Computed Tomography Diagnostic of Uncommon Case of Osteopetrosis in 80-Year-Old Man—Case Report
Abstract
1. Introduction
2. Materials and Methods
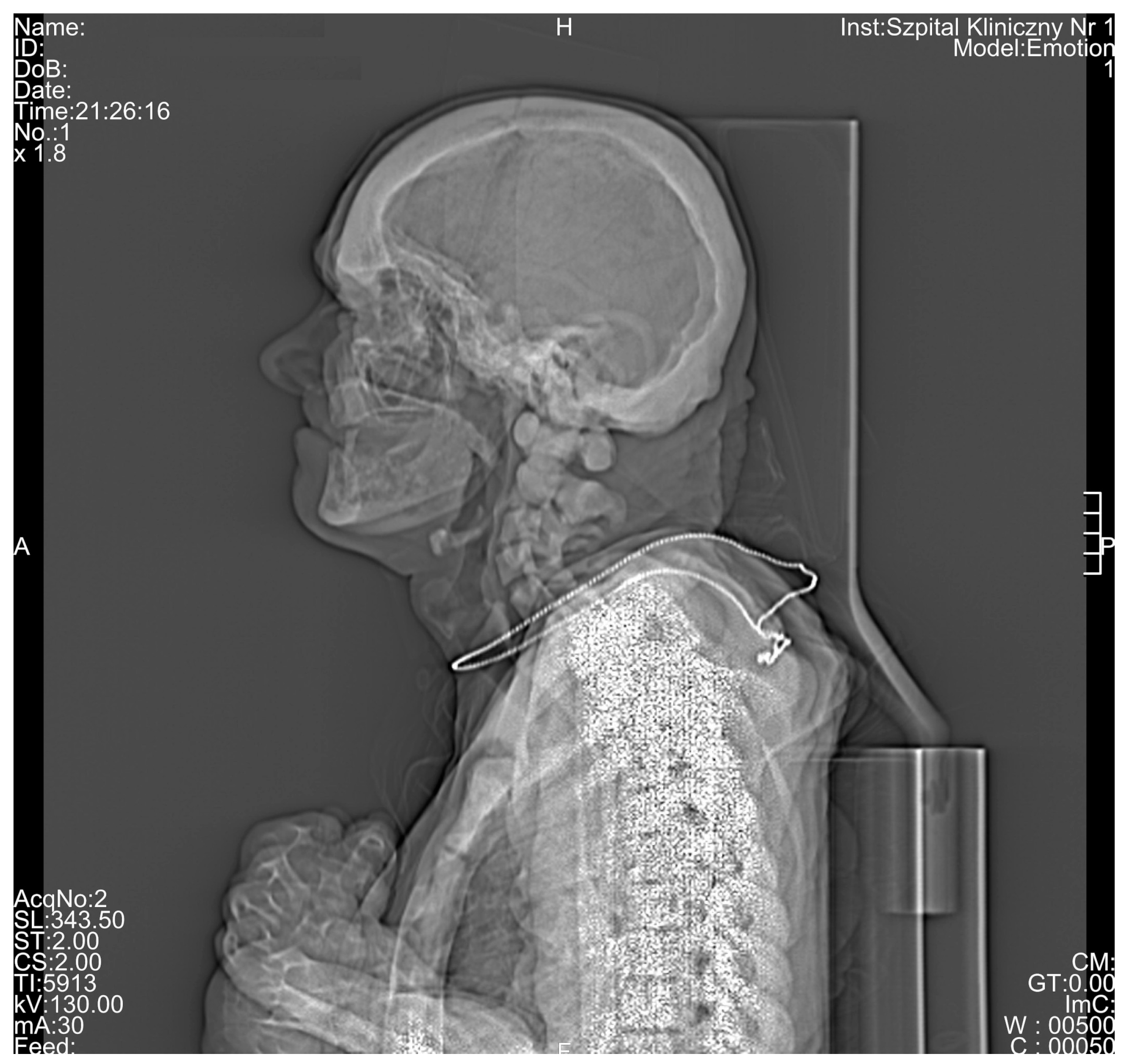
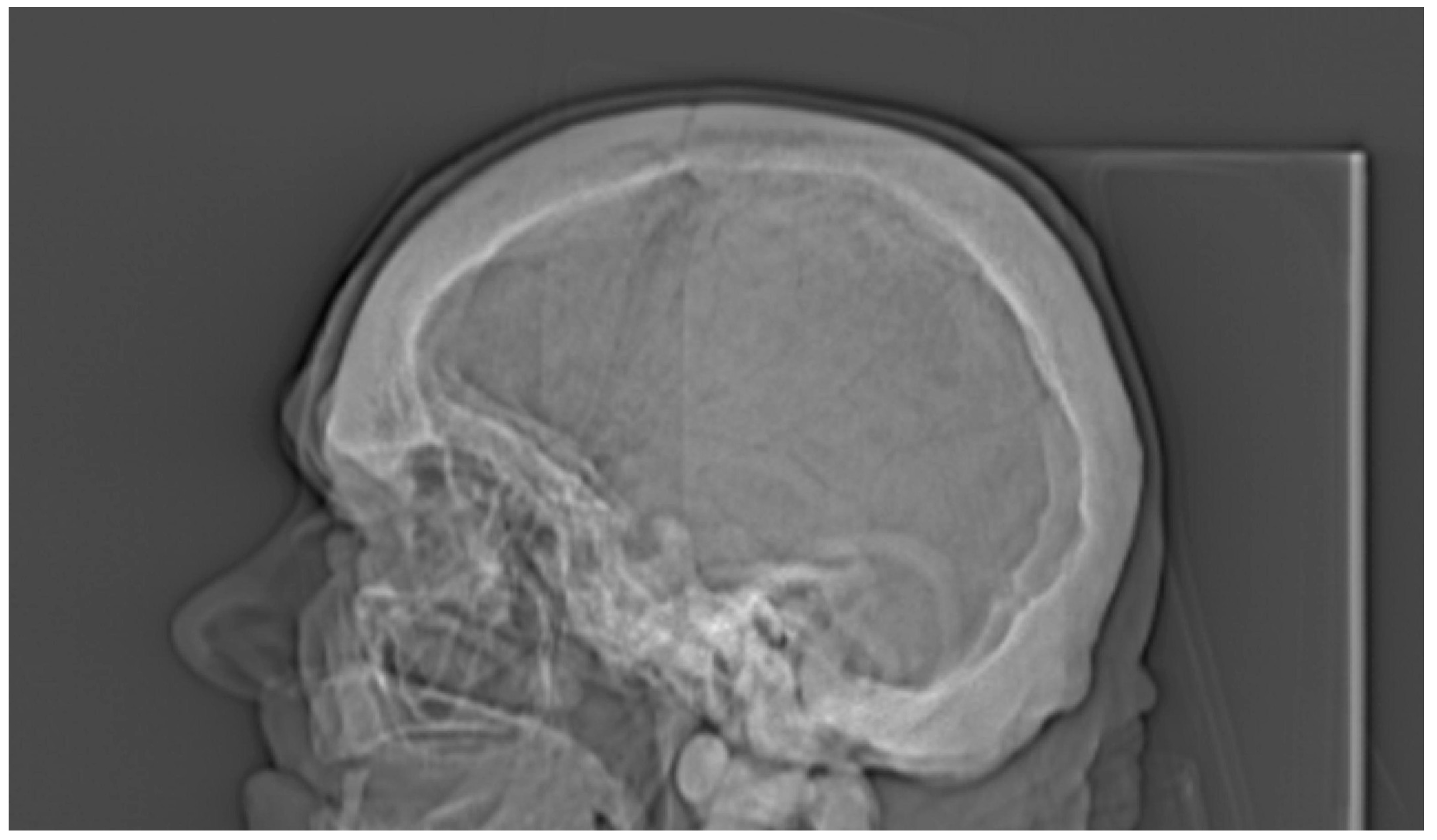
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Viguet-Carrin, S.; Garnero, P.; Delmas, P.D. The role of collagen in bone strength. Osteoporos. Int. 2006, 17, 319–336. [Google Scholar] [CrossRef]
- Teti, A. Bone development: Overview of bone cells and signaling. Curr. Osteoporos. Rep. 2011, 9, 264–273. [Google Scholar] [CrossRef]
- Seeman, E. Bone modeling and remodeling. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Sobacchi, C.; Frattini, A.; Guerrini, M.M.; Abinun, M.; Pangrazio, A.; Susani, L.; Bredius, R.; Mancini, G.; Cant, A.; Bishop, N.; et al. Osteoclast-poor human osteopetrosis due to mutations in the gene encoding RANKL. Nat. Genet. 2007, 39, 960–962. [Google Scholar] [CrossRef] [PubMed]
- Del Fattore, A.; Cappariello, A.; Teti, A. Genetics, pathogenesis and complications of osteopetrosis. Bone 2008, 42, 19–29. [Google Scholar] [CrossRef]
- Michou, L.; Brown, J.P. Genetics of bone diseases: Paget’s disease, fibrous dysplasia, osteopetrosis, and osteogenesis imperfecta. Joint Bone Spine 2011, 78, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Bollerslev, J.; Henriksen, K.; Nielsen, M.F.; Brixen, K.; Van Hul, W. Autosomal dominant osteopetrosis revisited: Lessons from recent studies. Eur. J. Endocrinol. 2013, 169, R39–R57. [Google Scholar] [CrossRef] [PubMed]
- Tolar, J.; Teitelbaum, S.L.; Orchard, P.J. Osteopetrosis. N. Engl. J. Med. 2004, 351, 2839–2849. [Google Scholar] [CrossRef]
- Waterval, J.J.; Borra, V.M.; Van Hul, W.; Stokroos, R.J.; Manni, J.J. Sclerosing bone dysplasias with involvement of the craniofacial skeleton. Bone 2014, 60, 48–67. [Google Scholar] [CrossRef]
- Wilson, C.J.; Vellodi, A. Autosomal recessive osteopetrosis: Diagnosis, management, and outcome. Arch. Dis. Child. 2000, 83, 449–452. [Google Scholar] [CrossRef]
- Curé, J.K.; Key, L.L.; Goltra, D.D.; VanTassel, P. Cranial MR imaging of osteopetrosis. Am. J. Neuroradiol. 2000, 21, 1110–1115. [Google Scholar] [PubMed]
- Steward, C.G. Neurological aspects of osteopetrosis. Neuropathol. Appl. Neurobiol. 2003, 29, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Dimai, H.P. Use of dual-energy X-ray absorptiometry (DXA) for diagnosis and fracture risk assessment; WHO-criteria, T- and Z-score, and reference databases. Bone 2017, 104, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Krupski, W.; Tatara, M.R.; Bury, P.; Szabelska, A.; Charuta, A.; Maciejewski, R.; Wallner, G.; Dąbrowski, A. Negative Effects of total gastrectomy on bone tissue metabolism and volumetric bone mineral density (vBMD) of lumbar spine in 1-year study in men. Medicine 2016, 95, e2817. [Google Scholar] [CrossRef]
- Kaste, S.C.; Kasow, K.A.; Horowitz, E.M. Quantitative bone mineral density assessment in malignant infantile osteopetrosis. Pediatr. Blood Cancer 2007, 8, 181–185. [Google Scholar] [CrossRef]
- Gregson, C.L.; Hardcastle, S.A.; Cooper, C.; Tobias, J.H. Friend or foe: High bone mineral density on routine bone density scanning, a review of causes and management. Rheumatology 2013, 52, 968–985. [Google Scholar] [CrossRef]
- Arruda, M.; Coelho, M.C.; Moraes, A.B.; de Paula Paranhos-Neto, F.; Madeira, M.; Farias, M.L.; Vieira Neto, L. Bone mineral density and microarchitecture in patients with autosomal dominant osteopetrosis: A report of two cases. J. Bone Miner. Res. 2016, 31, 657–662. [Google Scholar] [CrossRef]
- Lochmüller, E.M.; Bürklein, D.; Kuhn, V.; Glaser, C.; Müller, R.; Glüer, C.C.; Eckstein, F. Mechanical strength of thoracolumbar spine in the elderly: Prediction from in situ dual-energy X-ray absorptiometry, quantitative computed tomography (QCT), upper and lower limb peripheral QCT, and quantitative ultrasound. Bone 2002, 31, 77–84. [Google Scholar] [CrossRef]
- Waguespack, S.G.; Hui, S.L.; Dimeglio, L.A.; Econs, M.J. Autosomal dominant osteopetrosis: Clinical severity and natural history of 94 subjects with a chloride channel 7 gene mutation. J. Clin. Endocrinol. Metab. 2007, 92, 771–778. [Google Scholar] [CrossRef]
- Gasser, J.A. Assessing bone quantity by pQCT. Bone 1995, 17 (Suppl. 1), 45S–154S. [Google Scholar] [CrossRef]
- Peyrin, F.; Dong, P.; Pacureanu, A.; Langer, M. Micro- and nano-CT for the study of bone ultrastructure. Curr. Osteoporos. Rep. 2014, 12, 465–474. [Google Scholar] [CrossRef]
- Tatara, M.R.; Krupski, W.; Charuta, A. Quantitative computed tomography (QCT) applications in studies focused on quality determination of mineralized tissues in poultry. In Proceedings of the 10th Turkey Science and Production Conference, Chester, UK, 10–11 March 2016; pp. 36–47. [Google Scholar]
- Tatara, M.R.; Krupski, W.; Charuta, A.; Brodzki, A.; Jóźwik, A.; Strzałkowska, N.; Poławska, E.; Chmielowiec, K.; Horbańczuk, J.O. Morphological, densitometric and mechanical properties of pelvic limb bones in 14-month-old female ostriches (Struthio camelus). Poult. Sci. 2016, 95, 2421–2426. [Google Scholar] [CrossRef] [PubMed]
- Bollerslev, J.; Andersen, P.E., Jr. Radiological, biochemical and hereditary evidence of two types of autosomal dominant osteopetrosis. Bone 1988, 9, 7–13. [Google Scholar] [CrossRef]
- Bollerslev, J. Autosomal dominant osteopetrosis: Bone metabolism and epidemiological, clinical, and hormonal aspects. Endocr. Rev. 1989, 10, 45–67. [Google Scholar] [CrossRef] [PubMed]
- Grodum, E.; Gram, J.; Brixen, K.; Bollerslev, J. Autosomal dominant osteopetrosis: Bone mineral measurements of the entire skeleton of adults in two different subtypes. Bone 1995, 16, 431–434. [Google Scholar] [CrossRef]
- Boyle, A.; Subhas, N.; Sundaram, M. Radiologic case study. Osteopetrosis. Orthopedics 2012, 35, 723–724. [Google Scholar] [CrossRef]

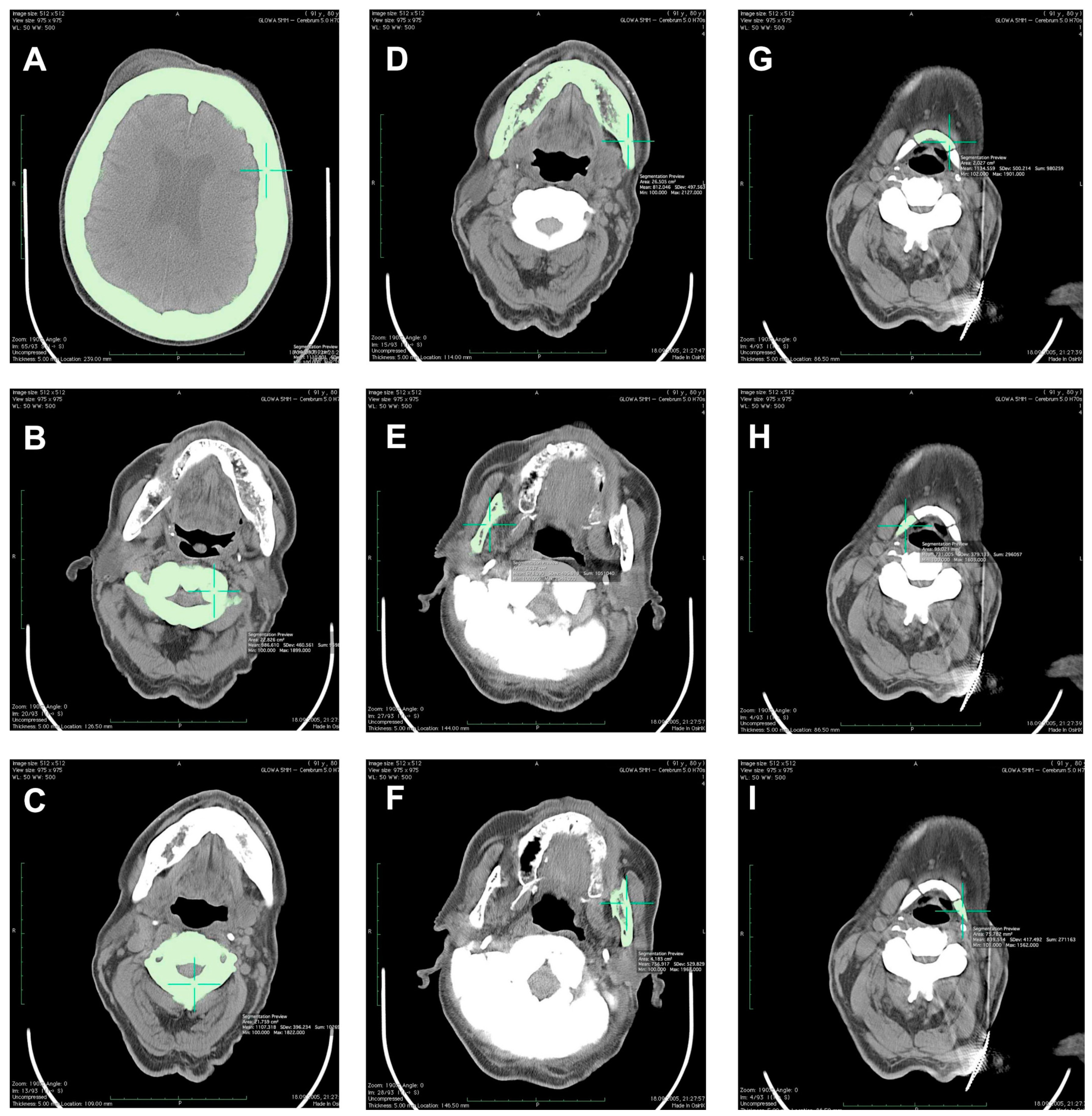



| Investigated Bone | TbCa-HA (mg Ca-HA/mL) | CbCa-HA (mg Ca-HA/mL) | T-Score | Z-Score | vBMD (g/cm3) |
|---|---|---|---|---|---|
| Spine | |||||
| Th12 vertebra | 834.1 | 800.3 | - | - | - |
| L1 vertebra | 918.5 | 813.5 | - | - | - |
| L2 vertebra | 927.6 | 861.5 | - | - | - |
| L3 vertebra | 860.6 | 816.4 | - | - | 1.936 |
| L4 vertebra | 865.7 | 846.6 | - | - | 1.943 |
| L5 vertebra | 1039.8 | 833.7 | - | - | 1.912 |
| Th12-L2 vertebrae | 893.4 | 825.1 | 27.12 | 31.00 | - |
| L3-L5 vertebrae | 922.0 | 832.2 | 28.20 | 32.08 | 1.930 |
| L1-L5 vertebrae | 922.4 | 834.3 | - | - | - |
| Reference value for 80-year-old man | 71.8 | - | - | - | - |
| C1 vertebra | - | - | - | - | 1.987 |
| C2 vertebra | - | - | - | - | 2.107 |
| C3 vertebra | - | - | - | - | 2.010 |
| Pelvis | |||||
| Right ilium | - | - | - | - | 1.954 |
| Left ilium | - | - | - | - | 1.924 |
| Cranium | |||||
| 50% of cranium height | - | - | - | - | 2.111 |
| Mandible | |||||
| Mandibular body | - | - | - | - | 1.812 |
| Right ramus | - | - | - | - | 1.678 |
| Left ramus | - | - | - | - | 1.757 |
| Hyoid | |||||
| Middle part | - | - | - | - | 2.135 |
| Right part | - | - | - | - | 1.731 |
| Left part | - | - | - | - | 1.840 |
| Radiological Feature | ADO Type I | ADO Type II | ADO Type III | Presented Case |
|---|---|---|---|---|
| General osteosclerosis | + | – | + | + |
| Skull vault osteosclerosis | + | – | + | + |
| Skull base osteosclerosis | – | + | – | + |
| Sandwich vertebrae (Ruger–Jersey spine) | – | + | – | + |
| Bone within bone | – | + | + | + |
| Hyoid sclerosis | – | – | – | + |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupski, W.; Kruk-Bachonko, J.; Tatara, M.R. Computed Tomography Diagnostic of Uncommon Case of Osteopetrosis in 80-Year-Old Man—Case Report. Medicina 2020, 56, 518. https://doi.org/10.3390/medicina56100518
Krupski W, Kruk-Bachonko J, Tatara MR. Computed Tomography Diagnostic of Uncommon Case of Osteopetrosis in 80-Year-Old Man—Case Report. Medicina. 2020; 56(10):518. https://doi.org/10.3390/medicina56100518
Chicago/Turabian StyleKrupski, Witold, Joanna Kruk-Bachonko, and Marcin R. Tatara. 2020. "Computed Tomography Diagnostic of Uncommon Case of Osteopetrosis in 80-Year-Old Man—Case Report" Medicina 56, no. 10: 518. https://doi.org/10.3390/medicina56100518
APA StyleKrupski, W., Kruk-Bachonko, J., & Tatara, M. R. (2020). Computed Tomography Diagnostic of Uncommon Case of Osteopetrosis in 80-Year-Old Man—Case Report. Medicina, 56(10), 518. https://doi.org/10.3390/medicina56100518






