Pain and Function in the Runner a Ten (din) uous Link
Abstract
:1. Introduction
2. Case Report
2.1. Case History
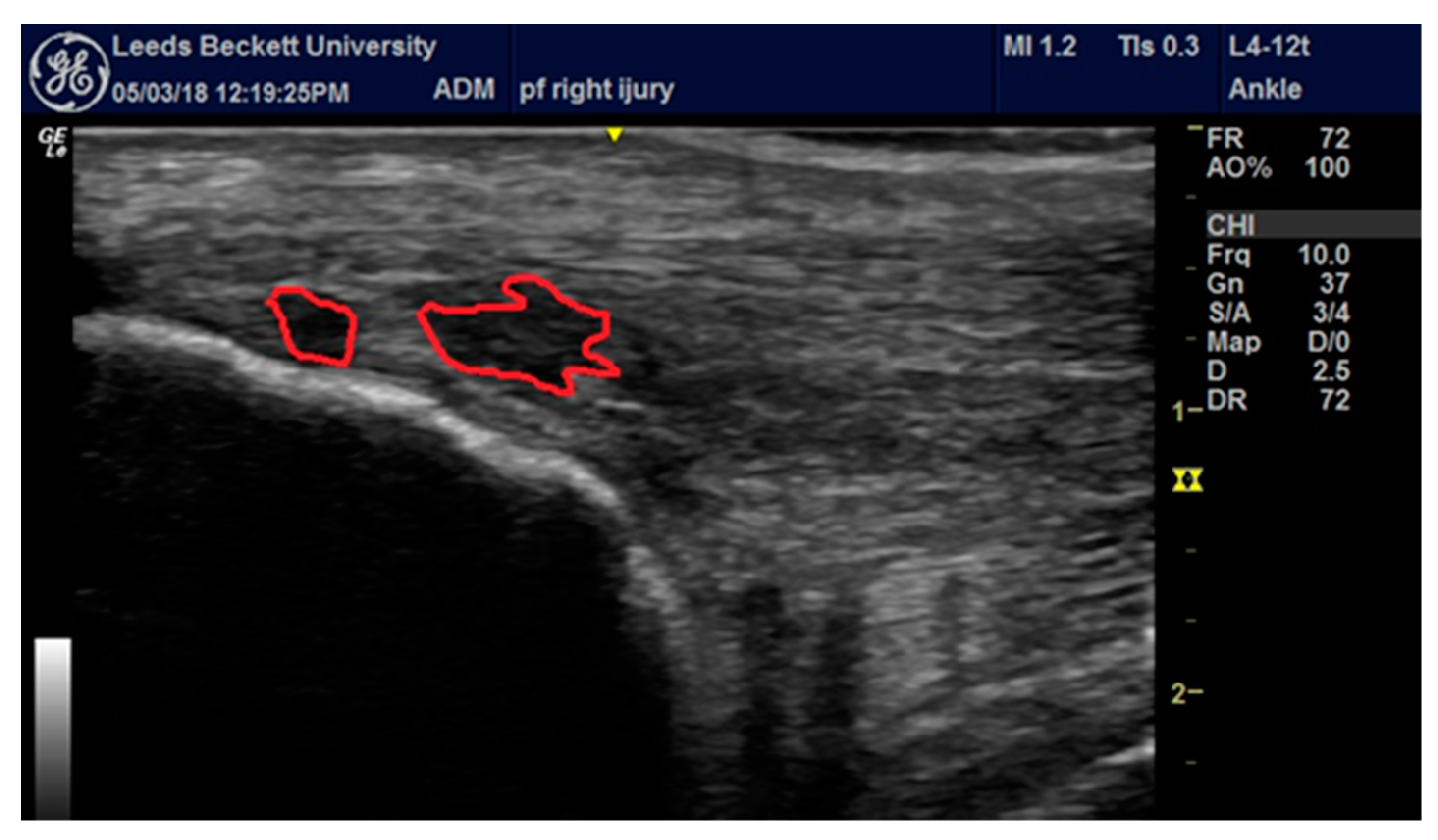
2.2. Assessment
2.3. Intervention
2.4. Outcomes
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Francis, P.; Whatman, C.; Sheerin, K.; Hume, P.; Johnson, M.I. The proportion of lower limb running injuries by gender, anatomical location and specific pathology: A systematic review. J. Sports Sci. Med. 2019, 18, 21–31. [Google Scholar] [PubMed]
- Lopes, A.D.; Hespanhol Junior, L.C.; Yeung, S.S.; Costa, L.O. What are the main running-related musculoskeletal injuries? A Systematic Review. Sports Med. 2012, 42, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L.; Stasinopoulos, D.; Brismee, J.M. Insertional and mid-substance Achilles tendinopathies: Eccentric training is not for everyone-updated evidence of non-surgical management. J. Man. Manip. Ther. 2018, 26, 119–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, J.L.; Purdam, C.R. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br. J. Sports Med. 2009, 43, 409–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, J.L.; Rio, E.; Purdam, C.R.; Docking, S.I. Revisiting the continuum model of tendon pathology: What is its merit in clinical practice and research? Br. J. Sports Med. 2016, 50, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.M.; Forster, B.B.; Robinson, J.; Cheong, Y.; Louis, L.; Maclean, L.; Taunton, J.E. Are ultrasound and magnetic resonance imaging of value in assessment of Achilles tendon disorders? A two year prospective study. Br. J. Sports Med. 2003, 37, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Silbernagel, K.G.; Thomee, R.; Eriksson, B.I.; Karlsson, J. Continued sports activity, using a pain-monitoring model, during rehabilitation in patients with Achilles tendinopathy: A randomized controlled study. Am. J. Sports Med. 2007, 35, 897–906. [Google Scholar] [CrossRef]
- Moseley, G.L.; Butler, D.S. Fifteen years of explaining pain: The past, present, and future. J. Pain 2015, 16, 807–813. [Google Scholar] [CrossRef] [Green Version]
- Bussin, E.R.; Cairns, B.; Bovard, J.; Scott, A. Randomised controlled trial evaluating the short-term analgesic effect of topical diclofenac on chronic Achilles tendon pain: A pilot study. BMJ Open 2017, 7, e015126. [Google Scholar] [CrossRef]
- Humphrey, J.; Chan, O.; Crisp, T.; Padhiar, N.; Morrissey, D.; Twycross-Lewis, R.; King, J.; Maffulli, N. The short-term effects of high volume image guided injections in resistant non-insertional Achilles tendinopathy. J. Sci. Med. Sport 2010, 13, 295–298. [Google Scholar] [CrossRef]
- Rio, E.; Kidgell, D.; Purdam, C.; Gaida, J.; Moseley, G.L.; Pearce, A.J.; Cook, J. Isometric exercise induces analgesia and reduces inhibition in patellar tendinopathy. Br. J. Sports Med. 2015, 49, 1277–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiegerinck, J.I.; Kerkhoffs, G.M.; van Sterkenburg, M.N.; Sierevelt, I.N.; van Dijk, C.N. Treatment for insertional Achilles tendinopathy: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Paquette, M.R.; Peel, S.A.; Smith, R.E.; Temme, M.; Dwyer, J.N. The impact of different cross-training modalities on performance and injury-related variables in high school cross country runners. J. Strength Cond. Res. 2018, 32, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Beyer, R.; Kongsgaard, M.; Hougs Kjaer, B.; Ohlenschlaeger, T.; Kjaer, M.; Magnusson, S.P. Heavy slow resistance versus eccentric training as treatment for achilles tendinopathy: A randomized controlled trial. Am. J. Sports Med. 2015, 43, 1704–1711. [Google Scholar] [CrossRef]
- Alfredson, H.; Pietila, T.; Jonsson, P.; Lorentzon, R. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am. J. Sports Med. 1998, 26, 360–366. [Google Scholar] [CrossRef] [Green Version]
- Woodley, B.L.; Newsham-West, R.J.; Baxter, G.D. Chronic tendinopathy: Effectiveness of eccentric exercise. Br. J. Sports Med. 2007, 41, 188–198. [Google Scholar] [CrossRef] [Green Version]
- Jonsson, P.; Alfredson, H.; Sunding, K.; Fahlstrom, M.; Cook, J. New regimen for eccentric calf-muscle training in patients with chronic insertional Achilles tendinopathy: Results of a pilot study. Br. J. Sports Med. 2008, 42, 746–749. [Google Scholar] [CrossRef]
- Rio, E.; Kidgell, D.; Moseley, G.L.; Gaida, J.; Docking, S.; Purdam, C.; Cook, J. Tendon neuroplastic training: Changing the way we think about tendon rehabilitation: A narrative review. Br. J. Sports Med. 2016, 50, 209–215. [Google Scholar] [CrossRef]
- O’Neill, S.; Watson, P.J.; Barry, S. Why are eccentric exercises effective for achilles tendinopathy? Int. J. Sports Phys. Ther. 2015, 10, 552–562. [Google Scholar]
- Robinson, J.M.; Cook, J.L.; Purdam, C.; Visentini, P.J.; Ross, J.; Maffulli, N.; Taunton, J.E.; Khan, K.M. The VISA-A questionnaire: A valid and reliable index of the clinical severity of Achilles tendinopathy. Br. J. Sports Med. 2001, 35, 335–341. [Google Scholar] [CrossRef]
- Martin, R.; Burdett, R.; Irrgang, J. Development of the foot and ankle disability index (FADI). J. Orthop. Sports Phys. Ther. 1999, 29, A32–A33. [Google Scholar]
- Hale, S.A.; Hertel, J. Reliability and sensitivity of the Foot and Ankle Disability Index in subjects with chronic ankle instability. J. Athl. Train. 2005, 40, 35–40. [Google Scholar]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63 (Suppl. S11), S240–S252. [Google Scholar]
- Borg, G.; Exertion, B.P.; Scales, P. Champaign; Human Kinetics: Champaign, IL, USA, 1998; pp. 1–104. [Google Scholar]
- Blazevich, A.J. Effects of physical training and detraining, immobilisation, growth and aging on human fascicle geometry. Sports Med. 2006, 36, 1003–1017. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.; Mc Cormack, W.; Toomey, C.; Norton, C.; Saunders, J.; Kerin, E.; Lyons, M.; Jakeman, P. Twelve weeks’ progressive resistance training combined with protein supplementation beyond habitual intakes increases upper leg lean tissue mass, muscle strength and extended gait speed in healthy older women. Biogerontology 2016, 18, 881–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seynnes, O.R.; de Boer, M.; Narici, M.V. Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training. J. Appl. Physiol. 2007, 102, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, D.A.; Kamen, G.; Frost, G. Neural adaptations to resistive exercise: Mechanisms and recommendations for training practices. Sports Med. 2006, 36, 133–149. [Google Scholar] [CrossRef]
- Hakkinen, K.; Pakarinen, A.; Kraemer, W.J.; Hakkinen, A.; Valkeinen, H.; Alen, M. Selective muscle hypertrophy, changes in EMG and force, and serum hormones during strength training in older women. J. Appl. Physiol. 2001, 91, 569–580. [Google Scholar] [CrossRef] [Green Version]
- Aagaard, P.; Andersen, J.L.; Dyhre-Poulsen, P.; Leffers, A.M.; Wagner, A.; Magnusson, S.P.; Halkjaer-Kristensen, J.; Simonsen, E.B. A mechanism for increased contractile strength of human pennate muscle in response to strength training: Changes in muscle architecture. J. Physiol. 2001, 534, 613–623. [Google Scholar] [CrossRef]
- Geremia, J.M.; Baroni, B.M.; Bobbert, M.F.; Bini, R.R.; Lanferdini, F.J.; Vaz, M.A. Effects of high loading by eccentric triceps surae training on Achilles tendon properties in humans. Eur. J. Appl. Physiol. 2018, 118, 1725–1736. [Google Scholar] [CrossRef]
- Kubo, K.; Kanehisa, H.; Ito, M.; Fukunaga, T. Effects of isometric training on the elasticity of human tendon structures in vivo. J. Appl. Physiol. 2001, 91, 26–32. [Google Scholar] [CrossRef]
- Langberg, H.; Ellingsgaard, H.; Madsen, T.; Jansson, J.; Magnusson, S.P.; Aagaard, P.; Kjaer, M. Eccentric rehabilitation exercise increases peritendinous type I collagen synthesis in humans with Achilles tendinosis. Scand. J. Med. Sci. Sports 2007, 17, 61–66. [Google Scholar] [CrossRef]
- Macgregor, L.J.; Hunter, A.M.; Orizio, C.; Fairweather, M.M.; Ditroilo, M. Assessment of Skeletal Muscle Contractile Properties by Radial Displacement: The Case for Tensiomyography. Sports Med. 2018, 48, 1607–1620. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, P.L.; Sanchez-Martinez, G.; Torrontegi, E.; Vazquez-Carrion, J.; Montalvo, Z.; Lucia, A. Comment on: Assessment of skeletal muscle contractile properties by radial displacement: The case for tensiomyography. Sports Med. 2019, 49, 973–975. [Google Scholar] [CrossRef]
- Wilson, H.V.; Johnson, M.I.; Francis, P. Repeated stimulation, inter-stimulus interval and inter-electrode distance alters muscle contractile properties as measured by Tensiomyography. PLoS ONE 2018, 13, e0191965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, H.V.; Jones, A.D.; Johnson, M.I.; Francis, P. The effect of inter-electrode distance on radial muscle displacement and contraction time of the biceps femoris, gastrocnemius medialis and biceps brachii, using Tensiomyography in healthy participants. Physiol. Meas. 2019, 40. [Google Scholar] [CrossRef]
- Franchi, M.V.; Atherton, P.J.; Reeves, N.D.; Fluck, M.; Williams, J.; Mitchell, W.K.; Selby, A.; Beltran Valls, R.M.; Narici, M.V. Architectural, functional and molecular responses to concentric and eccentric loading in human skeletal muscle. Acta Physiol. 2014, 210, 642–654. [Google Scholar] [CrossRef]
- Docking, S.I.; Ooi, C.C.; Connell, D. Tendinopathy: Is imaging telling us the entire story? J. Orthop. Sports Phys. Ther. 2015, 45, 842–852. [Google Scholar] [CrossRef] [Green Version]
- Kornaat, P.R.; Van de Velde, S.K. Bone marrow edema lesions in the professional runner. Am. J. Sports Med. 2014, 42, 1242–1246. [Google Scholar] [CrossRef]
- Stahl, R.; Luke, A.; Ma, C.B.; Krug, R.; Steinbach, L.; Majumdar, S.; Link, T.M. Prevalence of pathologic findings in asymptomatic knees of marathon runners before and after a competition in comparison with physically active subjects—A 3.0 T magnetic resonance imaging study. Skelet. Radiol. 2008, 37, 627–638. [Google Scholar] [CrossRef]
- Shaikh, Z.; Perry, M.; Morrissey, D.; Ahmad, M.; Del Buono, A.; Maffulli, N. Achilles tendinopathy in club runners. Int. J. Sports Med. 2012, 33, 390–394. [Google Scholar]
- Abdulhussein, H.; Chan, O.; Morton, S.; Kelly, S.; Padhiar, N.; Valle, X.; King, J.; Williams, S.; Morrissey, D. High Volume Image Guided Injections with or without steroid for mid-portion Achilles Tendinopathy: A Pilot Study. Clin. Res. Foot Ankle 2017, 5, 249. [Google Scholar] [CrossRef]
- Vulpiani, M.C.; Trischitta, D.; Trovato, P.; Vetrano, M.; Ferretti, A. Extracorporeal shockwave therapy (ESWT) in Achilles tendinopathy. A long-term follow-up observational study. J. Sports Med. Phys. Fit. 2009, 49, 171–176. [Google Scholar]
- Hausdorf, J.; Schmitz, C.; Averbeck, B.; Maier, M. Molecular basis for pain mediating properties of extracorporeal shock waves. Schmerz 2004, 18, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Maier, M.; Averbeck, B.; Milz, S.; Refior, H.J.; Schmitz, C. Substance P and prostaglandin E2 release after shock wave application to the rabbit femur. Clin. Orthop. Relat. Res. 2003, 406, 237–245. [Google Scholar] [CrossRef]
- Notarnicola, A.; Moretti, B. The biological effects of extracorporeal shock wave therapy (eswt) on tendon tissue. Muscles Ligaments Tendons J. 2012, 2, 33–37. [Google Scholar]
- O’Sullivan, P. It’s time for change with the management of non-specific chronic low back pain. Br. J. Sports Med. 2012, 46, 224–227. [Google Scholar] [CrossRef] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francis, P.; Thornley, I.; Jones, A.; I. Johnson, M. Pain and Function in the Runner a Ten (din) uous Link. Medicina 2020, 56, 21. https://doi.org/10.3390/medicina56010021
Francis P, Thornley I, Jones A, I. Johnson M. Pain and Function in the Runner a Ten (din) uous Link. Medicina. 2020; 56(1):21. https://doi.org/10.3390/medicina56010021
Chicago/Turabian StyleFrancis, Peter, Isobel Thornley, Ashley Jones, and Mark I. Johnson. 2020. "Pain and Function in the Runner a Ten (din) uous Link" Medicina 56, no. 1: 21. https://doi.org/10.3390/medicina56010021





