Development and Improvement of an Effective Method for Air and Surfaces Disinfection with Ozone Gas as a Decontaminating Agent
Abstract
:1. Introduction
1.1. Environmental and Human Health Risks
- Use the ozone sanitization cycle only in the absence of people;
- Do not use in the presence of flammable substances such as alcohol, petrol, hydrocarbons, bromine, hydrobromic acid, nitrogen oxides and nitroglycerin;
- Avoid exposure to UV rays produced by fluorescent lamps;
- Seal off the doors and windows of the environments before beginning ozone generation using proper sealing gummed papers in the door and window blows.
1.2. Sanitization Procedures
- Before using the ozone generator, check the correct positioning of the supply;
- Make sure that a differential switch automatically protects the power socket upstream;
- Connect the unit to a grounded power outlet;
- Place the ozone generator in the center of the room.
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- La Rosa, G.; Fratini, M.; Della Libera, S.; Iaconelli, M.; Muscillo, M. Viral infections acquired indoors through airborne, droplet or contact transmission. Ann. Dell’Ist. Super. Sanita 2013, 49, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Brosseau, L.M. Aerosol Transmission of Infectious Disease. J. Occup. Environ. Med. 2015, 57, 501–508. [Google Scholar] [CrossRef]
- Fernstrom, A.; Goldblatt, M. Aerobiology and Its Role in the Transmission of Infectious Diseases. J. Pathog. 2013, 6, 493960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinds, W.C. Aerosol Technology: Properties, Behavior and Measurement of Airborne Particles, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1999; Volume 504. [Google Scholar]
- Herfst, S.; Bohringer, M.; Karo, B.; Lawrence, P.; Lewis, N.S.; Mina, M.J.; Russell, C.J.; Steel, J.; De Swart, R.L.; Menge, C. Drivers of airborne human-to human pathogen transmission. Curr. Opin. Virol. 2017, 22, 22–29. [Google Scholar] [CrossRef] [PubMed]
- CDC. Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Health-Care Facilities, 1994; Morbidity and Mortality Weekly Report; CDC: Atlanta, GA, USA, 1994; Volume 43, pp. 1–132. [Google Scholar]
- Motta, O.; Zarrella, I.; Cucciniello, R.; Capunzo, M.; De Caro, F. A new strategy to control the proliferation of microorganisms in solid hospital waste and the diffusion of nosocomial infections. Infez. Med. 2018, 3, 210–215. [Google Scholar]
- Proto, A.; Zarrella, I.; Cucciniello, R.; Pironti, C.; De Caro, F.; Motta, O. Bactericidal and Fungicidal Activity in the Gas Phase of Sodium Dichloroisocyanurate (NaDCC). Curr. Microbiol. 2016, 73, 287–291. [Google Scholar] [CrossRef]
- Motta, O.; Zarrella, I.; Cucciniello, R.; Vigliotta, G.; Proto, A. Study of the antibacterial activity in the gas phase of a chemical formulation for household waste management. Lett. Appl. Microbiol. 2014, 60, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Alfano, D.; Albunia, A.R.; Motta, O.; Proto, A. Detection of diagenetic alterations by Spectroscopic Analysis on Archaeological Bones from the Necropolis of Poseidonia (Paestum): A case study. J. Cult. Herit. 2009, 10, 509–513. [Google Scholar] [CrossRef]
- Arnold, C. Rethinking sterile: The hospital microbiome. Environ. Health Perspect. 2014, 122, A182–A187. [Google Scholar] [CrossRef]
- Vigliotta, G.; Motta, O.; Guarino, F.; Iannece, P.; Proto, A. Assessment of perchlorate-reducing bacteria in a highly polluted river. Int. J. Hyg. Environ. Health 2010, 213, 437–444. [Google Scholar] [CrossRef]
- Scott, E.; Bloomfield, S.F. The survival and transfer of microbial contamination via cloths, hand and utensils. J. Appl. Bacteriol. 1990, 68, 271–278. [Google Scholar] [CrossRef]
- Hoy, R.H. Accidental systemic exposure to sodium hypochlorite (Clorox) during hemodialysis. Am. J. Hosp. Pharm. 1981, 38, 1512–1514. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, S.W.; Rajs, J.; Jonsson, J.A.; Persson, H. Poisoning with sodium hypochlorite solution. Report of a fatal case, supplemented with an experimental and clinico-epidemiological study. Am. J. Forensic Med. Pathol. 1991, 12, 320–327. [Google Scholar] [CrossRef]
- Gilbert, P.; McBain, A.J. Potential impact of increased use of biocides in consumer products on prevalence of antibiotic resistance. Clin. Microbiol. Rev. 2003, 16, 189–208. [Google Scholar] [CrossRef] [Green Version]
- Fraise, A.P. Choosing disinfectants. J. Hosp. Infect. 1999, 43, 255–264. [Google Scholar] [CrossRef]
- Pitten, F.A.; Werner, H.P.; Kramer, A. A standardized test to assess the impact of different organic challenges on the antimicrobial activity of antiseptics. J. Hosp. Infect. 2003, 55, 108–115. [Google Scholar] [CrossRef]
- Sousa, C.S.; Torres, L.M.; Azevedo, M.P.; de Camargo, T.C.; Graziano, K.U.; Lacerda, R.A.; Turrini, R.N. Sterilization with ozone in health care: An integrative literature review. Rev. Esc. Enferm. USP 2011, 45, 1243–1249. [Google Scholar] [CrossRef] [Green Version]
- Sauerbrei, A.; Schacke, M.; Gluck, B.; Bust, U.; Rabenau, H.F.; Wutzler, P. Does limited virucidal activity of biocides include duck hepatitis B virucidal action. BMC Infect. Dis. 2012, 12, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Drug Administration. GRAS status of ozone. Fed. Regist. 1982, 47, 50209–50210. [Google Scholar]
- Rubio-Romero, J.C.; Pardo-Ferreira, M.D.C.; Torrecilla-García, J.A.; Calero-Castro, S. Disposable masks: Disinfection and sterilization for reuse, and non-certified manufacturing, in the face of shortages during the COVID-19 pandemic. Saf. Sci. 2020, 129, 104830. [Google Scholar] [CrossRef]
- Dubuis, M.-E.; Dumont-Leblond, N.; Laliberté, C.; Veillette, M.; Turgeon, N.; Jean, J.; Duchaine, C. Ozone efficacy for the control of airborne viruses: Bacteriophage and norovirus models. PLoS ONE 2020, 15, e0231164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Sci. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matyssek, R.; Wieser, G.; Calfapietra, C.; De Vries, W.; Dizengremel, P.; Ernst, D.; Tuovinen, J.P. Forests under climate change and air pollution: Gaps in understanding and future directions for research. Environ. Pollut. 2012, 160, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Cucciniello, R.; Proto, A.; Rossi, F.; Marchettini, N.; Motta, O. An improved method for BTEX extraction from charcoal. Anal. Methods 2015, 7, 4811–4815. [Google Scholar] [CrossRef]
- Hwang, B.-F.; Chen, Y.-H.; Lin, Y.-T.; Wu, X.-T.; Lee, Y.-L. Relationship between exposure to fine particulates and ozone and reduced lung function in children. Environ. Res. 2015, 137, 382–390. [Google Scholar] [CrossRef]
- Lakey, P.S.J.; Morrison, G.C.; Won, Y.; Parry, K.M.; von Domaros, M.; Tobias, D.J.; Rim, D.; Shiraiwa, M. The impact of clothing on ozone and squalene ozonolysis products in indoor environments. Commun. Chem. 2019, 2, 56. [Google Scholar] [CrossRef] [Green Version]
- Salvador, C.M.; Bekö, G.; Weschler, C.J.; Morrison, G.; Le Breton, M.; Hallquist, M.; Ekberg, L.; Langer, S. Indoor ozone/human chemistry and ventilation strategies. Indoor Air 2019, 29, 913–925. [Google Scholar] [CrossRef] [Green Version]
- Fadeyi, M.O. Ozone in indoor environments: Research progress in the past 15 years. Sustain. Cities Soc. 2015, 18, 78–94. [Google Scholar] [CrossRef]
- Wisthaler, A.; Tamás, G.; Wyon, D.P.; Strøm-Tejsen, P.; Space, D.; Beauchamp, J.; Hansel, A.; Märk, T.D.; Weschler, C.J. Products of ozone-initiated chemistry in a simulated aircraft environment. Environ. Sci. Technol. 2005, 39, 4823–4832. [Google Scholar] [CrossRef]
- Saxena, P.; Sonwani, S. Remediation of ozone pollution by ornamental plants in indoor environment. Glob. J. Environ. Sci. Manag. 2020, 6, 497–508. [Google Scholar]
- Moccia, G.; Motta, O.; Pironti, C.; Proto, A.; Capunzo, M.; de Caro, F. A multidisciplinary approach for the decontamination of hospital settings. J. Infect. Public Health 2020. [Google Scholar] [CrossRef]
- Motta, O.; De Caro, F.; Quarto, F.; Proto, A.J. New FTIR methodology for the evaluation of 13C/12C isotope ratio in Helicobacter pylori infection diagnosis. J. Infect. 2009, 59, 90–94. [Google Scholar] [CrossRef]
- Hwang, S.H.; Park, W.M. Indoor air concentrations of carbon dioxide (CO2), nitrogen dioxide (NO2), and ozone (O3) in multiple healthcare facilities. Environ. Geochem. Health 2019, 42, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.R.; Hoffman, P.N.; Egan, K.; Jacobson, S.K.; Colville, A.; Spencer, W.; Larkin, S.; Jenks, P.J. Guidance for the decontamination of intracavity medical devices: The report of a working group of the Healthcare Infection Society. J. Hosp. Infect. 2019, 101, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.; Mohan, S.; Miller, R.; Tumin, D.; Uffman, J.C.; Tobias, J.D. Surface contamination in the operating room: Use of adenosine triphosphate monitoring. J. Anesth. 2019, 33, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob. Resist. Infect. Control 2016, 5, 5–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutala, W.A.; Weber, D.J. Best practices for disinfection of noncritical environmental surfaces and equipment in health care facilities: A bundle approach. Am. J. Infect. Control 2019, 47, A96–A105. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Disinfection, sterilization, and antisepsis: An overview. Am. J. Infect. Control 2019, 44, e1–e6. [Google Scholar] [CrossRef]
- Simmons, B.P. CDC guidelines for the prevention and control of nosocomial infections. Guideline for hospital environmental control. Am. J. Infect. Control 1983, 11, 97–120. [Google Scholar] [CrossRef]
- Alfa, M.J.; DeGagne, P.; Olson, N.; Puchalski, T. Comparison of ion plasma, vaporized hydrogen peroxide, and 100% ethylene oxide sterilizers to the 12/88 ethylene oxide gas sterilizer. Infect. Control Hosp. Epidemiol. 1996, 17, 92–100. [Google Scholar] [CrossRef]
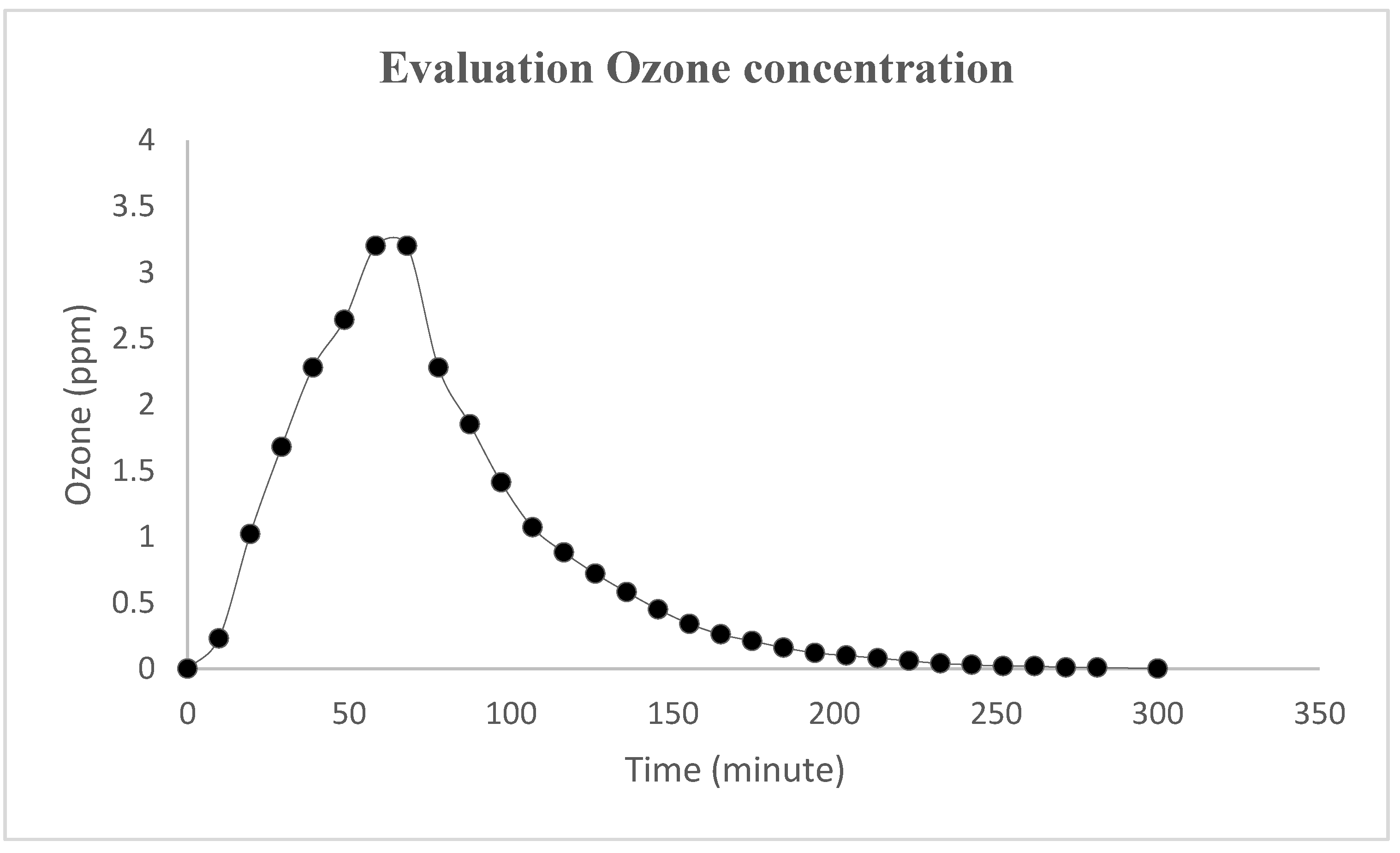
| Hygienic Surface Performance | Good | Satisfying | Unsatisfying | |
| CFU/plate | 0–25 | 26–50 | over 50 | |
| Hygienic Air Performance | A | B | C | D |
| CFU/m3 | <1 | 10 | 100 | 200 |
| AIR and Surface Analysed | Sabouraud Pre-Treatment | SD | Sabouraud Post Treatment | SD | (PCA) Pre-Treatent | SD | (PCA) Post Treatment | SD |
|---|---|---|---|---|---|---|---|---|
| AIR (CFU/m3) | 0 | 0 | 105 | ±4 | 1 | ±1 | ||
| DESK (CFU/plate) | 10 | ±1 | 5 | ±1 | 35 | ±3 | 5 | ±1 |
| TABLE (CFU/plate) | 10 | ±2 | 5 | ±1 | 18 | ±2 | 2 | ±2 |
| PRINTER (CFU/plate) | 14 | ±1 | 7 | ±2 | 10 | ±1 | 3 | ±1 |
| Air-conditioning (CFU/plate) | 10 | ±1 | 7 | ±1 | 12 | ±2 | 4 | ±1 |
| White coats (CFU/plate) | 0 | 0 | 36 | ±1 | 1 | ±1 |
| AIR and Surface Analysed | Sabouraud Pre-Treatment | Sabouraud Post Treatment | (PCA) Pre-Treatment | (PCA) Post Treatment |
|---|---|---|---|---|
| AIR (CFU/m3) | 0 | 0 | 5 | 1 |
| DESK (CFU/plate) | 0 | 0 | 2 | 0 |
| TABLE (CFU/plate) | 0 | 0 | 0 | 0 |
| Furniture (CFU/plate) | 0 | 0 | 1 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moccia, G.; De Caro, F.; Pironti, C.; Boccia, G.; Capunzo, M.; Borrelli, A.; Motta, O. Development and Improvement of an Effective Method for Air and Surfaces Disinfection with Ozone Gas as a Decontaminating Agent. Medicina 2020, 56, 578. https://doi.org/10.3390/medicina56110578
Moccia G, De Caro F, Pironti C, Boccia G, Capunzo M, Borrelli A, Motta O. Development and Improvement of an Effective Method for Air and Surfaces Disinfection with Ozone Gas as a Decontaminating Agent. Medicina. 2020; 56(11):578. https://doi.org/10.3390/medicina56110578
Chicago/Turabian StyleMoccia, Giuseppina, Francesco De Caro, Concetta Pironti, Giovanni Boccia, Mario Capunzo, Anna Borrelli, and Oriana Motta. 2020. "Development and Improvement of an Effective Method for Air and Surfaces Disinfection with Ozone Gas as a Decontaminating Agent" Medicina 56, no. 11: 578. https://doi.org/10.3390/medicina56110578
APA StyleMoccia, G., De Caro, F., Pironti, C., Boccia, G., Capunzo, M., Borrelli, A., & Motta, O. (2020). Development and Improvement of an Effective Method for Air and Surfaces Disinfection with Ozone Gas as a Decontaminating Agent. Medicina, 56(11), 578. https://doi.org/10.3390/medicina56110578








