A Pilot Study of Circulating Monocyte Subsets in Patients Treated with Stem Cell Transplantation for High-Risk Hematological Malignancies
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Healthy Controls
2.2. Blood Sampling, Flow-Cytometric Analysis of Monocyte Subsets and Analysis of Peripheral Blood Leukocytes
2.3. Statistical Analyses
3. Results
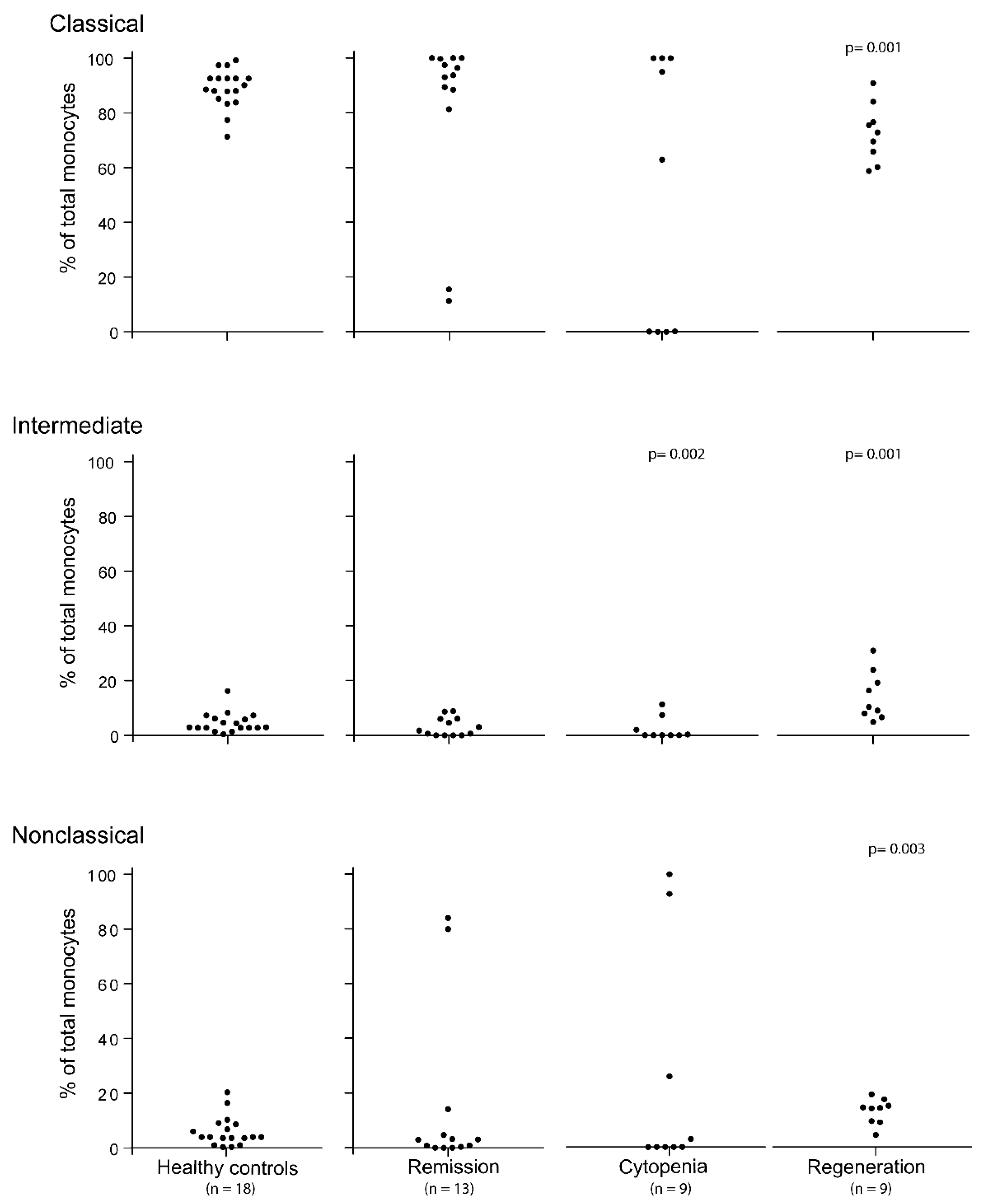
3.1. The Pretransplant Status of Our Patients with Hematological Malignancies; Monocyte Subsets for Patients in Complete Hematological Remission after Conventional Chemotherapy
3.2. The Peripheral Blood Levels of Various Monocyte Subsets Show Wide Variation during the Period of Severe Posttransplant Pancytopenia
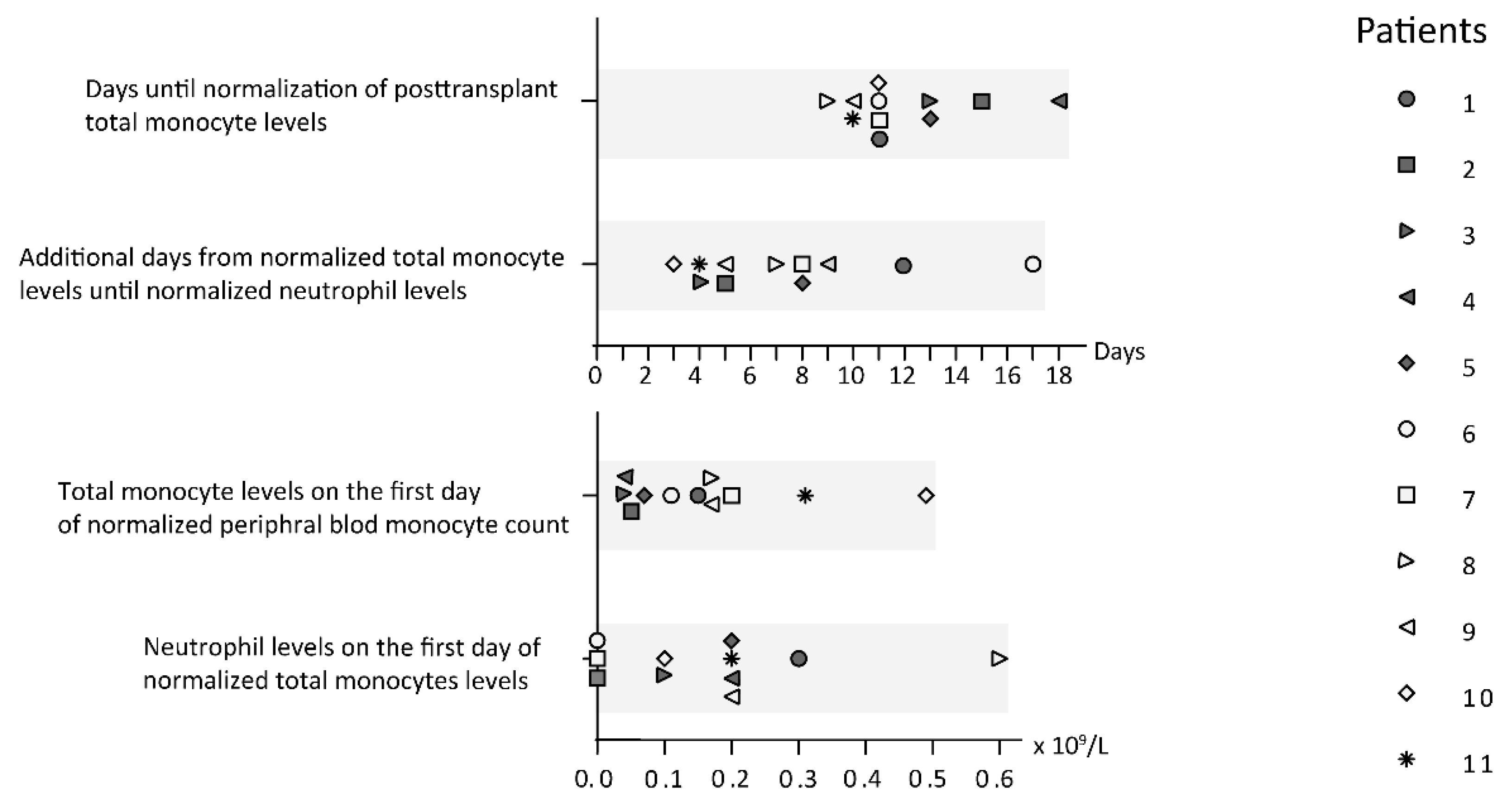
3.3. Stem Cell Transplant Recipients Show Early Posttransplant Total Monocyte Reconstitution
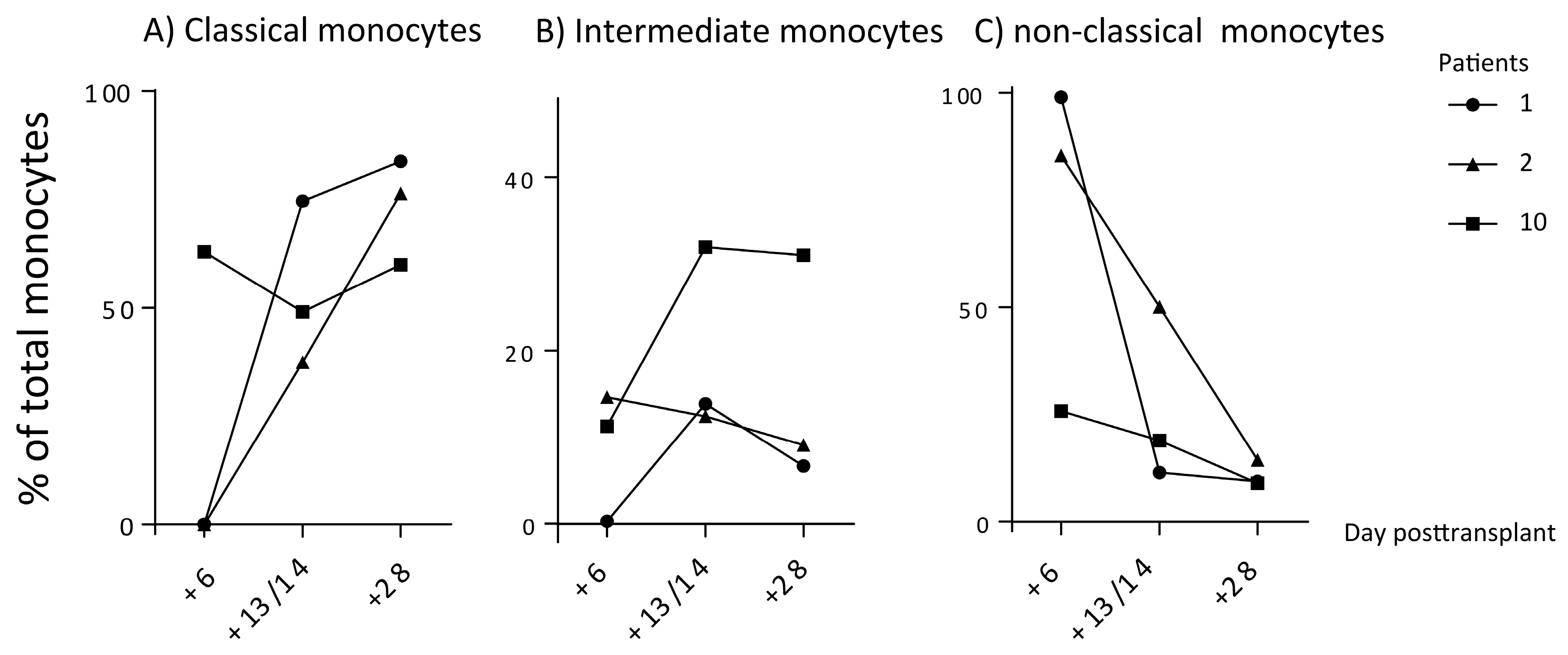
3.4. Early Posttransplant Monocyte Subset Regeneration after Stem Cell Transplantation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dohner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Buchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed]
- Wenzinger, C.; Williams, E.; Gru, A.A. Updates in the Pathology of Precursor Lymphoid Neoplasms in the Revised Fourth Edition of the WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues. Curr. Hematol. Malig. R 2018, 13, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Hasserjian, R.P. Myelodysplastic Syndrome Updated. Pathobiology 2019, 86, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Collignon, A.; Wanquet, A.; Maitre, E.; Cornet, E.; Troussard, X.; Aurran-Schleinitz, T. Prolymphocytic Leukemia: New Insights in Diagnosis and in Treatment. Curr. Oncol. Rep. 2017, 19. [Google Scholar] [CrossRef]
- Kantarjian, H.; Jabbour, E. Incorporating Immunotherapy Into the Treatment Strategies of B-Cell Adult Acute Lymphoblastic Leukemia: The Role of Blinatumomab and Inotuzumab Ozogamicin. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Krah, S.; Kolmar, H.; Becker, S.; Zielonka, S. Engineering IgG-Like Bispecific Antibodies-An Overview. Antibodies 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Guerra, V.A.; Jabbour, E.J.; Ravandi, F.; Kantarjian, H.; Short, N.J. Novel monoclonal antibody-based treatment strategies in adults with acute lymphoblastic leukemia. Ther. Adv. Hematol. 2019, 10, 2040620719849496. [Google Scholar] [CrossRef]
- Guerra, V.A.; DiNardo, C.; Konopleva, M. Venetoclax-based therapies for acute myeloid leukemia. Best Pract. Res. Clin. Haematol. 2019, 32, 145–153. [Google Scholar] [CrossRef]
- Tvedt, T.H.; Nepstad, I.; Bruserud, O. Antileukemic effects of midostaurin in acute myeloid leukemia—The possible importance of multikinase inhibition in leukemic as well as nonleukemic stromal cells. Expert Opin. Investig. Drugs 2017, 26, 343–355. [Google Scholar] [CrossRef]
- Liu, X.Y.; Gong, Y.P. Isocitrate dehydrogenase inhibitors in acute myeloid leukemia. Biomark. Res. 2019, 7. [Google Scholar] [CrossRef]
- Appelbaum, F.R. Maintenance therapy after allogeneic hematopoietic cell transplantation for acute myeloid leukemia. Best Pract. Res. Clin. Haematol. 2019, 32. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, J.J.; Gratwohl, A.; Schlenk, R.F.; Sierra, J.; Bornhauser, M.; Juliusson, G.; Racil, Z.; Rowe, J.M.; Russell, N.; Mohty, M.; et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: An integrated-risk adapted approach. Nat. Rev. Clin. Oncol. 2012, 9, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Juliusson, G.; Antunovic, P.; Derolf, A.; Lehmann, S.; Mollgard, L.; Stockelberg, D.; Tidefelt, U.; Wahlin, A.; Hoglund, M. Age and acute myeloid leukemia: Real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood 2009, 113, 4179–4187. [Google Scholar] [CrossRef] [PubMed]
- Versluis, J.; Hazenberg, C.L.E.; Passweg, J.R.; van Putten, W.L.J.; Maertens, J.; Biemond, B.J.; Theobald, M.; Graux, C.; Kuball, J.; Schouten, H.C.; et al. Post-remission treatment with allogeneic stem cell transplantation in patients aged 60 years and older with acute myeloid leukaemia: A time-dependent analysis. Lancet Haematol. 2015, 2, E427–E436. [Google Scholar] [CrossRef]
- Melve, G.K.; Ersvaer, E.; Kittang, A.O.; Bruserud, O. The chemokine system. in allogeneic stem-cell transplantation: A possible therapeutic target? Expert Rev. Hematol. 2011, 4, 563–576. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M.; Etzrodt, M.; Wildgruber, M.; Cortez-Retamozo, V.; Panizzi, P.; Figueiredo, J.L.; Kohler, R.H.; Chudnovskiy, A.; Waterman, P.; et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009, 325, 612–616. [Google Scholar] [CrossRef]
- Jakubzick, C.V.; Randolph, G.J.; Henson, P.M. Monocyte differentiation and antigen-presenting functions. Nat. Rev. Immunol. 2017, 17, 349–362. [Google Scholar] [CrossRef]
- Arderiu, G.; Espinosa, S.; Pena, E.; Crespo, J.; Aledo, R.; Bogdanov, V.Y.; Badimon, L. Tissue factor variants induce monocyte transformation and transdifferentiation into endothelial cell-like cells. J. Thromb. Haemost. 2017, 15, 1689–1703. [Google Scholar] [CrossRef]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef]
- Rommeley, M.; Spies-Weisshart, B.; Schilling, K.; Hochhaus, A.; Sayer, H.G.; Scholl, S. Reconstitution and functional analyses of neutrophils and distinct subsets of monocytes after allogeneic stem cell transplantation. J. Cancer Res. Clin. 2011, 137, 1293–1300. [Google Scholar] [CrossRef]
- Passlick, B.; Flieger, D.; Ziegler-Heitbrock, H.W. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 1989, 74, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.A.; Zhang, Y.; Fullerton, J.N.; Boelen, L.; Rongvaux, A.; Maini, A.A.; Bigley, V.; Flavell, R.A.; Gilroy, D.W.; Asquith, B.; et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J. Exp. Med. 2017, 214, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.; Liu, Y.J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef] [PubMed]
- Yeap, W.H.; Wong, K.L.; Shimasaki, N.; Teo, E.C.; Quek, J.K.; Yong, H.X.; Diong, C.P.; Bertoletti, A.; Linn, Y.C.; Wong, S.C. CD16 is indispensable for antibody-dependent cellular cytotoxicity by human monocytes. Sci. Rep. 2016, 6, 34310. [Google Scholar] [CrossRef]
- Wu, Z.H.; Zhang, Z.X.; Lei, Z.H.; Lei, P. CD14: Biology and role in the pathogenesis of disease. Cytokine Growth Factor Rev. 2019, 48, 24–31. [Google Scholar] [CrossRef]
- Boyette, L.B.; Macedo, C.; Hadi, K.; Elinoff, B.D.; Walters, J.T.; Ramaswami, B.; Chalasani, G.; Taboas, J.M.; Lakkis, F.G.; Metes, D.M. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS ONE 2017, 12, e0176460. [Google Scholar] [CrossRef]
- Thoma, M.D.; Huneke, T.J.; DeCook, L.J.; Johnson, N.D.; Wiegand, R.A.; Litzow, M.R.; Hogan, W.J.; Porrata, L.F.; Holtan, S.G. Peripheral blood lymphocyte and monocyte recovery and survival in acute leukemia postmyeloablative allogeneic hematopoietic stem cell transplant. Biol. Blood Marrow Transplant. 2012, 18, 600–607. [Google Scholar] [CrossRef][Green Version]
- Salzmann-Manrique, E.; Bremm, M.; Huenecke, S.; Stech, M.; Orth, A.; Eyrich, M.; Schulz, A.; Esser, R.; Klingebiel, T.; Bader, P.; et al. Joint Modeling of Immune Reconstitution Post Haploidentical Stem Cell Transplantation in Pediatric Patients With Acute Leukemia Comparing CD34(+)-Selected to CD3/CD19-Depleted Grafts in a Retrospective Multicenter Study. Front. Immunol. 2018, 9, 1841. [Google Scholar] [CrossRef]
- Rundgren, I.M.; Ersvaer, E.; Ahmed, A.B.; Ryningen, A.; Bruserud, O. Circulating monocyte subsets in multiple myeloma patients receiving autologous stem cell transplantation—A study of the preconditioning status and the course until posttransplant reconstitution for a consecutive group of patients. BMC Immunol. 2019, in press. [Google Scholar] [CrossRef]
- Pei, X.Y.; Zhao, X.Y.; Xu, L.P.; Wang, Y.; Zhang, X.H.; Chang, Y.J.; Huang, X.J. Immune reconstitution in patients with acquired severe aplastic anemia after haploidentical stem cell transplantation. Bone Marrow Transpl. 2017, 52, 1556–1562. [Google Scholar] [CrossRef]
- Turcotte, L.M.; Cao, Q.; Cooley, S.A.; Curtsinger, J.; Holtan, S.G.; Luo, X.H.; Yingst, A.; Weisdorf, D.J.; Blazar, B.R.; Miller, J.S.; et al. Monocyte Subpopulation Recovery as Predictors of Hematopoietic Cell Transplantation Outcomes. Biol. Blood Marrow Transplant. 2019, 25, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Ruutu, T.; Gratwohl, A.; Niederwieser, D.; de Witte, T.; van der Werf, S.; van Biezen, A.; Mohty, M.; Kroger, N.; Rambaldi, A.; McGrath, E.; et al. The EBMT-ELN working group recommendations on the prophylaxis and treatment of GvHD: A change-control analysis. Bone Marrow Transpl. 2017, 52, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.D.; Fentress, S.J.; Qiu, Y.F.; Yun, K.R.; Cox, J.S.; Chawla, A. Circadian Gene Bmal1 Regulates Diurnal Oscillations of Ly6C(hi) Inflammatory Monocytes. Science 2013, 341, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Schloss, M.J.; Hilby, M.; Nitz, K.; Prats, R.G.; Ferraro, B.; Leoni, G.; Soehnlein, O.; Kessler, T.; He, W.Y.; Luckow, B.; et al. Ly6C(high) Monocytes Oscillate in the Heart During Homeostasis and After Myocardial Infarction-Brief Report. Arterioscl. Throm. Vas. 2017, 37, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Smaaland, R.; Sothern, R.B.; Laerum, O.D.; Abrahamsen, J.F. Rhythms in human bone marrow and blood cells. Chronobiol. Int. 2002, 19, 101–127. [Google Scholar] [CrossRef] [PubMed]
- Smaaland, R.; Laerum, O.D.; Sothern, R.B.; Sletvold, O.; Bjerknes, R.; Lote, K. Colony-Forming Unit-Granulocyte-Macrophage and DNA-Synthesis of Human Bone-Marrow Are Circadian Stage-Dependent and Show Covariation. Blood 1992, 79, 2281–2287. [Google Scholar] [CrossRef]
- Rundgren, I.M.; Bruserud, O.; Ryningen, A.; Ersvaer, E. Standardization of sampling and sample preparation for analysis of human monocyte subsets in peripheral blood. J. Immunol. Methods 2018, 461, 53–62. [Google Scholar] [CrossRef]
- Wang, Y.T.; Zhao, X.Y.; Zhao, X.S.; Xu, L.P.; Zhang, X.H.; Wang, Y.; Liu, K.Y.; Chang, Y.J.; Huang, X.J. The impact of donor characteristics on the immune cell composition of mixture allografts of granulocytecolony-stimulating factor- mobilized marrow harvests and peripheral blood harvests. Transfusion 2015, 55, 2874–2881. [Google Scholar] [CrossRef]
- Arai, Y.; Kondo, T.; Yamazaki, H.; Takenaka, K.; Sugita, J.; Kobayashi, T.; Ozawa, Y.; Uchida, N.; Iwato, K.; Kobayashi, N.; et al. Allogeneic unrelated bone marrow transplantation from older donors results in worse prognosis in recipients with aplastic anemia. Haematologica 2016, 101, 644–652. [Google Scholar] [CrossRef]
- Lv, M.; Zhao, X.S.; Hu, Y.; Chang, Y.J.; Zhao, X.Y.; Kong, Y.; Zhang, X.H.; Xu, L.P.; Liu, K.Y.; Huang, X.J. Monocytic and promyelocytic myeloid-derived suppressor cells may contribute to G-CSF-induced immune tolerance in haplo-identical allogeneic hematopoietic stem cell transplantation. Am. J. Hematol. 2015, 90, E9–E16. [Google Scholar] [CrossRef]
- Porrata, L.F.; Inwards, D.J.; Ansell, S.M.; Micallef, I.N.; Johnston, P.B.; Hogan, W.J.; Markovic, S.N. Infused Autograft Lymphocyte to Monocyte Ratio and Survival in Diffuse Large B Cell Lymphoma. Biol. Blood Marrow Transplant. 2014, 20, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Ringden, O.; Labopin, M.; Gorin, N.C.; Le Blanc, K.; Rocha, V.; Gluckman, E.; Reiffers, J.; Arcese, W.; Vossen, J.M.; Jouet, J.P.; et al. Treatment with granulocyte colony-stimulating factor after allogeneic bone marrow transplantation for acute leukemia increases the risk of graft-versus-host disease and death. Biol. Blood Marrow Transplant. 2004, 10, 17. [Google Scholar] [CrossRef]
- Khoury, H.J.; Loberiza, F.R.; Ringden, O.; Barrett, A.J.; Bolwell, B.J.; Cahn, J.Y.; Champlin, R.E.; Gale, R.P.; Hale, G.A.; Urbano-Ispizua, A.; et al. Impact of posttransplantation G-CSF on outcomes of allogeneic hematopoietic stem cell transplantation. Blood 2006, 107, 1712–1716. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.S.; MacDonald, K.P.A.; Kuns, R.D.; Morris, H.M.; Banovic, T.; Don, A.L.J.; Rowe, V.; Wilson, Y.A.; Raffelt, N.C.; Engwerda, C.R.; et al. Induction of natural killer T cell-dependent alloreactivity by administration of granulocyte colony-stimulating factor after bone marrow transplantation. Nat. Med. 2009, 15, 436–441. [Google Scholar] [CrossRef]
- Ruutu, T.; Gratwohl, A.; de Witte, T.; Afanasyev, B.; Apperley, J.; Bacigalupo, A.; Dazzi, F.; Dreger, P.; Duarte, R.; Finke, J.; et al. Prophylaxis and treatment of GVHD: EBMT-ELN working group recommendations for a standardized practice. Bone Marrow Transpl. 2014, 49, 168–173. [Google Scholar] [CrossRef]
- Nunes, N.S.; Kanakry, C.G. Mechanisms of Graft-versus-Host Disease Prevention by Post-transplantation Cyclophosphamide: An Evolving Understanding. Front. Immunol. 2019, 10, 2668. [Google Scholar] [CrossRef]
- El Fakih, R.; Hashmi, S.K.; Ciurea, S.O.; Luznik, L.; Gale, R.P.; Aljurf, M. Post-transplant cyclophosphamide use in matched HLA donors: A review of literature and future application. Bone Marrow Transpl. 2020, 55, 40–47. [Google Scholar] [CrossRef]
- Cronstein, B.N. Low-dose methotrexate: A mainstay in the treatment of rheumatoid arthritis. Pharmacol. Rev. 2005, 57, 163–172. [Google Scholar] [CrossRef]
- Belgi, G.; Friedmann, P.S. Traditional therapies: Glucocorticoids, azathioprine, methotrexate, hydroxyurea. Clin. Exp. Dermatol. 2002, 27, 546–554. [Google Scholar] [CrossRef]
- Porrata, L.F. Autograft immune effector cells and survival in autologous peripheral blood hematopoietic stem cell transplantation. J. Clin. Apher. 2018, 33, 324–330. [Google Scholar] [CrossRef]
- Kholodnyuk, I.; Kadisa, A.; Svirskis, S.; Gravelsina, S.; Studers, P.; Spaka, I.; Sultanova, A.; Lejniece, S.; Lejnieks, A.; Murovska, M. Proportion of the CD19-Positive and CD19-Negative Lymphocytes and Monocytes within the Peripheral Blood Mononuclear Cell Set is Characteristic for Rheumatoid Arthritis. Medicina 2019, 55, 630. [Google Scholar] [CrossRef] [PubMed]
- Switonska, M.; Slomka, A.; Korbal, P.; Piekus-Slomka, N.; Sinkiewicz, W.; Sokal, P.; Zekanowska, E. Association of Neutrophil-to-Lymphocyte Ratio and Lymphocyte-to-Monocyte Ratio with Treatment Modalities of Acute Ischaemic Stroke: A Pilot Study. Medicina 2019, 55, 342. [Google Scholar] [CrossRef] [PubMed]
| Id | AGE | SEX | Diagnosis | Treatment | Treatment | CCC | REM | CSCT | REC |
|---|---|---|---|---|---|---|---|---|---|
| Conventional Induction and Consolidation Followed by Stem Cell Transplantation | |||||||||
| 1 | 29 | M | ALL | Remission induction followed by allotransplantation | Conditioning with busulfan plus cyclophosphamide | + | + | + | |
| 2 | 61 | F | MDS-HR2 | Remission induction followed by allotransplantation | Conditioning with fludarabine and treosulfan | + | + | + | |
| 3 | 67 | M | PLL-T | Remission induction followed by allotransplantation | Conditioning with fludarabine and treosulfan | + | |||
| 4 | 64 | M | MDS-HR2 | Remission induction followed by allotransplantation | Conditioning with fludarabine and treosulfan | + | + | ||
| 5 | 54 | F | AML-MDS | Remission induction followed by allotransplantation | Conditioning with busulfan plus cyclophosphamide | + | + | ||
| 6 | 72 | M | AML de novo | Remission induction followed by allotransplantation | Conditioning with fludarabine and treosulfan | + | + | + | |
| 7 | 53 | F | AML de novo | Remission induction followed by allotransplantation | Conditioning with busulfan plus cyclophosphamide | + | ++ | + | |
| 8 | 57 | M | AML de novo | Remission induction followed by allotransplantation | Conditioning with fludarabine and treosulfan | + | + | + | |
| 9 | 51 | M | AML de novo | Remission induction followed by autotransplantation | Conditioning with busulfan plus cyclophosphamide | + | |||
| 10 | 43 | M | APL relapse | Remission induction followed by autotransplantation | Conditioning with busulfan plus cyclophosphamide | + | + | + | |
| 11 | 34 | M | AML de novo | Remission induction followed by autotransplantation | Conditioning with busulfan plus cyclophosphamide | + | + | + | + |
| Conventional Induction and/or Consolidation Chemotherapy | |||||||||
| 12 | 65 | F | AML de novo | Induction chemotherapy, regeneration to remission | Induction with daunorubicin plus cytarabine. First consolidation: mitoxantrone, cytarabine. Second consolidation: etoposide, amsacrine and cytarabine | + | ++ | ||
| 13 | 63 | F | AML relapse | Conventional induction, regeneration to remission | Induction with etoposide, amsacrine and cytarabine | + | |||
| 14 | 68 | F | AML de novo | Conventional induction leading to remission; later consolidation chemotherapy | Induction: daunorubicin, cytarabine. First consolidation: mitoxantrone, cytarabine. | + | + | ||
| 15 | 18 | F | AML de novo | Induction chemotherapy, regeneration to remission | Daunorubicin plus cytarabine | + | |||
| 16 | 64 | M | AML relapse | Conventional induction leading to remission; later consolidation chemotherapy | Induction: daunorubicin, cytarabine. First consolidation: mitoxantrone, cytarabine. | + | + | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rundgren, I.M.; Ersvær, E.; Ahmed, A.B.; Ryningen, A.; Bruserud, Ø. A Pilot Study of Circulating Monocyte Subsets in Patients Treated with Stem Cell Transplantation for High-Risk Hematological Malignancies. Medicina 2020, 56, 36. https://doi.org/10.3390/medicina56010036
Rundgren IM, Ersvær E, Ahmed AB, Ryningen A, Bruserud Ø. A Pilot Study of Circulating Monocyte Subsets in Patients Treated with Stem Cell Transplantation for High-Risk Hematological Malignancies. Medicina. 2020; 56(1):36. https://doi.org/10.3390/medicina56010036
Chicago/Turabian StyleRundgren, Ida Marie, Elisabeth Ersvær, Aymen Bushra Ahmed, Anita Ryningen, and Øystein Bruserud. 2020. "A Pilot Study of Circulating Monocyte Subsets in Patients Treated with Stem Cell Transplantation for High-Risk Hematological Malignancies" Medicina 56, no. 1: 36. https://doi.org/10.3390/medicina56010036
APA StyleRundgren, I. M., Ersvær, E., Ahmed, A. B., Ryningen, A., & Bruserud, Ø. (2020). A Pilot Study of Circulating Monocyte Subsets in Patients Treated with Stem Cell Transplantation for High-Risk Hematological Malignancies. Medicina, 56(1), 36. https://doi.org/10.3390/medicina56010036





