
- Finamore, P.; Pedone, C.; Scarlata, S.; Di Paolo, A.; Grasso, S.; Santonico, M.; Pennazza, G.; Antonelli Incalzi, R. Validation of exhaled volatile organic compounds analysis using e-nose as index of COPD severity. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 1441–1448. [Google Scholar] [CrossRef]



| First Author (Year) [Reference] | OSAS | AHI | NO | Device | FeNO ppb | HC | NO ppb | p-Value |
|---|---|---|---|---|---|---|---|---|
| Zhang (2018) [20] | 75 | 28.1 e/h | FeNO (1) | NIOX MINO® | (1) 21.08 (8.79) | 30 | (1) 16.9 (6.86) | 0.02 |
| nNO (2) | 50 mL/s | (2) 487 (115.8) | (2) 413 (73.1) | |||||
| Przybylowski (2006) [21] | 66 | 40.3 e/h | FeNO | CA 45−55 | 23.1 (14.8) | 53 | 16.8 (9.8) | <0.05 |
| Petrosyan (2008) [22] | 26 | 63.7 e/h | FeNO (1) | LR2000 CA 250 mL/s | (1) 7.1 (4.6) | 9 O * 10NO † | (1) 5 (1.1) * (1) 4.2 (1.9) (2) 366 (169) * (2) 539 (264) † (3) 4.8 (1) * (3) 4.7 (1.2) † | <0.05 <0.05 <0.01 NS <0.05 <0.05 |
| nNO (2) | (2) 610 (222) | |||||||
| eCO (3) | (3) 6.4 (2.9) | |||||||
| Olopade (1997) [39] | 16 | 47.7 e/h | FeNO (1) | CA NA | (1) 6.6 (0.8) | 8 | (1) 6.8 (1.3) | NA |
| nNO | ||||||||
| JalilMirmohammadi (2014) [29] | 31 O * | 39.5 e/h | FeNO | NObreath® | 14.1, 3–31 * | 7 | 22.1, 5–58 | NS |
| 16 NO † | 40.1 e/h | 50 mL/s | 15.8, 2–31 † | |||||
| Gut (2016) [41] | 28 | 6.6 e/h | nNO | Eco Medics AG | 867 (371) | 23 | 644 (166) | 0.047 |
| Fortuna (2011) [23] | 30 | NA | FeNO (1) | NIOX | (1) 27.2 (18) | 30 | (1) 16.7 (8) | 0.0006 |
| >15 e/h | CaNO (2) | 50 mL/s | ||||||
| Foresi (2007) [30] | 34 | 31.3 e/h | FeNO | NOA 280 50,120,190, 250 e 300 mL/s | 21.8 (1.9) | 9 | 15.4 (1.7) | NS |
| Duarte (2019) [26] | 199 | 30.1 e/h | FeNO | NIOX MINO® 50 mL/s | 20.2 (14.5) | 30 | 16.9 (10.6) | 0.221 |
| Depalo (2008) [33] | 18 O | 59.1 e/h | FeNO (1) | CA | (1) 23.1 (2.1) | 15 O * | (1) 17.9 (2.1) * | NS |
| iNOS (2) | 45 mL/s | 10NO † | (1) 7.2 (0.6) † | <0.001 | ||||
| Culla (2010) [32] | 39 | NA | FeNO (1) | CA | (1) 23.1, 19−28 | 26 AS * 15 CR † 24 ‡ | (1) 40, 32−50 * (1) 22, 16−32 † (1) 11, 8−14 ‡ (2) 71, 56−91 * (2) 54, 40−73 † (2) 63, 59−73 ‡ | NS NS <0.001 0.015 0.009 <0.001 |
| >10 e/h | oNO (2) | 50 mL/s | (2) 104, 80−135 | |||||
| Carpagnano (2008) [31] | 30 O | 59.1 e/h | FeNO | CA | 31.6 (1.6) | 20 O * | 27.1 (1.8) * | NS |
| 45 mL/s | 10 NO † | 4.8 (0.7) † | <0.001 | |||||
| Duong-Quoy (2015) [25] | 52 | 25.6 e/h | FeNO (1) | FeNO+ 50,100,150,350 mL/s | (1) 16.7 (11.4) (2) 4 (1.7) | 30 | (1) 9.4 (6.6) | 0.003 |
| CaNO (2) | (2) 2.2 (0.7) | 0.001 | ||||||
| Barreto (2018) [28] | 17 CH mild * 17 CH mod/sev † | 2.3 e/h | FeNO | HyPair FENO | 11, 7.9−14.8 * | 20 | 13.5, 8.7−19.9 | NS |
| 8.6 e/h | 50 mL/s | 10, 6.5−16 † | ||||||
| Agustì (1999) [27] | 24 | 55 e/h | FeNO | CA | 22.2 (3) | 7 | 19.7 (3.2) | NS |
| NA | ||||||||
| Chua (2013) [24] | 75 | 40 e/h | FeNO | NIOX MINO® | 13.4 (6.5) | 29 | 6.5 (3.5) | <0.001 |
| 50 mL/s |
| First Author (Year) [Reference] | OSAS | AHI | Molecule | Standards | Value | HC | Value | p-Value |
|---|---|---|---|---|---|---|---|---|
| Carpagnano (2008) [31] | 30 OS | 59.1 e/h | pH | Volume collection X Tidal breathing ✔ Nose clip ✔ Storage ✔ Deaeration ✔ | 7.48 (0.07) | 20 ON * 10 NO † | 7.68 (0.08) * 7.99 (0.03) † | NS <0.01 |
| Carpagnano (2003) [52] | 18 | 59.2 e/h | 8-Isoprost. | Volume collection X Tidal breathing ✔ Nose clip ✔ Storage ✔ | 9.5 (1.9) pg/mL | 12 | 6.7 (0.2) pg/mL | <0.001 |
| Petrosyan (2008) [22] | 26 | 63.7 e/h | pH (1) | Volume collection ✔ Tidal breathing ✔ Nose clip ✔ Deaeration ✔ Storage ✔ | (1) 7.2 (0.69) | 9 O * 10NO † | (1) 7.79 (0.09) * (1) 7.77 (0.05) † (2) 4 (0.2) pg/mL * (2) 5 (1.9) pg/mL † (3) NA * (3) NA † (4) 1.2 (0.9) uM * (4) 0.3 (0.4) uM † | <0.01 <0.01 <0.001 <0.001 <0.001 <0.001 <0.05 <0.01 |
| 8-Isoprost.(2) | (2) 12 (6) pg/mL | |||||||
| Leuk.B4 (3) | (3) 8 (6) pg/mL | |||||||
| H2O2 (4) | (4) 5.8 (8.9) uM | |||||||
| Vlasic (2011) ▲ [55] | 17 | 3.54 e/h | Urates | Volume collection ✔ Tidal breathing ✔ Nose clip ✔ Storage X | 86, 28−113 µmol/L | 12 | 31, 23−42 µmol/L | 0.046 |
| Malakasioti (2012) ▲ [54] | 12 Mo-S (1) 22 Mild (2) | 13.6 e/h 2.8 e/h | log(H2O2) | Volume collection ✔ Tidal breathing ✔ Nose clip ✔ Storage ✔ | 0.4 (1.1) −0.9 (1.3) | 16 | −1.2 (1.2) | (1vs3) 0.003 (1vs2) 0.015 |
| Li (2009) [48] | 22 Mild * 22 Mo † 24 S ‡ | 14.1 e/h 29.7 e/h 70.1 e/h | 8-Isoprost.(1) IL−6 (2) TNF−α (3) IL−10 (4) | Volume collection X Tidal breathing ✔ Nose clip ✔ Storage ✔ | (1) 15.5 (2) pg/mL * (1) 18.8 (2) pg/mL † (1) 21.8 (2) pg/mL ‡ (2) 8.4 (1) pg/mL * (2) 13.9 (2) pg/mL † (2) 15.5 (2) pg/mL ‡ (3) 96.1 (8) pg/mL * (3) 116 (11) pg/mL † (3) 128.2 (8) pg/mL ‡ (4) 48.2 (6) pg/mL * (4) 31.2 (5) pg/mL † (4) 24 (4) pg/mL ‡ | 22 HNS ҂ 10 HS ‖ | (1) 12.6 (2) pg/mL ҂ (1) 16.8 (2) pg/mL ‖ (2) 6.8 (1) pg/mL ҂ (2) 10.9 (2) pg/mL ‖ (3) 83.7 (4) pg/mL ҂ (3) 97 (6) pg/mL ‖ (4) 56.8 (7) pg/mL ҂ (4) 38.6 (7) pg/mL ‖ | (1) <0.001 (2) <0.001 (3) <0.001 (4) <0.001 |
| Carpagnano (2002) [47] | 18 | 59.2 e/h | 8-Isoprost.(1) IL-6 (2) | Volume collection X Tidal breathing ✔ Nose clip X Storage ✔ | (1) 7.4 (0.7) pg/mL (2) 8.7 (0.3) pg/mL | 10 ON * 15 NO † | (1) 5 (0.3) pg/mL * (1) 4.5 (1) pg/mL † (2) 2.1(0.2) pg/m *l (2) 1.6(0.1) pg/mL † | 0.4 <0.005 <0.05 <0.001 |
| Goldbart (2006) ▲ [56] | 29 Mild * 21 Mo-S † | < 5 e/h > 5 e/h | Leuk.B4 (1) LeukTC4/D4/E4 (2) PGE2 (3) | Volume collection X Tidal breathing ✔ Nose clip X Storage ✔ | (1) 66.4 (4) pg/mL * (1) 97.6 (6) pg/mL † (2) 27.6 (8) pg/mL * (2) 45.1 (11) pg/mL † (3) ≈ 29 pg/mL * (3) ≈ 35 pg/mL † | NA | (1) 27.8 (4) pg/mL (2) 15.7 (8) pg/mL (3) ≈ 19 pg/mL | <0.001 <0.001 NS |
| Carpagnano (S 2010) [57] | 36 OS * 28 NOS † | 57.6 e/h 40.8 e/h | Leptin | Volume collection X Tidal breathing ✔ Nose clip ✔ Storage ✔ | 5.12, 3.8−6.6 ng/mL * 4.1, 3.9−5.2 ng/mL † | 24 ON ‡ 20 NO ҂ | 4.2, 3.6−5 ng/mL ‡ 3.2, 2.4−4 ng/mL ҂ | <0.05 |
| Barreto (2018) ▲ [28] | 17 CH mild * 17 CH Mo-S † | 2.3 e/h 8.6 e/h | 8-Isoprost. | Volume collection X Tidal breathing ✔ Nose clip ✔ Storage ✔ | 45, 30−88 pg/mL * 52, 39−130 pg/mL † | 20 | 19.2, 12−32 pg/mL | <0.01 <0.01 |
| Antonopoulou (2008) [46] | 45 | 39 e/h | pH (1) 8-Isoprost.(2) IL-6 (3) TNF-α (4) | Volume collection X Tidal breathing ✔ Nose clip? Storage ✔ Deaeration ? | (1) 7.44 (0.2) (2) 30.5 (19) pg/mL (3) 0.53 (0.3) pg/mL (4) 1.4 (0.9) pg/mL | 25 | (1) 7.46 (0.1) (2) 12 (3) pg/mL (3) 0.21 (0) pg/mL (4) 0.6 (0.3)pg/mL | 0.0009 <0.0001 0.03 0.0002 |
| Carpagnano (J 2010) [49] | 12 OS * 10 NO † | 48.8 e/h | IL-8 (1) ICAM-1 (2) | Volume collection X Tidal breathing ✔ Nose clip ✔ Storage ✔ | (1) 17.5 (2) pg/mL * (1) 14.8 (1.9) pg/mL † (2) 100 (3.6) pg/mL * (2) 88.6 (3.9) pg/mL † | 10 ON 8 NO | (1) 17 (0.7) pg/mL * (1) 7 (0.5) pg/mL † (2) 93 (2.6) pg/mL * (2) 51 (1.2) pg/mL † | NS <0.001 NS <0.001 |
| Karamanli (2014) [50] | 35 C-PAP | 3.8 vs. 45.6 | 8−Isoprost. (1) IL-6 (2) TNF-α (3) Peroxynitr.(4) | Volume collection X Tidal breathing ✔ Nose clip ✔ Storage ✔ | (1) 3 vs. 5.7 pg/mL (2) 0.3 vs. 1.1 pg/mL (3) 26.8 vs. 29 pg/mL (4) 4.6 vs. 17.3 pg/mL | - | - | 0.027 <0.001 <0.001 0.037 |
| Li (2008) [51] | 33 C-PAP† 28 UNT ‡ 2 OrAp ⁕ 5 SURG ҂ 22 HC | 24.7 vs. 45.7 32.5 vs. 31.4 12.9 vs. 38.6 28.8 vs. 32.7 | 8−Isoprost. (1) IL−6 (2) TNF−α (3) IL-10 (4) | Volume collection X Tidal breathing ✔ Nose clip ✔ Storage ✔ | (1) 15 vs. 20 pg/mL † (1) 17 vs. 17 pg/mL ‡(1) 12 vs. 18 pg/mL * (1) 13 vs. 20 pg/mL ҂ (2) 10 vs. 14 pg/mL † (2) 11 vs. 11 pg/mL ‡ (2) 8 vs. 11 pg/mL * (2) 9 vs. 13 pg/mL ҂ | (3) 97 vs. 118 pg/mL † (3) 108 vs. 108 pg/mL ‡ (3) 105 vs. 119 pg/mL * (3) 88 vs. 117 pg/mL ҂ (4) 42 vs. 21 pg/mL † (4) 38 vs. 38 pg/mL ‡ (4) 37 vs. 35 pg/mL * (4) 50 vs. 31 pg/mL ҂ | Unknown | |
| First Author (Year) [Reference] | OSAS | AHI | Device | Standards | Controls | Discriminative capacity | p-Value |
|---|---|---|---|---|---|---|---|
| Greulich (2013) [43] | 40 | 33.6 e/h | E-nose (Cyranose320) Disposable bags | Internal cross-validation ✔ External validation set ✔ | 20 | AUROC 0.85 (95%CI 0.74−0.96) | - |
| Dragonieri (2016) [65] | 13 (6 validation set) | 44.8 e/h | E-nose (Cyranose320) Disposable bags | Internal cross-validation ✔ External validation set ✔ | 15 COPD (6 validation set) 13 OVS. (6 validation set) | AUROC OSAS vs. OVS.: 1 AUROC OSAS vs. COPD: 0.83 | <0.001 <0.01 |
| Kunos (2015) [66] | 17 OSAS 9 habitual snorers | 29.8 e/h | E-nose Mylar bags | Internal cross-validation ✔ External validation set X | 10 | Accuracy OSAS vs. HC (morning): 77% | <0.001 |
| Antonelli Incalzi (2015) [67] | 50 C-PAP | 41.8 e/h | E-nose (BIONOTE) Pneumopipe + TenaxGR | Internal cross-validation ✔ External validation set X | 29 consonant change 21 discordant change | ||
| Dragonieri (2015) [61] | 19 OS | 27.8 e/h | GC-MS (1) E-nose (2) (Cyranose320) Tedlar bags Carboxen and Carbopack cartridges | Internal cross-validation ✔ External validation set X | 14 ON 20 NO | (1) Accuracy OS vs. ON: 91% (2) AUC OS vs. NO: 1 (2) AUC OS vs. ON:0.7 | |
| Scarlata (2017) [63] | 20 hypo 20 non-hypo | 13.6 e/h 2.8 e/h | E-nose (BIONOTE) Pneumopipe + TenaxGR | Internal cross-validation ✔ External validation set X | 56 NO 20 non-hypo COPD 20 ON | Accuracy OSA vs. HC: 0.99 Accuracy OSAS vs. COPD: 0.75 | |
| Benedek (2013) [64] | 18 | 2 e/h | E-nose (Cyranose320) Mylar bags | Internal cross-validation X External validation set X | 10 habitual snoring | AUROC: 0.84 | <0.003 |
| Greulich (2018) [60] | 15 | 26 e/h | Ion mobility mass spectrometry (1) E-nose (Cyranose320) (2) | Internal cross-validation ✔ External validation set X | 15 | (1) AUROC 0.79 (2) AUROC 0.9 | 0.004 <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finamore, P.; Scarlata, S.; Cardaci, V.; Antonelli Incalzi, R. Exhaled Breath Analysis in Obstructive Sleep Apnea Syndrome: A Review of the Literature. Medicina 2019, 55, 538. https://doi.org/10.3390/medicina55090538
Finamore P, Scarlata S, Cardaci V, Antonelli Incalzi R. Exhaled Breath Analysis in Obstructive Sleep Apnea Syndrome: A Review of the Literature. Medicina. 2019; 55(9):538. https://doi.org/10.3390/medicina55090538
Chicago/Turabian StyleFinamore, Panaiotis, Simone Scarlata, Vittorio Cardaci, and Raffaele Antonelli Incalzi. 2019. "Exhaled Breath Analysis in Obstructive Sleep Apnea Syndrome: A Review of the Literature" Medicina 55, no. 9: 538. https://doi.org/10.3390/medicina55090538
APA StyleFinamore, P., Scarlata, S., Cardaci, V., & Antonelli Incalzi, R. (2019). Exhaled Breath Analysis in Obstructive Sleep Apnea Syndrome: A Review of the Literature. Medicina, 55(9), 538. https://doi.org/10.3390/medicina55090538






