Organised Versus Opportunistic Cervical Cancer Screening in Urban and Rural Regions of Lithuania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Sample
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef]
- Arbyn, M.; Raifu, A.O.; Weiderpass, E.; Bray, F.; Anttila, A. Trends of cervical cancer mortality in the member states of the European Union. Eur. J. Cancer 2009, 45, 2640–2648. [Google Scholar] [CrossRef]
- Arbyn, M.; Anttila, A.; Jordan, J.; Ronco, G.; Schenck, U.; Segnan, N.; Wiener, H.; Herbert, A.; von Karsa, L. European Guidelines for Quality Assurance in Cervical Cancer Screening. Second edition—Summary document. Ann. Oncol. 2010, 21, 448–458. [Google Scholar] [CrossRef]
- Altobelli, E.; Lattanzi, A. Cervical carcinoma in the European Union: An update on disease burden, screening program state of activation, and coverage as of March 2014. Int. J. Gynecol. Cancer 2015, 25, 474–483. [Google Scholar] [CrossRef]
- Ferroni, E.; Camilloni, L.; Jimenez, B.; Furnari, G.; Borgia, P.; Guasticchi, G.; Giorgi Rossi, P.; Methods to increase participation Working Group. How to increase uptake in oncologic screening: A systematic review of studies comparing population-based screening programs and spontaneous access. Prev. Med. 2012, 55, 587–596. [Google Scholar] [CrossRef]
- Salo, H.; Nieminen, P.; Kilpi, T.; Auranen, K.; Leino, T.; Vänskä, S.; Tiihonen, P.; Lehtinen, M.; Anttila, A. Divergent coverage, frequency and costs of organised and opportunistic Pap testing in Finland. Int. J. Cancer 2014, 135, 204–213. [Google Scholar] [CrossRef]
- Serraino, D.; Gini, A.; Taborelli, M.; Ronco, G.; Giorgi-Rossi, P.; Zappa, M.; Crocetti, E.; Franzo, A.; Falcini, F.; Visioli, C.B.; et al. Changes in cervical cancer incidence following the introduction of organized screening in Italy. Prev. Med. 2015, 75, 56–63. [Google Scholar] [CrossRef]
- Oscarsson, M.G.; Benzein, E.G.; Wijma, B.E. Reasons for non-attendance at cervical screening as reported by non-attendees in Sweden. J. Psychosom. Obstet. Gynecol. 2008, 29, 23–31. [Google Scholar] [CrossRef]
- Waller, J.; Bartoszek, M.; Marlow, L.; Wardle, J. Barriers to cervical cancer screening attendance in England: A population-based survey. J. Med Screen. 2009, 16, 199–204. [Google Scholar] [CrossRef]
- Bosgraaf, R.P.; Ketelaars, P.J.; Verhoef, V.M.; Massuger, L.F.; Meijer, C.J.; Melchers, W.J.; Bekkers, R.L. Reasons for non-attendance to cervical screening and preferences for HPV self-sampling in Dutch women. Prev. Med. 2014, 64, 108–113. [Google Scholar] [CrossRef]
- The Order of the Lithuanian Minister of Health No V-482. Valstybes Zinios, 3 July 2004, No. 104-3856. Available online: https://www.e-tar.lt/portal/en/legalActEditions/TAR.8FD6BCF64FD4 (accessed on 25 June 2019).
- Anttila, A.; Ronco, G.; Working Group on the Registration and Monitoring of Cervical Cancer Screening Programmes in the European Union; within the European Network for Information on Cancer (EUNICE). Description of the national situation of cervical cancer screening in the member states of the European Union. Eur. J. Cancer 2009, 45, 2685–2708. [Google Scholar]
- Solomon, D.; Davey, D.; Kurman, R.; Moriarty, A.; O’Connor, D.; Prey, M.; Raab, S.; Sherman, M.; Wilbur, D.; Wright, T., Jr.; et al. Forum Group Members; Bethesda 2001 Workshop. The 2001 Bethesda System: Terminology for reporting results of cervical cytology. Jama 2002, 287, 2114–2119. [Google Scholar] [CrossRef]
- Everett, T.; Bryant, A.; Griffin, M.F.; Martin-Hirsch, P.P.; Forbes, C.A.; Jepson, R.G. Interventions targeted at women to encourage the uptake of cervical screening. Cochrane Database Syst. Rev. 2011, 5, CD002834. [Google Scholar] [CrossRef]
- Kurtinaitienė, R.; Rimienė, J.; Labanauskaitė, I.; Lipunova, N.; Smailytė, G. Increasing attendance in a cervical cancer screening programme by personal invitation: Experience of a Lithuanian primary health care centre. Acta Med. Litu. 2016, 23, 180–184. [Google Scholar] [CrossRef]
- Eaker, S.; Adami, H.-O.; Granath, F.; Wilander, E.; Sparén, P. A large population-based randomized controlled trial to increase attendance at screening for cervical cancer. Cancer Epidemiol. Biomark. Prev. 2004, 13, 346–354. [Google Scholar]
- Camilloni, L.; Ferroni, E.; Cendales, B.J.; Pezzarossi, A.; Furnari, G.; Borgia, P.; Guasticchi, G.; Giorgi Rossi, P.; Methods to increase participation Working Group. Methods to increase participation in organised screening programs: A systematic review. BMC Public Health 2013, 13, 464. [Google Scholar] [CrossRef]
- Virtanen, A.; Anttila, A.; Luostarinen, T.; Malila, N.; Nieminen, P. Improving cervical cancer screening attendance in Finland. Int. J. Cancer 2015, 136, E677–E684. [Google Scholar] [CrossRef]
- Broberg, G.; Jonasson, J.M.; Ellis, J.; Gyrd-Hansen, D.; Anjemark, B.; Glantz, A.; Söderberg, L.; Ryd, M.L.; Holtenman, M.; Milsom, I.; et al. Increasing participation in cervical cancer screening: Telephone contact with long-term non-attendees in Sweden. Results from RACOMIP, a randomized controlled trial. Int. J. Cancer 2013, 133, 164–171. [Google Scholar] [CrossRef]
- Haguenoer, K.; Sengchanh, S.; Gaudy-Graffin, C.; Boyard, J.; Fontenay, R.; Marret, H.; Goudeau, A.; De Laroche, N.P.; Rusch, E.; Giraudeau, B. Vaginal self-sampling is a cost-effective way to increase participation in a cervical cancer screening programme: A randomised trial. Br. J. Cancer 2014, 111, 2187–2196. [Google Scholar] [CrossRef]
- Espinas, J.A.; Aliste, L.; Fernández, E.; Argimon, J.M.; Tresserras, R.; Borras, J.M. Narrowing the Equity Gap: The Impact of Organized versus Opportunistic Cancer Screening in Catalonia (Spain). J. Med Screen. 2011, 18, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Radde, K.; Gottschalk, A.; Bussas, U.; Schülein, S.; Schriefer, D.; Seifert, U.; Neumann, A.; Kaiser, M.; Blettner, M.; Klug, S.J. Invitation to cervical cancer screening does increase participation in Germany: Results from the MARZY study. Int. J. Cancer 2016, 139, 1018–1030. [Google Scholar] [CrossRef] [Green Version]
- Lancucki, L.; Fender, M.; Koukari, A.; Mai, V.; Onysko, J.; Ronco, G.; Patnick, J.; Lynge, E.; Mancini, E.; Tornberg, S.; et al. A Fall-off in Cervical Screening Coverage of Younger Women in Developed Countries. J. Med. Screen. 2010, 17, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Albrow, R.; Blomberg, K.; Kitchener, H.; Brabin, L.; Patnick, J.; Tishelman, C.; Törnberg, S.; Sparén, P.; Widmark, C. Interventions to improve cervical cancer screening uptake amongst young women: A systematic review. Acta Oncol. 2014, 53, 445–451. [Google Scholar] [CrossRef]
- Virtanen, A.; Nieminen, P.; Luostarinen, T.; Anttila, A. Self-sample HPV Tests as an Intervention for Nonattendees of Cervical Cancer Screening in Finland: A Randomized Trial. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1960–1969. [Google Scholar] [CrossRef]
- Petkeviciene, J.; Ivanauskiene, R.; Klumbiene, J. Sociodemographic and lifestyle determinants of non-attendance for cervical cancer screening in Lithuania, 2006–2014. Public Health 2018, 156, 79–86. [Google Scholar] [CrossRef]
- Hansen, B.T.; Hukkelberg, S.S.; Haldorsen, T.; Eriksen, T.; Skare, G.B.; Nygård, M. Factors associated with non-attendance, opportunistic attendance and reminded attendance to cervical screening in an organized screening program: A cross-sectional study of 12,058 Norwegian women. BMC Public Health 2011, 11, 264. [Google Scholar] [CrossRef]
- Virtanen, A.; Nieminen, P.; Niironen, M.; Luostarinen, T.; Anttila, A. Self-sampling experiences among non-attendees to cervical screening. Gynecol. Oncol. 2014, 135, 487–494. [Google Scholar] [CrossRef]
- Everatt, R.; Intaitė, B. Trends in cervical cancer mortality rates in Lithuania, 1987–2016. Cancer Epidemiol. 2018, 57, 85–89. [Google Scholar] [CrossRef]
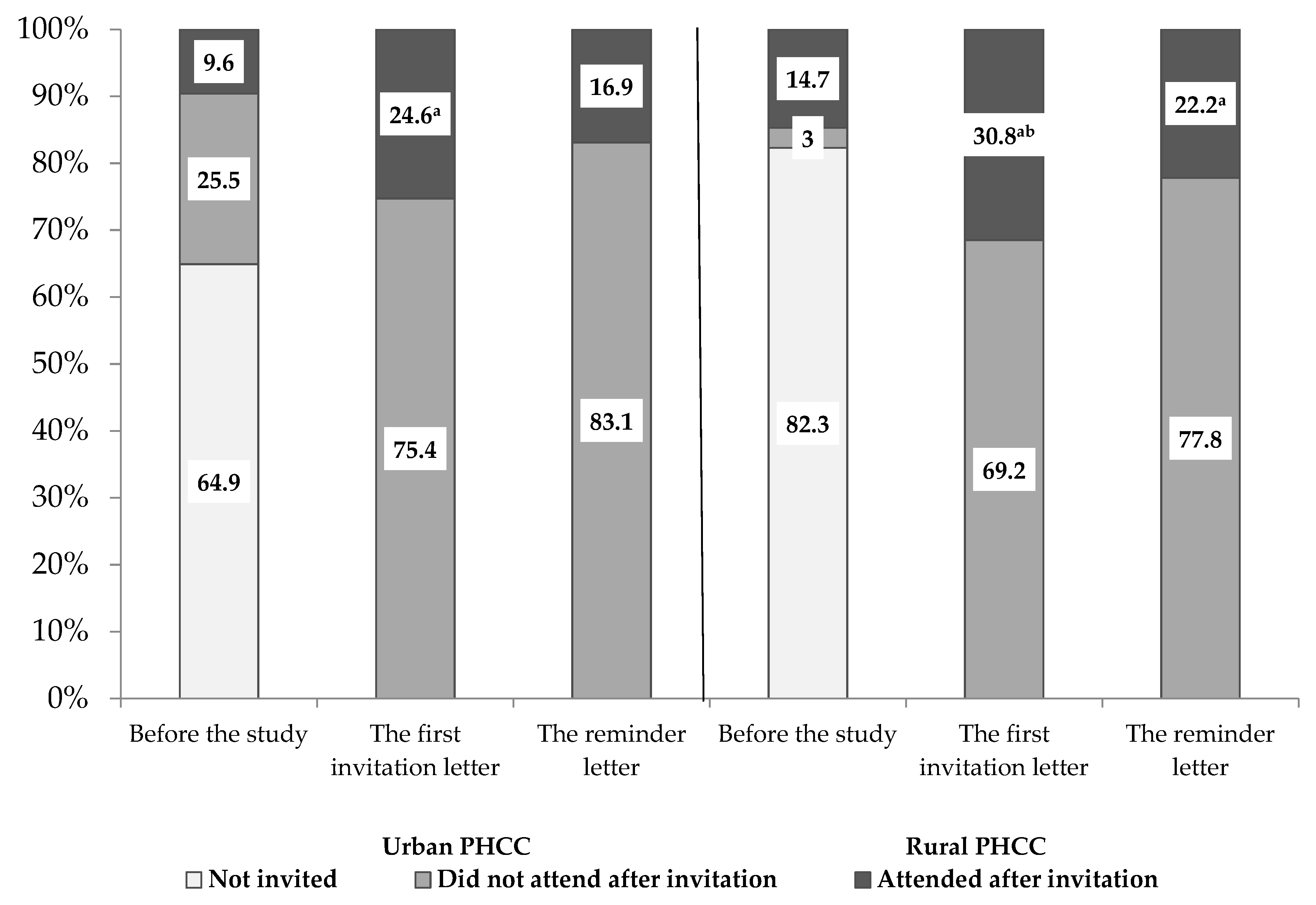
| Urban PHCC | Rural PHCC | |||||||
|---|---|---|---|---|---|---|---|---|
| The First Invitation Letter | The Reminder Letter | The First Invitation Letter | The Reminder Letter | |||||
| Invited | Attended | Invited | Attended | Invited | Attended | Invited | Attended | |
| N | N (%) | N | N (%) | N | N (%) | N | N (%) | |
| Age (years) | ||||||||
| 25–34 | 350 | 66 (18.9) * | 245 | 48 (19.6) | 428 | 99 (23.1) * | 221 | 47 (21.3) |
| 35–44 | 438 | 124 (28.3) | 281 | 47 (16.7) | 401 | 143 (35.7) | 194 | 54 (27.8) |
| 45–54 | 546 | 129 (23.6) | 382 | 61 (16.0) | 619 | 202 (32.6) | 328 | 72 (22.0) |
| 55–60 | 257 | 72 (28.0) | 134 | 20 (14.9) | 395 | 123 (31.1) | 186 | 33 (17.7) |
| Total | 1591 | 391 (24.6) | 1042 | 176 (16.9) | 1843 | 567 (30.8) ** | 929 | 206 (22.2) |
| Pap smear test results | ||||||||
| Unknown result a | 20 (5.1) | 4 (2.2) | 34 (6.0) | 5 (2.4) | ||||
| Normal | 270 (69.1) | 133 (75.6) | 353 (62.3) | 152 (73.8) | ||||
| Abnormal b | 101 (25.8) | 39 (22.2) | 180 (31.7) *** | 49 (23.8) | ||||
| Variable | Participation Rate | Abnormal Pap Smear Tests | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| The First Invitation Letter | The Reminder Letter | The First Invitation Letter | The Reminder Letter | |||||||||
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Study area | ||||||||||||
| Urban | 1 | 1 | 1 | 1 | ||||||||
| Rural | 1.39 | 1.19–1.61 | <0.001 | 1.44 | 1.15–1.80 | 0.002 | 1.39 | 1.04–1.85 | 0.027 | 1.10 | 0.68–1.78 | 0.710 |
| Age (years) | ||||||||||||
| 25–34 | 1 | 1 | 1 | 1 | ||||||||
| 35–44 | 1.78 | 1.42–2.23 | <0.001 | 1.08 | 0.79–1.48 | 0.630 | 1.40 | 0.92–2.13 | 0.117 | 1.59 | 0.80–3.16 | 0.190 |
| 45–54 | 1.49 | 1.20–1.84 | <0.001 | 0.90 | 0.67–1.21 | 0.501 | 0.91 | 0.60–1.37 | 0.644 | 1.63 | 0.85–3.13 | 0.141 |
| 55–60 | 1.56 | 1.23–1.99 | <0.001 | 0.75 | 1.15–1.80 | 0.122 | 0.77 | 0.48–1.23 | 0.266 | 1.06 | 0.44–2.51 | 0.904 |
| Coverage of Cervical Cancer Screening | |||
|---|---|---|---|
| N | % (95% CI) | Increase % | |
| Urban PHCC (N = 1760) | |||
| Before the study (invitation by a family doctor) | 169 | 9.6 (8.2–11.0) | - |
| 1st invitation letter | 560 | 31.8 (29.6–34.0) | +231.3 |
| 2nd invitation letter | 736 | 41.8 (39.5–44.1) | +31.4 |
| Total increase | +335.4 | ||
| Rural PHCC (N = 2160) | |||
| Before the study (invitation by a family doctor) | 317 | 14.7 (13.2–16.2) | - |
| 1st invitation letter | 884 | 40.9 (39.9–42.0) | +178.2 |
| 2nd invitation letter | 1090 | 50.5 (49.4–51.5) | +23.5 |
| Total increase | +243.5 | ||
| Urban PHCC (N = 55) | Rural PHCC (N = 38) | |
|---|---|---|
| Attitudinal and emotional barriers | % | % |
| Intends to attend for a Pap smear test but faces various obstacles | 52.7 | 36.1 |
| Worries that a Pap smear test might be unpleasant | 34.6 | 36.8 |
| Believes that she is not at risk of cervical cancer | 21.2 | 25.0 |
| Is afraid to be diagnosed with cervical cancer | 20.8 | 27.8 |
| Negative experience during a Pap test in the past | 20.0 | 8.3 |
| Feels healthy and sees no need for a Pap test | 15.4 | 22.2 |
| Sexually inactive for a long time and sees no need to attend | 15.1 | 16.2 |
| Doesn’t trust the efficiency of a Pap test | 9.3 | 13.5 |
| Practical barriers | % | % |
| The long waiting-time for doctor’s appointment | 49.1 | 34.3 |
| Lack of time due to long working hours or family duties | 41.5 | 29.7 |
| Has a regular gynaecological examination | 24.5 | 24.3 |
| Inconvenient appointment time | 23.6 | 10.8 |
| A family doctor doesn’t invite to participate in the screening | 11.3 | 24.3 |
| Has never heard of a Pap test | 7.5 | 16.2 |
| Has never been invited to have a Pap test | 7.5 | 22.2 |
| The clinic is too far away from women’s living place | 1.9 | 22.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulauskiene, J.; Ivanauskiene, R.; Skrodeniene, E.; Petkeviciene, J. Organised Versus Opportunistic Cervical Cancer Screening in Urban and Rural Regions of Lithuania. Medicina 2019, 55, 570. https://doi.org/10.3390/medicina55090570
Paulauskiene J, Ivanauskiene R, Skrodeniene E, Petkeviciene J. Organised Versus Opportunistic Cervical Cancer Screening in Urban and Rural Regions of Lithuania. Medicina. 2019; 55(9):570. https://doi.org/10.3390/medicina55090570
Chicago/Turabian StylePaulauskiene, Justina, Rugile Ivanauskiene, Erika Skrodeniene, and Janina Petkeviciene. 2019. "Organised Versus Opportunistic Cervical Cancer Screening in Urban and Rural Regions of Lithuania" Medicina 55, no. 9: 570. https://doi.org/10.3390/medicina55090570





