Changes in Bond Strength and Topography for Y-TZP Etched with Hydrofluoric Acid Depending on Concentration and Temperature Conditions
Abstract
:1. Introduction
2. Materials and Methods
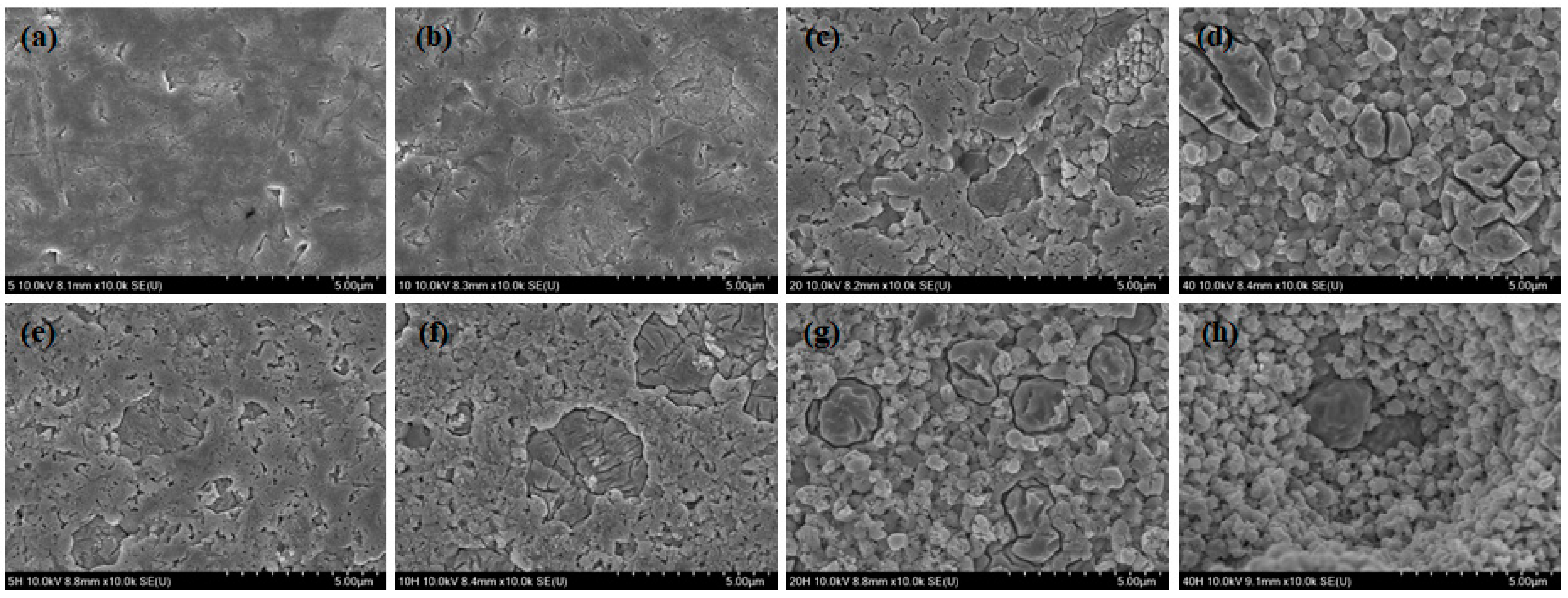
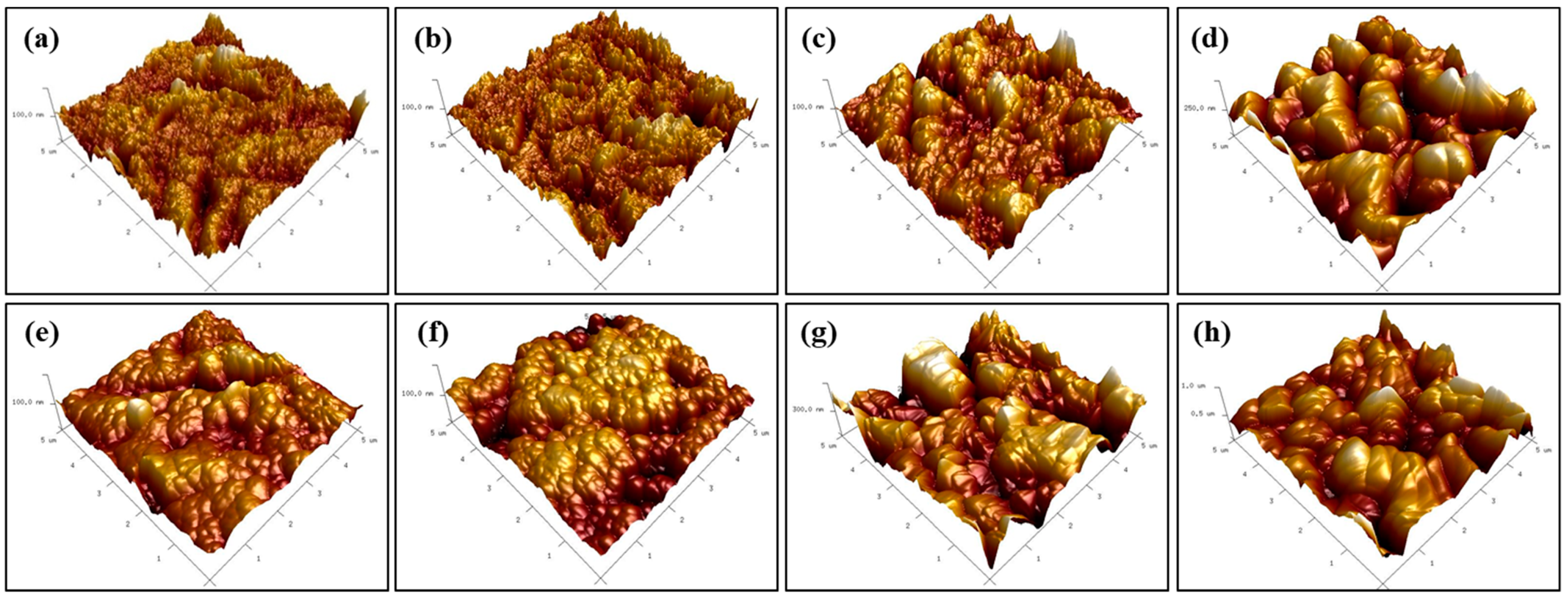
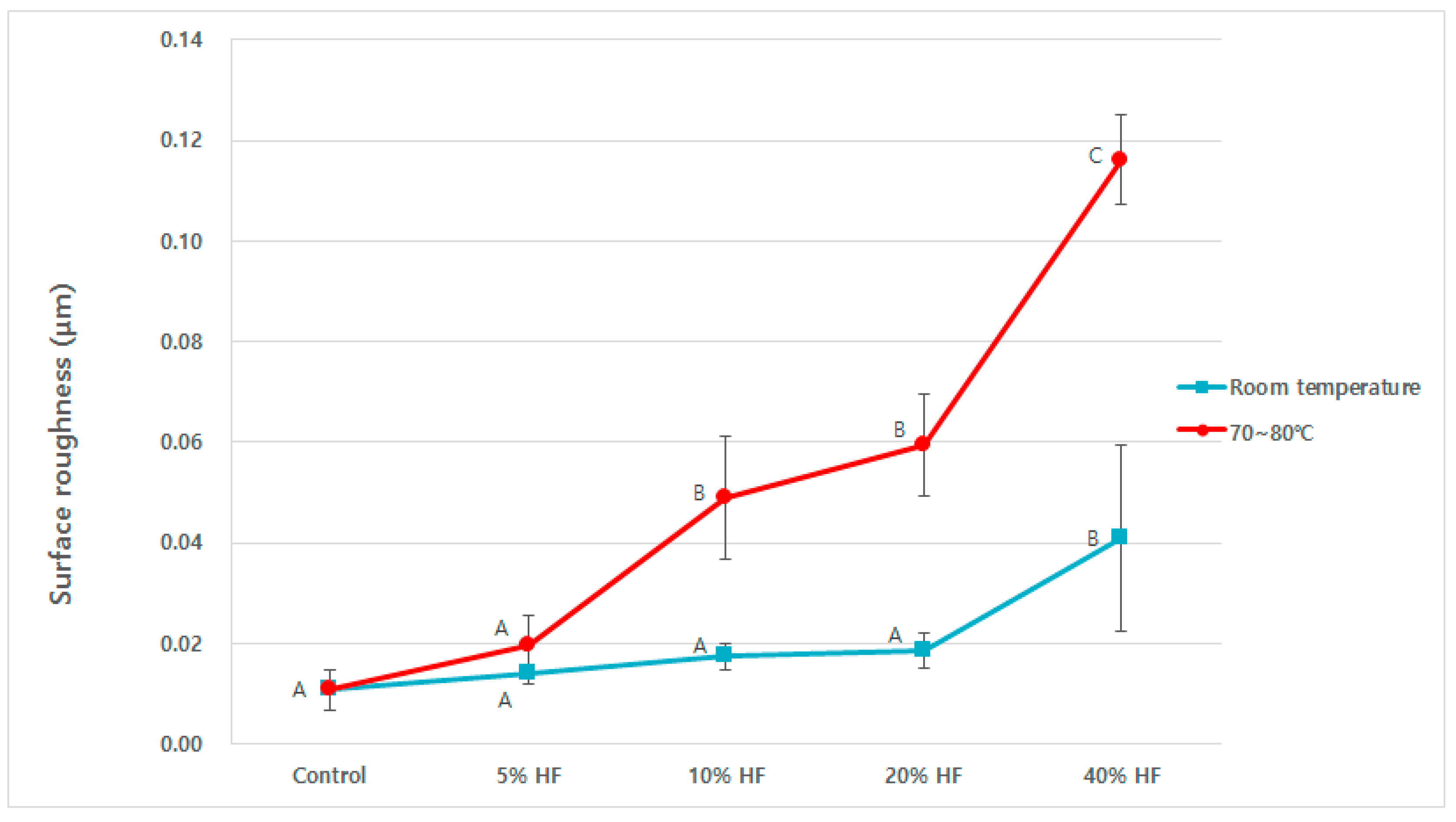
3. Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Denry, I.; Kelly, J.R. State of the art of zirconia for dental applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, M.; Vallittu, P.K. Effect of surface conditioning methods on the bond strength of luting cement to ceramics. Dent. Mater. 2003, 19, 725–731. [Google Scholar] [CrossRef] [Green Version]
- Manicon, P.F.; Iommetti, P.R.; Raffaelli, L. An overview of zirconia ceramics: Basic properties and clinical applications. J. Dent. 2007, 35, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Beuer, F.; Schweiger, J.; Edelhoff, D. Digital dentistry: An overview of recent developments for CAD/CAM generated restorations. Br. Dent. J. 2008, 204, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Blatz, M.B.; Sadan, A.; Kern, M. Resin-ceramic bonding: A review of the literature. J. Prosthet. Dent. 2003, 89, 268–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derand, T.; Molin, M.; Kvam, K. Bond strength of composite luting cement to zirconia ceramic surfaces. Dent. Mater. 2005, 21, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.Y.; Stoner, B.R.; Piascik, J.R.; Smith, R. Adhesion/cementation to zirconia and other non-silicate ceramics: Where are we now? Dent. Mater. 2011, 27, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Burke, F.J.; Fleming, G.J.; Nathanson, D.; Marquis, P.M. Are adhesive technologies needed to support ceramics? An assessment of the current evidence. J. Adhes Dent. 2002, 4, 7–22. [Google Scholar]
- Yoshida, K.; Yamashita, M.; Atsuta, M. Zirconate coupling agent for bonding resin luting cement to pure zirconium. Am. J. Dent. 2004, 17, 249–252. [Google Scholar]
- Yoshida, K.; Tsuo, Y.; Atsuta, M. Bonding of dual-cured resin cement to zirconia ceramic using phosphate acid ester monomer and zirconate coupler. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 77, 28–33. [Google Scholar] [CrossRef] [Green Version]
- Kitayama, S.; Nikaido, T.; Takahashi, R.; Zhu, L.; Ikeda, M.; Foxton, R.M.; Sadr, A.; Tagami, J. Effect of primer treatment on bonding of resin cements to zirconia ceramic. Dent. Mater. 2010, 26, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, N.; Yoshihara, K.; Feitosa, V.P.; Tamada, Y.; Irie, M.; Yoshida, Y.; Van Meerbeek, B.; Hayakawa, S. Chemical interaction mechanism of 10-MDP with zirconia. Sci. Rep. 2017, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Lim, M.J.; Yu, M.K.; Lee, K.W. The effect of continuous application of MDP-containing primer and luting resin cement on bond strength to tribochemical silica-coated Y-TZP. Restor. Dent. Endod. 2018, 43, e19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attia, A. Bond strength of three luting agents to zirconia ceramic-influence of surface treatment and thermocycling. J. Appl. Oral Sci. 2011, 19, 388–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, R.; Fujishima, A.; Shibata, Y.; Manabe, A.; Miyazaki, T. Cooperation of phosphate monomer and silica modification on zirconia. J. Dent. Res. 2008, 87, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Rüttermann, S.; Fries, L.; Raab, W.H.; Janda, R. The effect of different bonding techniques on ceramic/resin shear bond strength. J. Adhes Dent. 2008, 10, 197–203. [Google Scholar] [PubMed]
- Piwowarczyk, A.; Lauer, H.C.; Sorensen, J.A. The shear bond strength between luting cements and zirconia ceramics after two pre-treatments. Oper. Dent. 2005, 30, 382–388. [Google Scholar]
- Cavalcanti, A.N.; Foxton, R.M.; Watson, T.F.; Oliveira, M.T.; Giannini, M.; Marchi, G.M. Bond strength of resin cements to a zirconia ceramic with different surface treatments. Oper. Dent. 2009, 34, 280–287. [Google Scholar] [CrossRef] [Green Version]
- Guazzato, M.; Albakry, M.; Quach, L.; Swain, M.V. Influence of surface and heat treatments on the flexural strength of a glass-infiltrated alumina/zirconia-reinforced dental ceramic. Dent. Mater. 2005, 21, 454–463. [Google Scholar] [CrossRef]
- Passos, S.P.; Linke, B.; Major, P.W.; Nychka, J.A. The effect of air-abrasion and heat treatment on the fracture behavior of Y-TZP. Dent. Mater. 2015, 31, 1011–1021. [Google Scholar] [CrossRef]
- Aboushelib, M.N.; Kleverlaan, C.J.; Feilzer, A.J. Selective infiltration-etching technique for a strong and durable bond of resin cements to zirconia-based materials. J. Prosthet. Dent. 2007, 98, 379–388. [Google Scholar] [CrossRef]
- Casucci, A.; Osorio, E.; Osorio, R.; Monticelli, F.; Toledano, M.; Mazzitelli, C.; Ferrari, M. Influence of different surface treatments on surface zirconia frameworks. J. Dent. 2009, 37, 891–897. [Google Scholar] [CrossRef]
- Sriamporn, T.; Thamrongananskul, N.; Busabok, C.; Poolthong, S.; Uo, M.; Tagami, J. Dental zirconia can be etched by hydrofluoric acid. Dent. Mater. J. 2014, 33, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaiyabutr, Y.; McGowan, S.; Phillips, K.M.; Kois, J.C.; Giordano, R.A. The effect of hydrofluoric acid surface treatment and bond strength of a zirconiaveneering ceramic. J. Prosthet. Dent. 2008, 100, 194–202. [Google Scholar] [CrossRef]
- Genuino, H.C.; Opembe, N.N.; Njagi, E.C.; McClain, S.; Suib, S.L. A review of hydrofluoric acid and its use in the car wash industry. J. Ind. Eng. Chem. 2012, 18, 1529–1539. [Google Scholar] [CrossRef]
- Ozcan, M.; Allahbeickaraghi, A.; Dundar, M. Possible hazardous effects of hydrofluoric acid and recommendations for treatment approach: A review. Clin. Oral Investig. 2012, 16, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Alex, G. Preparing porcelain surfaces for optimal bonding. Compend. Contin. Educ. Dent. 2008, 29, 324–335. [Google Scholar]
- Callister, W.D., Jr.; Rethwisch, D.G. Materials Science and Engineering: An Introduction, 8th ed.; John Wiley SonsInc: New York, NY, USA, 2010. [Google Scholar]
- Flamant, Q.; Marro, F.G.; Rovira, J.J.R.; Anglada, M. Hydrofluoric acid etching of dental zirconia. Part 1: Etching mechanism and surface characterization. J. Eur Ceram. Soc. 2016, 36, 1211–1234. [Google Scholar] [CrossRef] [Green Version]
- Lowalekar, V.; Raghavan, S. Etching of zirconium oxide, hafnium oxide, and hafnium silicates in dilute hydrofluoric acid solutions. J. Mater. Res. 2004, 19, 1149–1156. [Google Scholar] [CrossRef]
- Yu, M.K.; Lim, M.J.; Na, N.R.; Lee, K.W. Effect of hydrofluoric acid-based etchant at an elevated temperature on the bond strength and surface topography of Y-TZP ceramics. Restor. Dent. Endod. 2019, 45, e6. [Google Scholar] [CrossRef]
- Hawkinson, T.E.; Korpela, D.B. Chemical hazards in semiconductor operations. In Semiconductor Safety Handbook: Safety and Health in the Semiconductor Industry; William Andrew: New York, NY, USA, 1997. [Google Scholar]
- Smielak, B.; Klimek, L. Effect of hydrofluoric acid concentration and etching duration on select surface roughness parameters for zirconia. J. Prosthet. Dent. 2015, 113, 596–602. [Google Scholar] [CrossRef]
- Lughi, V.; Sergo, V. Low temperature degradation–Aging-of zirconia: A critical review of the relevant aspects in dentistry. Dent. Mater. 2010, 26, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, J.; Gremillart, L.; Deville, S. Low-Temperature Degradation of Zirconia and Implications for Biomedical Implants. Annu. Rev. Mater. Res. 2007, 37, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.H.; Son, J.S.; Kim, K.H.; Kwon, T.Y. Improved resin-zirconia bonding by room temperature hydrofluoric acid etching. Materials 2015, 8, 8508–8566. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Material/Trade Name | Manufacturer | Main Components |
|---|---|---|
| Hydrofluoric acid (HF) /Hydrofluoric acid | Merck KGaA, Darmstadt, Germany | 48 wt% in H2O |
| Zirconia Primer /Z-prime Plus | Bisco Inc., Schaumbrug, IL, USA | BPDM, HEMA, ethanol |
| MDP-containing resin cement /G-CEM LinkAce | GC Corp., Tokyo, Japan | Paste A: Fluoro-alumino-silicate glass, UDMA, dimethacrylate, Silicon dioxide Paste B: Phosphoric acid ester monomer (MDP), silicon dioxide, UDMA, dimethacrylate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-E.; Lim, M.-J.; Yu, M.-K.; Lee, K.-W. Changes in Bond Strength and Topography for Y-TZP Etched with Hydrofluoric Acid Depending on Concentration and Temperature Conditions. Medicina 2020, 56, 568. https://doi.org/10.3390/medicina56110568
Kim H-E, Lim M-J, Yu M-K, Lee K-W. Changes in Bond Strength and Topography for Y-TZP Etched with Hydrofluoric Acid Depending on Concentration and Temperature Conditions. Medicina. 2020; 56(11):568. https://doi.org/10.3390/medicina56110568
Chicago/Turabian StyleKim, Hyo-Eun, Myung-Jin Lim, Mi-Kyung Yu, and Kwang-Won Lee. 2020. "Changes in Bond Strength and Topography for Y-TZP Etched with Hydrofluoric Acid Depending on Concentration and Temperature Conditions" Medicina 56, no. 11: 568. https://doi.org/10.3390/medicina56110568
APA StyleKim, H.-E., Lim, M.-J., Yu, M.-K., & Lee, K.-W. (2020). Changes in Bond Strength and Topography for Y-TZP Etched with Hydrofluoric Acid Depending on Concentration and Temperature Conditions. Medicina, 56(11), 568. https://doi.org/10.3390/medicina56110568




