TET2/IDH1/2/WT1 and NPM1 Mutations Influence the RUNX1 Expression Correlations in Acute Myeloid Leukemia
Abstract
1. Introduction
2. Material and Methods
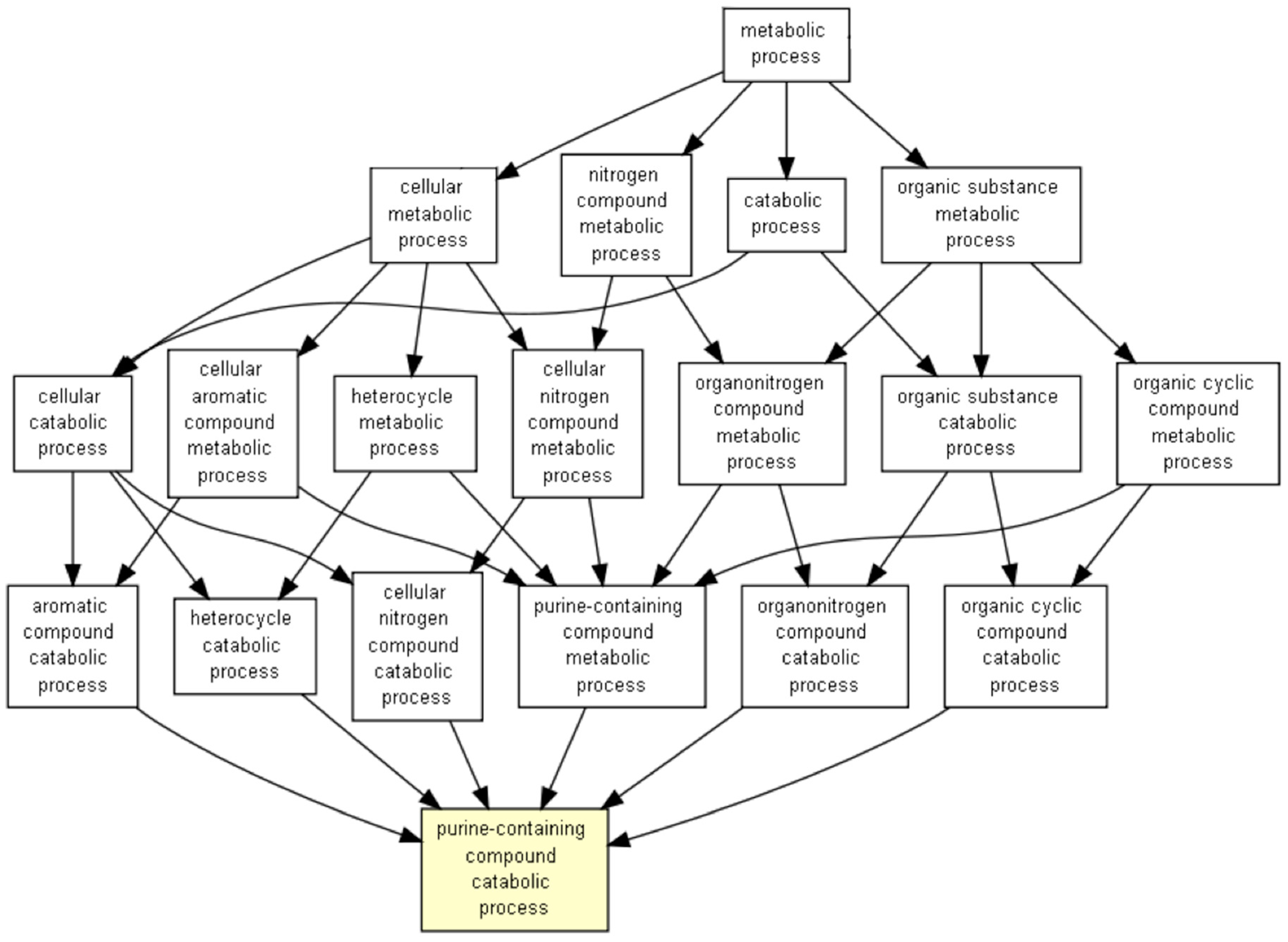
3. Results
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khwaja, A.; Bjorkholm, M.; Gale, R.E.; Levine, R.L.; Jordan, C.T.; Ehninger, G.; Bloomfield, C.D.; Estey, E.; Burnett, A.; Cornelissen, J.J.; et al. Acute myeloid leukaemia. Nat. Rev. Dis. Primers 2016, 2, 16010. [Google Scholar] [CrossRef] [PubMed]
- Pasca, S.; Turcas, C.; Jurj, A.; Teodorescu, P.; Iluta, S.; Hotea, I.; Bojan, A.; Selicean, C.; Fetica, B.; Petrushev, B.; et al. The Influence of Methylating Mutations on Acute Myeloid Leukemia: Preliminary Analysis on 56 Patients. Diagnostics 2020, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Bowman, R.L.; Levine, R.L. TET2 in Normal and Malignant Hematopoiesis. Cold Spring Harb. Perspect. Med. 2017, 7, a026518. [Google Scholar] [CrossRef] [PubMed]
- Amaya, M.L.; Pollyea, D.A. Targeting the IDH2 Pathway in Acute Myeloid Leukemia. Clin. Cancer Res. 2018, 24, 4931–4936. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, M.; Chen, X.; Chen, L.; Xu, Y.; Lv, L.; Wang, P.; Yang, H.; Ma, S.; Lin, H.; et al. WT1 Recruits TET2 to Regulate Its Target Gene Expression and Suppress Leukemia Cell Proliferation. Mol. Cell 2015, 57, 662–673. [Google Scholar] [CrossRef]
- Moran-Crusio, K.; Reavie, L.; Shih, A.; Abdel-Wahab, O.; Ndiaye-Lobry, D.; Lobry, C.; Figueroa, M.E.; Vasanthakumar, A.; Patel, J.; Zhao, X.; et al. Tet2 Loss Leads to Increased Hematopoietic Stem Cell Self-Renewal and Myeloid Transformation. Cancer Cell 2011, 20, 11–24. [Google Scholar] [CrossRef]
- Figueroa, M.E.; Lugthart, S.; Li, Y.; Erpelinck-Verschueren, C.; Deng, X.; Christos, P.J.; Schifano, E.; Booth, J.; van Putten, W.; Skrabanek, L.; et al. DNA Methylation Signatures Identify Biologically Distinct Subtypes in Acute Myeloid Leukemia. Cancer Cell 2010, 17, 13–27. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data: Figure 1. Cancer Dis. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- The Gene Ontology Consortium. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019, 47, D330–D338. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Eden, E.; Navon, R.; Steinfeld, I.; Lipson, D.; Yakhini, Z. GOrilla: A tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinform. 2009, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yan, J.; Matheny, C.J.; Corpora, T.; Bravo, J.; Warren, A.J.; Bushweller, J.H.; Speck, N.A. Energetic Contribution of Residues in the Runx1 Runt Domain to DNA Binding. J. Biol. Chem. 2003, 278, 33088–33096. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Ebrahem, Q.; Mahfouz, R.Z.; Hasipek, M.; Enane, F.; Radivoyevitch, T.; Rapin, N.; Przychodzen, B.; Hu, Z.; Balusu, R.; et al. Leukemogenic nucleophosmin mutation disrupts the transcription factor hub that regulates granulomonocytic fates. J. Clin. Investig. 2018, 128, 4260–4279. [Google Scholar] [CrossRef]
- Cameron, S.; Taylor, D.S.; TePas, E.C.; Speck, N.A.; Mathey-Prevot, B. Identification of a critical regulatory site in the human interleukin-3 promoter by in vivo footprinting. Blood 1994, 83, 2851–2859. [Google Scholar] [CrossRef]
- Cai, Z.; de Bruijn, M.; Ma, X.; Dortland, B.; Luteijn, T.; Downing, J.R.; Dzierzak, E. Haploinsufficiency of AML1 Affects the Temporal and Spatial Generation of Hematopoietic Stem Cells in the Mouse Embryo. Immunity 2000, 13, 423–431. [Google Scholar] [CrossRef]
- Robin, C.; Ottersbach, K.; Durand, C.; Peeters, M.; Vanes, L.; Tybulewicz, V.; Dzierzak, E. An Unexpected Role for IL-3 in the Embryonic Development of Hematopoietic Stem Cells. Dev. Cell 2006, 11, 171–180. [Google Scholar] [CrossRef]
- Borriello, F.; Galdiero, M.; Varricchi, G.; Loffredo, S.; Spadaro, G.; Marone, G. Innate Immune Modulation by GM-CSF and IL-3 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 834. [Google Scholar] [CrossRef]
- Weber, G.F.; Chousterman, B.G.; He, S.; Fenn, A.M.; Nairz, M.; Anzai, A.; Brenner, T.; Uhle, F.; Iwamoto, Y.; Robbins, C.S.; et al. Interleukin-3 amplifies acute inflammation and is a potential therapeutic target in sepsis. Science 2015, 347, 1260–1265. [Google Scholar] [CrossRef]
- Robbins, C.S.; Chudnovskiy, A.; Rauch, P.J.; Figueiredo, J.-L.; Iwamoto, Y.; Gorbatov, R.; Etzrodt, M.; Weber, G.F.; Ueno, T.; van Rooijen, N.; et al. Extramedullary Hematopoiesis Generates Ly-6C high Monocytes That Infiltrate Atherosclerotic Lesions. Circulation 2012, 125, 364–374. [Google Scholar] [CrossRef]
- Testa, U.; Riccioni, R.; Militi, S.; Coccia, E.; Stellacci, E.; Samoggia, P.; Latagliata, R.; Mariani, G.; Rossini, A.; Battistini, A.; et al. Elevated expression of IL-3Rα in acute myelogenous leukemia is associated with enhanced blast proliferation, increased cellularity, and poor prognosis. Blood 2002, 100, 2980–2988. [Google Scholar] [CrossRef] [PubMed]
- Vergez, F.; Green, A.S.; Tamburini, J.; Sarry, J.-E.; Gaillard, B.; Cornillet-Lefebvre, P.; Pannetier, M.; Neyret, A.; Chapuis, N.; Ifrah, N.; et al. High levels of CD34+CD38low/-CD123+ blasts are predictive of an adverse outcome in acute myeloid leukemia: A Groupe Ouest-Est des Leucemies Aigues et Maladies du Sang (GOELAMS) study. Haematologica 2011, 96, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Jordan, C.; Upchurch, D.; Szilvassy, S.; Guzman, M.; Howard, D.; Pettigrew, A.; Meyerrose, T.; Rossi, R.; Grimes, B.; Rizzieri, D.; et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia 2000, 14, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Shimizu, Y.; Furuhata, E.; Maeda, S.; Kishima, M.; Nishimura, H.; Enomoto, S.; Hayashizaki, Y.; Suzuki, H. RUNX1 regulates site specificity of DNA demethylation by recruitment of DNA demethylation machineries in hematopoietic cells. Blood Adv. 2017, 1, 1699–1711. [Google Scholar] [CrossRef]
- Lacaud, G. Runx1 is essential for hematopoietic commitment at the hemangioblast stage of development in vitro. Blood 2002, 100, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Estey, E.H. Acute myeloid leukemia: 2019 update on risk-stratification and management. Am. J. Hematol. 2018, 93, 1267–1291. [Google Scholar] [CrossRef] [PubMed]
- Robak, T. Purine Nucleoside Analogues in the Treatment of Myleoid Leukemias. Leuk. Lymphoma 2003, 44, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Freyer, C.W.; Gupta, N.; Wetzler, M.; Wang, E.S. Revisiting the role of cladribine in acute myeloid leukemia: An improvement on past accomplishments or more old news? Cladribine in Acute Myeloid Leukemia. Am. J. Hematol. 2015, 90, 62–72. [Google Scholar] [CrossRef]
- Park, H.; Youk, J.; Kim, I.; Yoon, S.-S.; Park, S.; Lee, J.-O.; Bang, S.-M.; Koh, Y. Comparison of cladribine- and fludarabine-based induction chemotherapy in relapsed or refractory acute myeloid leukaemia. Ann. Hematol. 2016, 95, 1777–1786. [Google Scholar] [CrossRef]
- Wierzbowska, A.; Robak, T.; Pluta, A.; Wawrzyniak, E.; Cebula, B.; Hołowiecki, J.; Kyrcz-Krzemień, S.; Grosicki, S.; Giebel, S.; Skotnicki, A.B.; et al. Cladribine combined with high doses of arabinoside cytosine, mitoxantrone, and G-CSF (CLAG-M) is a highly effective salvage regimen in patients with refractory and relapsed acute myeloid leukemia of the poor risk: A final report of the Polish Adult Leukem: CLAG-M in refractory and relapsed AML. Eur. J. Haematol. 2007, 80, 115–126. [Google Scholar] [CrossRef]
- Zhou, A.; Han, Q.; Song, H.; Zi, J.; Ma, J.; Ge, Z. Efficacy and toxicity of cladribine for the treatment of refractory acute myeloid leukemia: A meta-analysis. DDDT 2019, 13, 1867–1878. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H. Acute myeloid leukemia-Major progress over four decades and glimpses into the future: Progress of acute myeloid leukemia. Am. J. Hematol. 2016, 91, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Kadia, T.M.; Cortes, J.; Ravandi, F.; Jabbour, E.; Konopleva, M.; Benton, C.B.; Burger, J.; Sasaki, K.; Borthakur, G.; DiNardo, C.D.; et al. Cladribine and low-dose cytarabine alternating with decitabine as front-line therapy for elderly patients with acute myeloid leukaemia: A phase 2 single-arm trial. Lancet Haematol. 2018, 5, e411–e421. [Google Scholar] [CrossRef]
- Costa, Y.; Ding, J.; Theunissen, T.W.; Faiola, F.; Hore, T.A.; Shliaha, P.V.; Fidalgo, M.; Saunders, A.; Lawrence, M.; Dietmann, S.; et al. NANOG-dependent function of TET1 and TET2 in establishment of pluripotency. Nature 2013, 495, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Guilhamon, P.; Eskandarpour, M.; Halai, D.; Wilson, G.A.; Feber, A.; Teschendorff, A.E.; Gomez, V.; Hergovich, A.; Tirabosco, R.; Fernanda Amary, M.; et al. Meta-analysis of IDH-mutant cancers identifies EBF1 as an interaction partner for TET2. Nat. Commun. 2013, 4, 2166. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, K.; Shinoda, A.; Kano, F.; Sato, R.; Shirahige, K.; Murata, M. PPARγ-induced PARylation promotes local DNA demethylation by production of 5-hydroxymethylcytosine. Nat. Commun. 2013, 4, 2262. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.; An, J.; Bandukwala, H.S.; Chavez, L.; Äijö, T.; Pastor, W.A.; Segal, M.F.; Li, H.; Koh, K.P.; Lähdesmäki, H.; et al. Modulation of TET2 expression and 5-methylcytosine oxidation by the CXXC domain protein IDAX. Nature 2013, 497, 122–126. [Google Scholar] [CrossRef] [PubMed]






| Mutated Gene | OR | Lower 95% CI | Upper 95% CI | p-Value |
|---|---|---|---|---|
| TP53 | 0.097 | 0.002 | 0.659 | 0.006 |
| RUNX1 | 3.428 | 1.089 | 11.961 | 0.032 |
| FLT3 | 0.482 | 0.221 | 1.014 | 0.043 |
| ASXL1 | 6.904 | 0.663 | 346.497 | 0.068 |
| MUC16 | 0 | 0 | 1.779 | 0.158 |
| STAG2 | 3.431 | 0.475 | 39.006 | 0.200 |
| DNMT3A | 1.355 | 0.646 | 2.820 | 0.387 |
| TTN | 1.689 | 0.303 | 9.430 | 0.477 |
| KRAS | 1.689 | 0.303 | 9.430 | 0.477 |
| PHF6 | 1.678 | 0.218 | 12.937 | 0.674 |
| U2AF1 | 0.537 | 0.051 | 3.129 | 0.711 |
| SMC1A | 0.651 | 0.060 | 4.126 | 0.711 |
| SMC3 | 0.651 | 0.060 | 4.126 | 0.711 |
| NPM1 | 0.852 | 0.407 | 1.753 | 0.734 |
| CEBPA | 0.717 | 0.154 | 2.712 | 0.768 |
| NRAS | 0.811 | 0.207 | 2.764 | 0.787 |
| PTPN11 | 0.818 | 0.128 | 4.000 | 1 |
| KIT | 0.990 | 0.148 | 5.299 | 1 |
| FCGBP | 1.103 | 0.090 | 9.915 | 1 |
| BRINP3 | 1.103 | 0.090 | 9.915 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasca, S.; Jurj, A.; Tomuleasa, C.; Zdrenghea, M. TET2/IDH1/2/WT1 and NPM1 Mutations Influence the RUNX1 Expression Correlations in Acute Myeloid Leukemia. Medicina 2020, 56, 637. https://doi.org/10.3390/medicina56120637
Pasca S, Jurj A, Tomuleasa C, Zdrenghea M. TET2/IDH1/2/WT1 and NPM1 Mutations Influence the RUNX1 Expression Correlations in Acute Myeloid Leukemia. Medicina. 2020; 56(12):637. https://doi.org/10.3390/medicina56120637
Chicago/Turabian StylePasca, Sergiu, Ancuta Jurj, Ciprian Tomuleasa, and Mihnea Zdrenghea. 2020. "TET2/IDH1/2/WT1 and NPM1 Mutations Influence the RUNX1 Expression Correlations in Acute Myeloid Leukemia" Medicina 56, no. 12: 637. https://doi.org/10.3390/medicina56120637
APA StylePasca, S., Jurj, A., Tomuleasa, C., & Zdrenghea, M. (2020). TET2/IDH1/2/WT1 and NPM1 Mutations Influence the RUNX1 Expression Correlations in Acute Myeloid Leukemia. Medicina, 56(12), 637. https://doi.org/10.3390/medicina56120637







