Mesenchymal Stromal Cell Therapy in the Management of Perianal Fistulas in Crohn’s Disease: An Up-To-Date Review
Abstract
:1. Introduction
2. Mesenchymal Stromal Cells
3. Surgical Management
4. State of the Art
4.1. Mesenchymal Stromal Cells in Perianal Fistulizing Crohn Disease
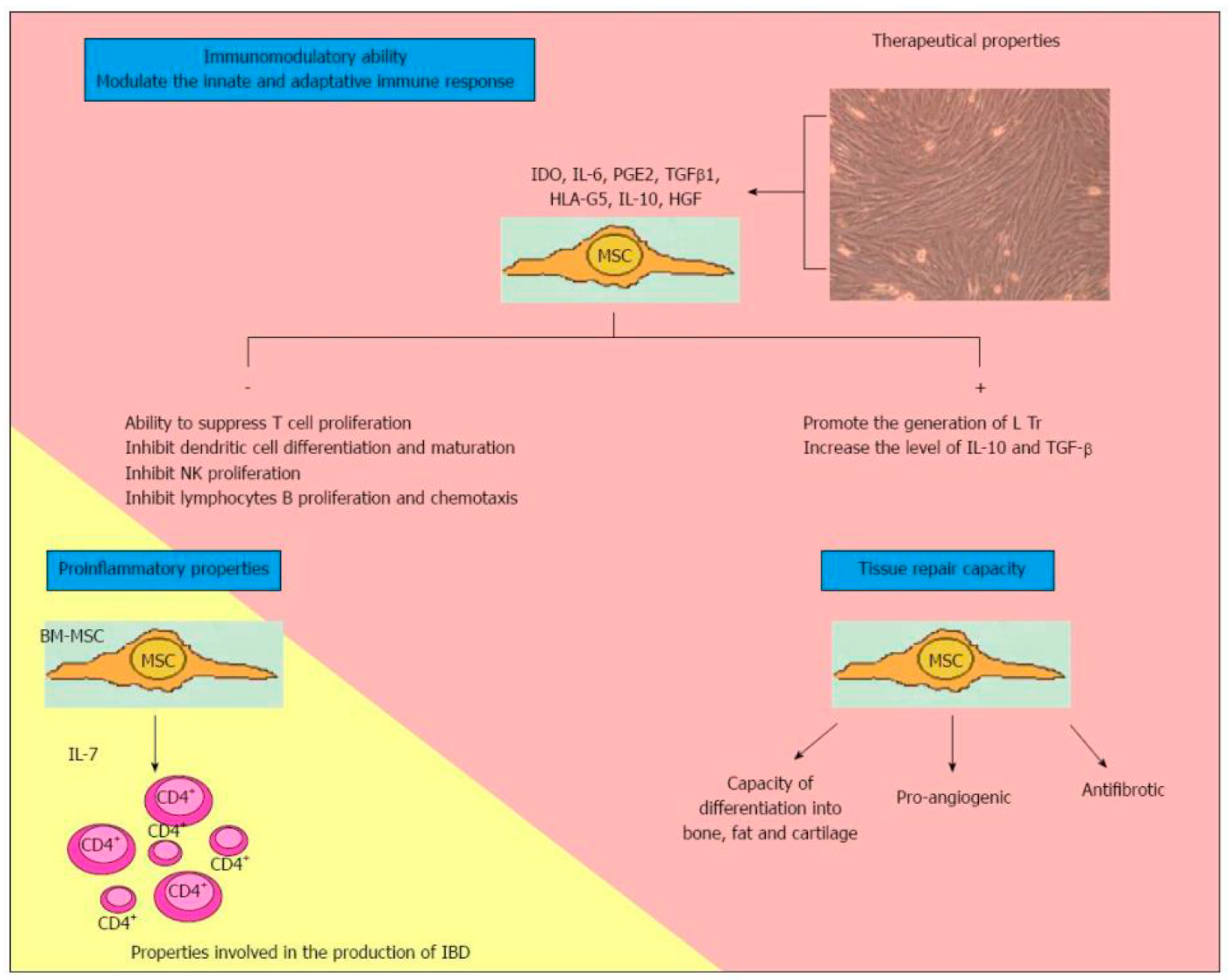
- Their migration into the inflammation site or tissue injury [51];
4.2. Literature Review
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cosnes, J.; Gower-Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Parks, A.G.; Gordon, P.H.; Hardcastle, J.D. A classification of fistula-in-ano. Br. J. Surg. 1976, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Odze, R.; Goldblum, J. Odze and Goldblum Surgical Pathology of the GI Tract, Liver, Biliary Tract, and Pancreas; Elsevier, Saunders: Philadelphia, PA, USA, 2015; ISBN 978-032-331-495-4. [Google Scholar]
- Bataille, F.; Klebl, F.; Rummele, P.; Schroeder, J.; Farkas, S.; Wild, P.J.; Fürst, A.; Hofstädter, F.; Schölmerich, J.; Herfarth, H.; et al. Morphological characterisation of Crohn’s disease fistulae. Gut 2004, 53, 1314–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, D.A.; Loftus, E.V., Jr.; Tremaine, W.J.; Panaccione, R.; Harmsen, W.S.; Zinsmeister, A.R.; Sandborn, W.J. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology 2002, 122, 875–880. [Google Scholar] [CrossRef]
- Lightner, A.L.; Ashburnb, J.H.; Mantaj, S.B.; Carvello, M.; Chandrasinghe, P.; de Buck van Overstraeten, A.; Fleshner, P.R.; Gallo, G.; Kotze, P.G.; Holubar, S.D.; et al. Fistulizing Crohn’s disease. Curr. Probl. Surg. 2020. [Google Scholar] [CrossRef]
- Penner, A.; Crohn, B.B. Perianal fistulae as a complication of regional ileitis. Ann. Surg. 1938, 108, 867–873. [Google Scholar] [CrossRef]
- Hellers, G.; Bergstrand, O.; Ewerth, S.; Holmström, B. Occurrence and outcome after primary treatment of anal fistulae in Crohn’s disease. Gut 1980, 21, 525–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasparek, M.S.; Glatzle, J.; Temeltcheva, T.; Mueller, M.H.; Koenigsrainer, A.; Kreis, M.E. Long-term quality of life in patients with Crohn’s disease and perianal fistulas: Influence of fecal diversion. Dis. Colon Rectum 2007, 50, 2067–2074. [Google Scholar] [CrossRef]
- Guadalajara, H.; García-Arranz, M.; Herreros, M.D.; Borycka-Kiciak, K.; Lightner, A.L.; García-Olmo, D. Mesenchymal stem cells in perianal Crohn’s disease. Tech. Coloproctol. 2020, 24, 883–889. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Bieback, K.; Kluter, H. Mesenchymal Stromal Cells from Umbilical Cord Blood. Curr. Stem Cell Res. 2007, 2, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Jin, J.; Chen, L.; Zhu, J.; Huang, W.; Zhao, J.; Qian, H.; Zhang, X. Isolation of mesenchymal stem cells from human placenta: Comparison with human bone marrow mesenchymal stem cells. Cell Biol. Int. 2006, 30, 681–687. [Google Scholar] [CrossRef] [PubMed]
- In’t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; de Groot-Swings, G.M.J.S.; Claas, F.H.J.; Fibbe, W.E.; Kanhai, H.H.H. Isolation of Mesenchymal Stem Cells of Fetal or Maternal Origin from Human Placenta. Stem Cells 2004, 22, 1338–1345. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Helder, M.N.; Knippenberg, M.; Klein-Nulend, J.; Wuisman, P.I.J.M. Stem Cells from Adipose Tissue Allow Challenging New Concepts for Regenerative Medicine. Tissue. Eng. 2017, 13, 1799–1808. [Google Scholar] [CrossRef]
- Lin, K.; Matsubara, Y.; Masuda, Y.; Togashi, K.; Ohno, T.; Tamura, T.; Toyoshima, Y.; Sugimachi, K.; Toyoda, M.; Marc, H.; et al. Characterization of adipose tissue-derived cells isolated with the Celution system. Cytotherapy 2008, 10, 417–426. [Google Scholar] [CrossRef]
- Tremolada, C.; Colombo, V.; Ventura, C. Adipose Tissue and Mesenchymal Stem Cells: State of the Art and Lipogems® Technology Development. Curr. Stem Cell Rep. 2016, 2, 304–312. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Montiel, Mdel, P.; Gómez-Gómez, G.J.; Flores, A.I. Therapy with stem cells in inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 1211–1227. [CrossRef]
- Wang, H.-S.; Hung, S.-C.; Peng, S.-T.; Huang, C.-C.; Wei, H.-M.; Guo, Y.-J.; Fu, Y.-S.; Lai, M.-C.; Chen, C.-C. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells 2004, 22, 1330–1337. [Google Scholar] [CrossRef] [Green Version]
- English, K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol. Cell Biol. 2013, 91, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Mayne, C.G.; Williams, C.B. Induced and natural regulatory T cells in the development of inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 1772–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakaguchi, S. Naturally arising Foxp3-expressing CD25+ CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 2005, 6, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Ponte, A.L.; Marais, E.; Gallay, N.; Langonné, A.; Delorme, B.; Hérault, O.; Charbord, P.; Domenech, J. The In Vitro Migration Capacity of Human Bone Marrow Mesenchymal Stem Cells: Comparison of Chemokine and Growth Factor Chemotactic Activities. Stem Cells 2007, 25, 1737–1745. [Google Scholar] [CrossRef]
- Salem, H.K.; Thiemermann, C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells 2010, 28, 585–596. [Google Scholar] [CrossRef] [Green Version]
- Gold, S.L.; Cohen-Mekelburg, S.; Schneider, Y.; Steinlauf, A. Perianal Fistulas in Patients with Crohn’s Disease, Part 2. Gastroenterol. Hepatol. 2018, 14, 521–528. [Google Scholar]
- Sandborn, W.J.; Fazio, V.W.; Feagan, B.G.; Hanauer, S.B. AGA technical review on perianal Crohn’s disease. Gastroenterology 2003, 125, 1508–1530. [Google Scholar] [CrossRef]
- Hyder, S.A.; Travis, S.P.L.; Jewell, D.P.; Neil, J.; George, B.D. Fistulating anal Crohn’s disease: Results of combined surgical and infliximab treatment. Dis. Colon Rectum 2006, 49, 1837–1841. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, W.B.; Decanini, A.; Mellgren, A.; Lowry, A.C.; Goldberg, S.M.; Madoff, R.D.; Spencer, M.P. Does infliximab infusion impact results of operative treatment for Crohn’s perianal fistulas? Dis. Colon Rectum 2007, 50, 1754–1760. [Google Scholar] [CrossRef]
- Bubbers, E.J.; Cologne, K.G. Management of complex anal fistulas. Clin. Colon Rectal. Surg. 2016, 29, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, R.D.; Sackier, J.M.; Hodde, J.P. Incontinence rates after cutting seton treatment for anal fistula. Colorectal Dis. 2009, 11, 564–571. [Google Scholar] [CrossRef]
- Mizrahi, N.; Wexner, S.D.; Zmora, O.; Da Silva, G.; Efron, J.; Weiss, E.G.; Vernava, A.M., 3rd; Nogueras, J.J. Endorectal advancement flap: Are there predictors of failure? Dis. Colon Rectum 2002, 45, 1616–1621. [Google Scholar] [CrossRef]
- Sonoda, T.; Hull, T.; Piedmonte, M.R.; Fazio, V.W. Outcomes of primary repair of anorectal and rectovaginal fistulas using the endorectal advancement flap. Dis. Colon Rectum 2002, 45, 1622–1628. [Google Scholar] [CrossRef] [PubMed]
- Makowiec, F.; Jehle, E.C.; Becker, H.D.; Starlinger, M. Clinical course after transanal advancement flap repair of perianal fistula in patients with Crohn’s disease. Br. J. Surg. 1995, 82, 603–606. [Google Scholar] [CrossRef]
- Joo, J.S.; Weiss, E.G.; Nogueras, J.J.; Wexner, S.D. Endorectal advancement flap in perianal Crohn’s disease. Am. Surg. 1998, 64, 147–150. [Google Scholar]
- Grimaud, J.C.; Munoz-Bongrand, N.; Siproudhis, L.; Abramowitz, L.; Sénéjoux, A.; Vitton, V.; Gambiez, L.; Flourié, B.; Hébuterne, X.; Louis, E.; et al. Fibrin glue is effective healing perianal fistulas in patients with Crohn’s disease. Gastroenterology 2010, 138, 2275–2281. [Google Scholar] [CrossRef]
- Loungnarath, R.; Dietz, D.W.; Mutch, M.G.; Birnbaum, E.H.; Kodner, I.J.; Fleshman, J.W. Fibrin glue treatment of complex anal fistulas has low success rate. Dis. Colon Rectum 2004, 47, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Limura, E.; Giordano, P. Modern management of anal fistula. World J. Gastroenterol. 2015, 21, 12–20. [Google Scholar] [CrossRef]
- Rojanasakul, A. LIFT procedure: A simplified technique for fistula-in-ano. Tech. Coloproctol. 2009, 13, 237–240. [Google Scholar] [CrossRef]
- Kotze, P.G.; Shen, B.; Lightner, A.; Yamamoto, T.; Spinelli, A.; Ghosh, S.; Panaccione, R. Modern management of perianal fistulas in Crohn’s disease: Future directions. Gut 2018, 67, 1181–1194. [Google Scholar] [CrossRef]
- Singh, S.; Ding, N.S.; Mathis, K.L.; Dulai, P.S.; Farrell, A.M.; Pemberton, J.H.; Hart, A.L.; Sandborn, W.J.; Loftus, E.V., Jr. Systematic review with meta-analysis: Faecal diversion for management of perianal Crohn’s disease. Aliment. Pharm. Ther. 2015, 42, 783–792. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Piatetzky-Shapiro, I.I.; Petrakova, K.V. Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 1966, 16, 381–390. [Google Scholar]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of bone marrow: Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef] [PubMed]
- Owen, M. Marrow stromal stem cells. J. Cell Sci. Suppl. 1988, 10, 63–76. [Google Scholar] [CrossRef] [Green Version]
- Sale, G.E.; Storb, R. Bilateral diffuse pulmonary ectopic ossification after marrow allograft in a dog: Evidence for allotransplantation of hemopoietic and mesenchymal stem cells. Exp. Hematol. 1983, 11, 961–966. [Google Scholar]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Tyndall, A.; Walker, U.A.; Cope, A.; Dazzi, F.; De Bari, C.; Fibbe, W.; Guiducci, S.; Jones, S.; Jorgensen, C.; Feldmann, M.; et al. Immunomodulatory properties of mesenchymal stem cells: A review based on an interdisciplinary meeting held at the Kennedy Institute of Rheumatology Division, London, UK, 31 October 2005. Arthritis Res. 2007, 9, 301. [Google Scholar] [CrossRef] [Green Version]
- Pittenger, M.F.; Martin, B.J. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ. Res. 2004, 95, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiyama, T.; Kohara, H.; Noda, M.; Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006, 25, 977–988. [Google Scholar] [CrossRef] [Green Version]
- Dai, W.; Hale, S.L.; Martin, B.J.; Kuang, J.Q.; Dow, J.S.; Wold, L.E.; Kloner, R.A. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: Short- and long-term effects. Circulation 2005, 112, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Ryan, J.M.; Barry, F.; Murphy, J.M.; Mahon, B.P. Interferon-γ does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin. Exp. Immunol. 2007, 149, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Meisel, R.; Zibert, A.; Laryea, M.; Göbel, U.; Däubener, W.; Dilloo, D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 2004, 103, 4619–4621. [Google Scholar] [CrossRef] [Green Version]
- Horton, J.A.; Hudak, K.E.; Chung, E.J.; White, A.O.; Scroggins, B.T.; Burkeen, J.F.; Citrin, D.E. Mesenchymal stem cells inhibit cutaneous radiation-induced fibrosis by suppressing chronic inflammation. Stem Cells 2013, 31, 2231–2241. [Google Scholar] [CrossRef]
- Bartholomew, A.; Sturgeon, C.; Siatskas, M.; Ferrer, K.; McIntosh, K.; Patil, S.; Hardy, W.; Devine, S.; Ucker, D.; Deans, R.; et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002, 30, 42–48. [Google Scholar] [CrossRef]
- Le Blanc, K.; Frassoni, F.; Ball, L.; Locatelli, F.; Roelofs, H.; Lewis, I.; Lanino, E.; Sundberg, B.; Bernardo, M.E.; Remberger, M.; et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet 2008, 371, 1579–1586. [Google Scholar] [CrossRef]
- Ryan, J.M.; Barry, F.P.; Murphy, J.M.; Mahon, B.P. Mesenchymal stem cells avoid allogeneic rejection. J. Inflamm. 2005, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Wang, D.; Liang, J.; Zhang, H.; Feng, X.; Wang, H.; Hua, B.; Liu, B.; Ye, S.; Hu, X.; et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010, 62, 2467–2475. [Google Scholar] [CrossRef]
- Lee, R.H.; Pulin, A.A.; Seo, M.J.; Kota, D.J.; Ylostalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous hMSCs Improve Myocardial Infarction in Mice because Cells Embolized in Lung Are Activated to Secrete the Anti-inflammatory Protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Yamout, B.; Hourani, R.; Salti, H.; Barada, W.; El-Hajj, T.; Al-Kutoubi, A.; Herlopian, A.; Baz, E.K.; Mahfouz, R.; Khalil-Hamdan, R.; et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: A pilot study. J. Neuroimmunol. 2010, 227, 185–189. [Google Scholar] [CrossRef]
- Gallo, G.; La Torre, M.; Pietroletti, R.; Bianco, F.; Altomare, D.F.; Pucciarelli, S.; Gagliardi, G.; Perinotti, R. Italian society of colorectal surgery recommendations for good clinical practice in colorectal surgery during the novel coronavirus pandemic. Tech. Coloproctol. 2020, 24, 501–505. [Google Scholar] [CrossRef] [Green Version]
- Lightner, A.L.; García-Olmo, D. Mesenchymal Stem Cell Therapy Can Transcend Perianal Crohn’s Disease: How Colorectal Surgeons Can Help in the Coronavirus Disease 2019 Crisis. Dis. Colon Rectum 2020, 63, 874–878. [Google Scholar] [CrossRef]
- Mizushima, T.; Takahashi, H.; Takeyama, H.; Naito, A.; Haraguchi, N.; Uemura, M.; Nishimura, J.; Hata, T.; Takemasa, I.; Yamamoto, H.; et al. A clinical trial of autologous adipose-derived regenerative cell transplantation for a postoperative enterocutaneous fistula. Surg. Today 2016, 46, 835–842. [Google Scholar] [CrossRef]
- García-Olmo, D.; García-Arranz, M.; Herreros, D.; Pascual, I.; Peiro, C.; Rodríguez-Montes, J.A. A phase I clinical trial of the treatment of crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis. Colon Rectum 2005, 48, 1416–1423. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, M.Y.; Feng, T.; Feng, R.; Mao, R.; Chen, B.L.; He, Y.; Zeng, Z.R.; Zhang, S.H.; Chen, M.H. Systematic review with meta-analysis: The efficacy and safety of stem cell therapy for Crohn’s disease. Stem Cell Res. 2017, 8, 136. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.H.; Chang, M.C.; Tsai, K.S.; Hung, M.C.; Chen, H.L.; Hung, S.C. Mesenchymal stem cells promote growth and angiogenesis of tumors in mice. Oncogene 2013, 32, 4343–4354. [Google Scholar] [CrossRef] [Green Version]
- Tsai, K.; Yang, S.; Lei, Y.; Tsai, C.; Chen, H.; Hsu, C.; Chen, L.; Wang, H.; Miller, S.A.; Chiou, S.; et al. Mesenchymal stem cells promote formation of colorectal tumors in mice. Gastroenterology 2011, 141, 1046–1056. [Google Scholar] [CrossRef] [Green Version]
- Panés, J.; Garcia-Olmo, D.; van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Danese, S.; et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: A phase 3 randomised, double-blind controlled trial. Lancet 2016, 388, 1281–1290. [Google Scholar] [CrossRef]
- Sasaki, M.; Abe, R.; Fujita, Y.; Ando, S.; Inokuma, D.; Shimizu, H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J. Immunol. 2008, 180, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xiang, L.X.; Shao, J.Z.; Pan, R.L.; Wang, Y.X.; Dong, X.J.; Zhang, G.R. Recruitment of endogenous bone marrow mesenchymal stem cells towards injured liver. J. Cell Mol. Med. 2010, 14, 1494–1508. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.; Dong, C.; Chen, X.; Fang, Z.; Xu, J.; Liu, M.; Zhang, X.; Gu, D.S.; Wang, D.; Han, Z.C.; et al. Human umbilical cord mesenchymal stem cells ameliorate mice trinitrobenzene sulfonic acid (TNBS)-induced colitis. Cell Transpl. 2011, 20, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Kraitchman, D.L.; Tatsumi, M.; Gilson, W.D.; Ishimori, T.; Kedziorek, D.; Walczak, P.; Segars, W.P.; Chen, H.H.; Fritzges, D.; Bulte, J.W.M.; et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation 2005, 112, 1451–1461. [Google Scholar] [CrossRef] [Green Version]
- Anjos-Afonso, F.; Siapati, E.K.; Bonnet, D. In vivo contribution of murine mesenchymal stem cells into multiple cell-types under minimal damage conditions. J. Cell Sci. 2004, 117, 5655–5664. [Google Scholar] [CrossRef] [Green Version]
- Ruster, B.; Gottig, S.; Ludwig, R.J.; Bistrain, R.; Muller, S.; Seifried, E.; Gille, J.; Henschler, R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood 2006, 108, 3938–3944. [Google Scholar] [CrossRef]
- Garcia-Olmo, D.; Herreros, D.; Pascual, I.; Pascual, J.A.; Del-Valle, E.; Zorrilla, J.; De-La-Quintana, P.; Garcia-Arranz, M.; Pascual, M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: A phase II clinical trial. Dis. Colon Rectum 2009, 52, 79–86. [Google Scholar] [CrossRef]
- Garcia-Olmo, D.; Guadalajara-Labajo, H. Stem Cell Application in Fistula Disease. In Anal Fistula; Abcarian, H., Ed.; Springer: New York, NY, USA, 2014; pp. 129–138. [Google Scholar] [CrossRef]
- Molendijk, I.; Bonsing, B.A.; Roelofs, H.; Peeters, K.C.M.J.; Wasser, M.N.J.M.; Dijkstra, G.; Van Der Woude, C.J.; Duijvestein, M.; Veenendaal, R.A.; Zwaginga, J.J. Allogeneic Bone Marrow—Derived Mesenchymal Stromal Cells Promote Healing of Refractory Perianal Fistulas in Patients With Crohn’s Disease. Gastroenterology 2015, 149, 918–927. [Google Scholar] [CrossRef] [Green Version]
- Molendijk, I.; van der Meulen-de Jong, A.E.; Verspaget, H.W.; Veenendaal, R.A.; Hommes, D.W.; Bonsing, B.A.; Peeters, K.C.M.J. Standardization of mesenchymal stromal cell therapy for perianal fistulizing Crohn’s disease. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1148–1154. [Google Scholar] [CrossRef]
- Garcia-Olmo, D.; Herreros, D.; Pascual, M.; Pascual, I.; De-La-Quintana, P.; Trebol, J.; Garcia-Arranz, M. Treatment of enterocutaneous fistula in Crohn’s Disease with adipose-derived stem cells: A comparison of protocols with and without cell expansion. Int. J. Colorectal Dis. 2009, 24, 27–30. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.; Cho, H.; Bae, Y.; Suh, K.; Jung, J. Direct comparison of human mesench-ymal stem cells derived from adipose tissues and bone marrow inmediating neovascularization in response to vascular ischemia. Cell Physiol. Biochem. 2007, 20, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative analysis of mesench-ymal stem cells from bone marrow, umbilical cord blood, or adi-pose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Izadpanah, R.; Trygg, C.; Patel, B.; Kriedt, C.; Dufour, J.; Gimble, J.M.; Bunnell, B.A. Biologic properties of mesench-ymal stem cells derived from bone marrow and adipose tissue. J. Cell Biochem. 2006, 99, 1285–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolic, M.; Stift, A.; Reinisch, W.; Vogelsang, H.; Matic, A.; Müller, C.; von Strauss und Torney, M.; Riss, S. Allogeneic expanded-adipose derived stem cells in the treatment of rectovaginal fistulas in Crohn’s disease. Colorectal Dis. 2020. [Google Scholar] [CrossRef]
- Nie, Y.; Lau, C.; Lie, A.; Chan, G.; Mok, M. Defective phenotype of mesenchymalstem cells in patients with systemic lupus erythematosus. Lupus 2010, 19, 850–859. [Google Scholar] [CrossRef]
- Choudhery, M.S.; Khan, M.; Mahmood, R.; Mehmood, A.; Khan, S.N.; Riazuddin, S. Bone marrow derivedmesenchymal stem cells from aged mice have reduced woundhealing, angiogenesis, proliferation and anti-apoptosiscapabilities. Cell Biol. Int. 2012, 36, 747–753. [Google Scholar] [CrossRef]
- Georgiev-Hristov, T.; Guadalajara, H.; Herreros, M.D.; Lightner, A.L.; Dozois, E.J.; García-Arranz, M.; García-Olmo, D. A Step-By-Step Surgical Protocol for the Treatment of Perianal Fistula with Adipose-Derived Mesenchymal Stem Cells. J. Gastrointest. Surg. 2018, 22, 2003–2012. [Google Scholar] [CrossRef]
- Chudy-Onwugaje, K.O.; Christian, K.E.; Farraye, F.A.; Cross, R.K. A State-of-the-Art Review of New and Emerging Therapies for the Treatment of IBD. Inflamm. Bowel Dis. 2019, 25, 820–830. [Google Scholar] [CrossRef]
- Scott, L.J. Darvadstrocel: A Review in Treatment-Refractory Complex Perianal Fistulas in Crohn’s Disease. BioDrugs 2018, 32, 627–634. [Google Scholar] [CrossRef]
- Kotze, P.G.; Spinelli, A.; Warusavitarne, J.; Di Candido, F.; Sahnan, K.; Adegbola, S.O.; Danese, S. Darvadstrocel for the treatment of patients with perianal fistulas in Crohn’s disease. Drugs Today 2019, 55, 95–105. [Google Scholar] [CrossRef] [PubMed]
- CHMP. European Medicines Agency: EMA/CHMP/64055/2018 Committee for Medicinal Products for Human Use (CHMP) Assessment Report Alofisel [Internet]; CHMP: London, UK, 2017; Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/alofisel (accessed on 3 December 2019).
- Carvello, M.; Lightner, A.; Yamamoto, T.; Kotze, P.G.; Spinelli, A. Mesenchymal Stem Cells for Perianal Crohn’s Disease. Cells 2019, 8, 764. [Google Scholar] [CrossRef] [Green Version]
- Bislenghi, G.; Wolthuis, A.; Van Assche, G.; Vermeire, S.; Ferrante, M.; D’Hoore, A. Expert Opinion on Biological Therapy Cx601 (darvadstrocel) for the treatment of perianal fistulizing Crohn’s disease. Expert Opin. Biol. 2019, 19, 607–616. [Google Scholar] [CrossRef]
- Herreros, M.D.; Garcia-Olmo, D.; Guadalajara, H.; Georgiev-Hristov, T.; Brandariz, L.; Garcia-Arranz, M. Stem Cell Therapy: A Compassionate Use Program in Perianal Fistula. Stem Cells Int. 2019, 2019, 6132340. [Google Scholar] [CrossRef] [Green Version]
- Panés, J.; García-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Danese, S. Long-term Efficacy and Safety of Stem Cell Therapy (Cx601) for Complex Perianal Fistulas in Patients With Crohn’s Disease. Gastroenterology 2018, 154, 1334–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sands, B.E.; Anderson, F.H.; Bernstein, C.N.; Chey, W.Y.; Feagan, B.G.; Fedorak, R.N.; Kamm, M.A.; Korzenik, J.R.; Lashner, B.A.; Rutgeerts, P. Infliximab Maintenance Therapy for Fistulizing Crohn’s Disease. N. Engl. J. Med. 2004, 350, 876–885. [Google Scholar] [CrossRef]
- Lee, W.Y.; Park, K.J.; Cho, Y.B.; Yoon, S.N.; Song, K.H.; Kim, D.S.; Jung, S.H.; Kim, M.; Yoo, H.W.; Kim, I.; et al. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for crohn’s fistula. Stem Cells 2013, 31, 2575–2581. [Google Scholar] [CrossRef]
- Vaegler, M.; Maerz, J.; Amend, B.; Silva, L.; Mannheim, J.; Fuchs, K.; Will, S.; Sievert, K.; Stenzl, A.; Hart, M.; et al. Labelling and Tracking of Human Mesenchymal Stromal Cells in Preclinical Studies and Large Animal Models of Degenerative Diseases. Curr. Stem Cell Res. 2014, 9, 444–450. [Google Scholar] [CrossRef]
- Dietz, A.B.; Dozois, E.J.; Fletcher, J.G.; Butler, G.W.; Radel, D.; Lightner, A.L.; Dave, M.; Friton, J.; Nair, A.; Faubion, W.A.; et al. Autologous Mesenchymal Stem Cells, Applied in a Bioabsorbable Matrix, for Treatment of Perianal Fistulas in Patients With Crohn’s Disease. Gastroenterology 2017, 153, 59–62. [Google Scholar] [CrossRef]
- Dige, A.; Hougaard, H.T.; Agnholt, J.; Pedersen, B.G.; Tencerova, M.; Kassem, M.; Krogh, K.; Lundbyet, L. Efficacy of Injection of Freshly Collected Autologous Adipose Tissue into Perianal Fistulas in Patients with Crohn’s Disease. Gastroenterology 2019, 156, 2208–2216. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Li, M.; Zhang, Y.; Ni, M.; Wang, Y.; Xu, D.; Shi, Y.; Zhang, B.; Chen, Y.; Huang, Y.; et al. Autologous adipose-derived stem cells for the treatment of Crohn’s fistula-in-ano: An open-label, controlled trial. Stem Cell Res. 2020, 11, 124. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Jeon, B.G.; Chae, G.; Lee, S.J. The clinical efficacy of stem cell therapy for complex perianal fistulas: A meta-analysis. Tech. Coloproctol. 2019, 23, 411–427. [Google Scholar] [CrossRef]
- Sanz-Baro, R.; García-Arranz, M.; Guadalajara, H.; de la Quintana, P.; Herreros, M.D.; García-Olmo, D. First-in-Human Case Study: Pregnancy in Women with Crohn’s Perianal Fistula Treated With Adipose-Derived Stem Cells: A Safety Study. Stem Cells Transl. Med. 2015, 4, 598–602. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Bernardo, M.E.; Sgarella, A.; Maccario, R.; Avanzini, M.A.; Ubezio, C.; Minelli, A.; Alvisi, C.; Vanoli, A.; Corazza, G.R.; et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulizing Crohn’s disease. Gut 2011, 60, 788–798. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Gallia, A.; Sgarella, A.; Kruzliak, P.; Gobbi, P.G.; Corazza, G.R. Long-term follow-up of Crohn disease fistulas after local injections of bone marrow-derived mesenchymal stem cells. Mayo Clin. Proc. 2015, 90, 747–755. [Google Scholar] [CrossRef]
- Cho, Y.B.; Lee, W.Y.; Park, K.J.; Kim, M.; Yoo, H.W.; Yu, C.S. Autologous adipose tissue-derived stem cells for the treatment of Crohn’s fistula: A Phase I clinical study. Cell Transpl. 2013, 22, 279–285. [Google Scholar] [CrossRef] [Green Version]
- De La Portilla, F.; Alba, F.; Garcia-Olmo, D.; Herrerias, J.M.; Gonzalez, F.X.; Galindo, A. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: Results from a multicenter Phase I/IIa clinical trial. Int. J. Colorectal Dis. 2013, 28, 313–323. [Google Scholar] [CrossRef]
- Park, K.J.; Ryoo, S.B.; Kim, J.S.; Kim, T.I.; Baik, S.H.; Lee, K.Y.; Kim, M.; Kim, W.H. Allogeneic adipose-derived stem cells for the treatment of perianal fistula in Crohn’s disease: A pilot clinical trial. Colorectal Dis. 2016, 18, 468–476. [Google Scholar] [CrossRef]
- Cho, Y.B.; Park, K.J.; Yoon, S.N.; Song, K.H.; Kim, D.S.; Jung, S.H.; Kim, M.; Jeong, H.Y.; Yu, C.S. Long-term results of adipose-derived stem cell therapy for the treatment of Crohn’s fistula. Stem Cells Transl. Med. 2015, 4, 532–537. [Google Scholar] [CrossRef]
- Barnhoorn, M.C.; Wasser, M.N.J.M.; Roelofs, H.; Maljaars, P.W.J.; Ilse Molendijk, I.; Bonsing, B.A.; Liesbeth, E.M.; Oosten, L.E.M.; Dijkstra, G.; van der Woude, C.J.; et al. Long-term evaluation of allogeneic bone marrow-derived mesenchymal stromal cell therapy for Crohn’s disease perianal fistulas. J. Crohn’s Colitis 2020, 14, 64–70. [Google Scholar] [CrossRef]
- Garcia-Olmo, D.; Guadalajara, H.; Rubio-Perez, I.; Herreros, M.D.; de-la-Quintana, P.; Garcia-Arranz, M. Recurrent anal fistulae: Limited surgery supported by stem cells. World J. Gastroenterol. 2015, 21, 3330–3336. [Google Scholar] [CrossRef] [Green Version]
- Wainstein, C.; Quera, R.; Fluxá, D.; Kronberg, U.; Conejero, A.; López-Köstner, F.; Jofre, C.; Zarate, A.J. Stem Cell Therapy in Refractory Perineal Crohn’s Disease: Long-term Follow-up. Colorectal Dis. 2018. [Google Scholar] [CrossRef]
| Authors | Year | n Patients (Missing) | Cell Type | Intervention | Time-Point | Healing (%) | Follow-Up | Recurrence % |
|---|---|---|---|---|---|---|---|---|
| Garcia-Olmo et al. [64] | 2005 | 9 fistulas in 4 patients with CD: -1 PF (Suprasphincteric) -3 Rectovaginal -5 Enterocutaneous | Adipose autologous | Local injection of 3 × 106 stem cells | 8 weeks | 6/8 (75%) -1/1 -2/3 -3/4 (1 NA) | NA | NA |
| Wainstein et al. [111] | 2008 | 11 fistulas in 8 patients with CD 8 PF (Trans-sphincteric) 1 PF (Inter-sphincteric) 2 Pouch-vaginal | ASCs + Platelet-rich plasma (PRP) | Local injection of 100–120 million ASCs + PRP | 21–37 months | 10/11 (91%) CH 1/11 (9%) PH | 37 months | 0% |
| Garcia-Olmo et al. [75] | 2009 | 49(1) patients (of which 14 with CD) -24 fistulas of which 7 PF + CD -25 fistulas of which 7 PF + CD | Adipose autologous | Local injection of 2 × 106 of Stem cells + Fibrin glue Fibrin glue alone | 8 weeks | -17/24 (70.8%) of which 5/7 (71%) with CD -4/25 (16%) of which 1/7 (14%) with CD | 52 weeks | (17.6%) |
| Ciccocioppo et al. [103,104] | 2011 | 10 patients with CD: -9 PF (NS) -1Enterocutaneous | Bone marrow autologous | Local injection of 1.5–3 × 107 | 52 weeks | 7/10 (70%) -6/9 -1/1 | 52 weeks | 0% |
| de la Portilla et al. [106] | 2012 | 24 (8) patients with PF in CD: 17 PF (Trans-sphincteric) 5 PF (Inter-sphincteric) 1 PF (Extra-sphincteric) 1 PF (Supra-sphincteric) | Adipose allogeneic | Local injection of 2 × 107 (+4 × 107) | 24 weeks | 8/16 (50%) | 24 weeks | 20.0% |
| Cho et al. [105] | 2013 | 9 (1) patients with CD: -3 PF -3 PF -3 PF of which: 5 (Trans-sphincteric) 4 (Supra-sphincteric) 1 (Extra-sphincteric) | Adipose autologous | Local injection of 1 × 107 or 2 × 107 or 4 × 107 | 8 weeks | 3/9 (33.3%) -0/3 -2/3 -1/3 | 8 months | 0% |
| Lee et al. [96,108] | 2013 | 33 (17) patients with PF -24 PF (Trans-sphincteric) -4 PF (Inter-sphincteric) -5 PF (Extrasphincteric) | Adipose autologous | Local injection 3 × 107 or 6 × 107 | 8 weeks | 27/33 (81.8%) | 1 year/2 years After 1 year 23/26 (88.5%) CH After 2 years 20/24 (83.3%) CH | 8 weeks:11.1% 1 year: 11.5% 2 years:16.7% |
| Molendijk et al. [77] | 2015 | 21 patients (with 23 PF): -5 patients with: 3 Trans-sphincteric 1 Inter-sphincteric 1 Supra-sphincteric -5 patients with: 2 Trans-sphincteric 1 Inter-sphincteric 2 Extra-sphincteric -5 patients with: 5 Trans-sphincteric -6 patients with: 5 Trans-sphincteric 1 Inter-sphincteric 1 Superficial 1 Extrasphinteric | Bone marrow alogeneic | Local injection: -1 × 107 -3 × 107 -9 × 107 -NaCl (Placebo) | 24 weeks | -4/5 (80%) -4/5 (80%) -1/5 (20%) -2/6 (33.3%) | 24 weeks | Only 1 Extrasphinteric fistula in placebo group recurred 1/23 = 0.04% |
| Garcia-Olmo-Guadalajara [110] | 2015 | 10 patients with PF (7 non-CD; 3 CD) Type of PF: NS | Autologous ASCs or Allogenic ASCs or SVF | Local injection of 2–3 × 104 cells | 8 weeks | 6/10 (60%) CH 3/10 (30%) PH Of 3 patients with CD: 1 with CD: CH 1 with CD: PH 1 with CD: NO healing | 1 year 60.0% CH | 40.0% |
| Park et al. [107] | 2015 | 6 patients with CD: -3 PF (Suprasphincteric) -3 Fistulas: 1 PF (Suprasphincteric) 1 PF (Trans-sphincteric) 1 Rectovaginal | Adipose allogeneic | Local injection of 4.33 × 107 17 × 107 | 34 weeks | 3/6 (50%) -2/3 -1/3 | NA | |
| Panes et al. [68,94] | 2016 | 171(41) patients with PF: -88 (19) PF -83 (22) PF Type of PF: NS | Adipose allogeneic | Local injection: -12 × 107 -Placebo | 24 weeks | 53/107(49.5%) 36/105(34.3%) | 52 weeks | (25%) (44.1%) |
| Dietz et al. [98] | 2017 | 12 patients with PF in CD: 8 PF (Trans-sphincteric) 3 PF (Inter-sphincteric) 1 PF (Suprasphincteric) | Adipose autologous | Local injection of 2 × 106 on GoreBioA plug | 26 weeks | 10/12 (83.3%) | NA | |
| Herreros et al. [93] | 2019 | 45 patients (52 fistulas): 18 PF in CD (NS) 24 PF non-CD (NS) 7 Rectovaginal 1 Urethrorectal 1 Sacral 1 Hidradenitis suppurativa | Autologous ASCs or Allogenic ASCs or SVF | Local injection of around 48 million cells: SVF (31/52) 60% Allo-ASCs (12/52) 23% Auto-ASCs (9/52) 17% | 26 weeks | 49/52 (94.2%) PH–CH 24 /52 (46.2%) CH Of 18 CD fistulas: 10/18 (55.5%) CH | 1 year | 0% |
| Barnhoorn et al. [109] | 2020 | 21(5) patients with PF: -5(1) patients -5 (1) patients -5 patients -6 (3) patients Type of PF: NS | Bone marrow alogeneic | Local injection: -1 × 107 -3 × 107 -9 × 107 -Placebo | 4 years | -3/4(75%) -4/4(100%) -1/5(20%) -0/3(0%) | 4 years | Only in placebo-group all fistulas recurred after 4 year. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, G.; Tiesi, V.; Fulginiti, S.; De Paola, G.; Vescio, G.; Sammarco, G. Mesenchymal Stromal Cell Therapy in the Management of Perianal Fistulas in Crohn’s Disease: An Up-To-Date Review. Medicina 2020, 56, 563. https://doi.org/10.3390/medicina56110563
Gallo G, Tiesi V, Fulginiti S, De Paola G, Vescio G, Sammarco G. Mesenchymal Stromal Cell Therapy in the Management of Perianal Fistulas in Crohn’s Disease: An Up-To-Date Review. Medicina. 2020; 56(11):563. https://doi.org/10.3390/medicina56110563
Chicago/Turabian StyleGallo, Gaetano, Vincenzo Tiesi, Serena Fulginiti, Gilda De Paola, Giuseppina Vescio, and Giuseppe Sammarco. 2020. "Mesenchymal Stromal Cell Therapy in the Management of Perianal Fistulas in Crohn’s Disease: An Up-To-Date Review" Medicina 56, no. 11: 563. https://doi.org/10.3390/medicina56110563
APA StyleGallo, G., Tiesi, V., Fulginiti, S., De Paola, G., Vescio, G., & Sammarco, G. (2020). Mesenchymal Stromal Cell Therapy in the Management of Perianal Fistulas in Crohn’s Disease: An Up-To-Date Review. Medicina, 56(11), 563. https://doi.org/10.3390/medicina56110563






