Assessment of Absorption of Glycated Nail Proteins in Patients with Diabetes Mellitus and Diabetic Retinopathy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Sample Description
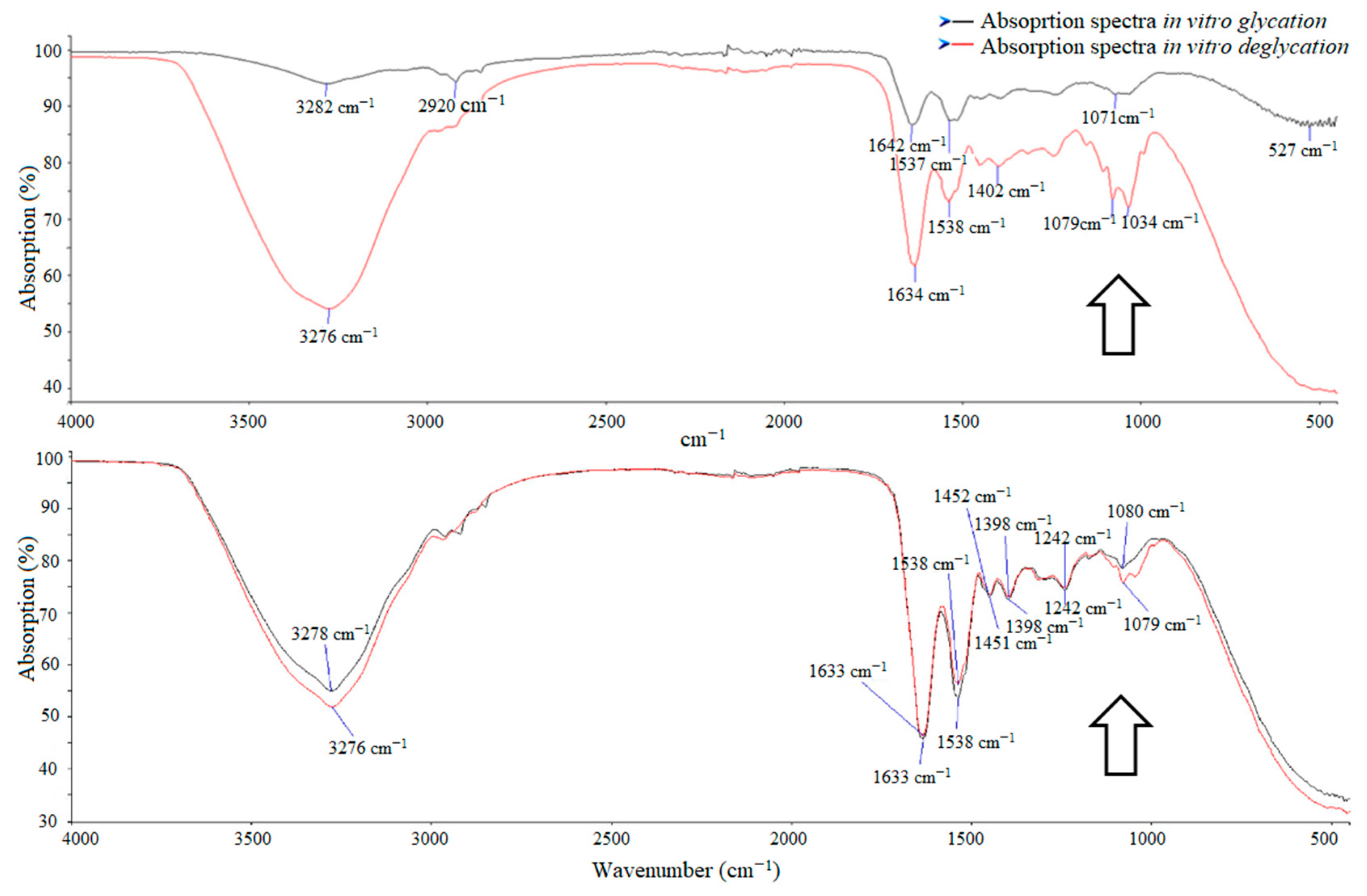
3.2. Absorption in Diabetic Patients and Control Group
3.3. HbA1c Level and Absorption of Glycated Nail Protein
3.4. Absorption of Glycated Nail Protein according to Severity of Diabetic Retinopathy
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roden, M. Diabetes mellitus—Definition, klassifikation und diagnose. Wien Klin. Wochenschr. 2016, 128, 37–40. [Google Scholar] [CrossRef]
- Mauricio, D.; Alonso, N.; Gratacòs, M. Chronic diabetes complications: The need to move beyond classical concepts. Trends Endocrinol. Metab. 2020, 31, 287–295. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Johnson, E.J.; Tuttle, K.R. Inflammatory mechanisms as new biomarkers and therapeutic targets for diabetic kidney disease. Adv. Chronic Kidney Dis. 2018, 25, 181–191. [Google Scholar] [CrossRef]
- Dankner, R.; Agay, N.; Olmer, L.; Murad, H.; Boker, L.K.; Balicer, R.D.; Freedman, L.S. Metformin treatment and cancer risk: Cox regression analysis, with time–dependent covariates, of 320,000 persons with incident diabetes mellitus. Am. J. Epidemiol. 2019, 188, 1794–1800. [Google Scholar] [CrossRef] [Green Version]
- Nentwich, M.M.; Ulbig, M.W. Diabetic retinopathy—Ocular complications of diabetes mellitus. World J. Diabetes 2015, 6, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Sayin, N.; Kara, N.; Pekel, G. Ocular complications of diabetes mellitus. World J. Diabetes 2015, 6, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Winocour, P.; Farrington, K. Erythropoietic stress and anemia in diabetes mellitus. Nat. Rev. Endocrinol. 2009, 5, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.P.; Mahadi, F.; Roy, A.; Sharma, P. Reactive oxygen species, reactive nitrogen species and antioxidants in etiopathogenesis of diabetes mellitus type-2. Indian J. Clin. Biochem. 2009, 24, 324–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corcóstegui, B.; Durán, S.; González-Albarrán, M.O.; Hernández, C.; Ruiz-Moreno, J.M.; Salvador, J.; Udaondo, P.; Simó, R. Update on diagnosis and treatment of diabetic retinopathy: A consensus guideline of the Working Group of Ocular Health (Spanish society of diabetes and Spanish vitreous and retina society). J. Ophthalmol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [Green Version]
- Moore, T.C.; Moore, J.E.; Kaji, Y.; Frizzell, N.; Usui, T.; Poulaki, V.; Campbell, I.L.; Stitt, A.W.; Gardiner, T.A.; Archer, D.B.; et al. The role of advanced glycation end products in retinal microvascular leukostasis. Invest. Ophthalmol. Vis. Sci. 2003, 44, 4457–4464. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, S.; Lee, T.D.; Baker, J.A.; Rabinowitz, L.T.; Asmerom, Y.; Legesse, K.; Ranney, H.M. Reverse phase high–performance liquid chromatography and secondary ion mass spectrometry. A strategy for identification of ten human hemoglobin variants. Hemoglobin 1986, 10, 379–400. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, E.; Taylor, A. Too sweet: Problems of protein glycation in the eye. Exp. Eye Res. 2019, 178, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Kishabongo, A.S.; Katchunga, P.; Van Aken, E.H.; Speeckaert, R.; Lagniau, S.; Coopman, R.; Speeckaert, M.M.; Delanghe, J.R. Glycation of nail proteins: From basic biochemical findings to a representative marker for diabetic glycation–associated target organ damage. PLoS ONE 2015, 10, e0120112. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.I.A.; de Souza, E.; de Oliveira Pedrosa, F.; Rea, R.R.; da Silva Couto Alves, A.; Picheth, G.; Rego, F.G. RAGE receptor and its soluble isoforms in diabetes mellitus complications. J. Bras. Patol. Med. Lab. 2013, 49, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Uchiki, T.; Weikel, K.A.; Jiao, W.; Shang, F.; Caceres, A.; Pawlak, D.; Handa, J.T.; Brownlee, M.; Nagaraj, R.; Taylor, A. Glycation-altered proteolysis as a pathobiologic mechanism that links dietary glycemic index, aging, and age-related disease (in nondiabetics). Aging Cell 2012, 11, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, A.; Takabatake, Y.; Kimura, T.; Maejima, I.; Namba, T.; Yamamoto, T.; Matsuda, J.; Minami, S.; Kaimori, J.Y.; Matsui, I.; et al. Autophagy inhibits the accumulation of advanced glycation end products by promoting lysosomal biogenesis and function in the kidney proximal tubules. Diabetes 2017, 66, 1359–1372. [Google Scholar] [CrossRef] [Green Version]
- Aragonès, G.; Dasuri, K.; Olukorede, O.; Francisco, S.G.; Renneburg, C.; Kumsta, C.; Hansen, M.; Kageyama, S.; Komatsu, M.; Rowan, S.; et al. Autophagic receptor p62 protects against glycation-derived toxicity and enhances viability. Aging Cell 2020. [Google Scholar] [CrossRef]
- Odani, H.; Matsumoto, Y.; Shinzato, T.; Usami, J.; Maeda, K. Mass spectrometric study on the protein chemical modification of uremic patients in advanced Maillard reaction. J. Chromatogr. B Biomed. Sci. Appl. 1999, 731, 131–140. [Google Scholar] [CrossRef]
- Miyazawa, T.; Nakagawa, K.; Shimasaki, S.; Nagai, R. Lipid glycation and protein glycation in diabetes and atherosclerosis. Amino Acids 2012, 42, 1163–1170. [Google Scholar] [CrossRef]
- Smith, M.A.; Taneda, S.; Richey, P.L.; Miyata, S.; Yan, S.D.; Stern, D.; Sayre, L.M.; Monnier, V.M.; Perry, G. Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc. Natl. Acad. Sci. USA 1994, 91, 5710–5714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez/García, L.C.; de Cádiz Villarreal, A.G.; Rivas, J.P.; González, J.J.M.; Álvarez, G.G.; Salazar, M.T.A. Implantación del cribado de retinopatía diabética mediante retinografía digital en atención primaria. Aten. Primaria 2013, 45, 149–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peer, N.; Kengne, A.P.; Motala, A.A.; Mbanya, J.C. Diabetes in the Africa region: An update. Diabetes Res. Clin. Pract. 2014, 103, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Greene, R.A.; Scher, R.K. Nail changes associated with diabetes mellitus. J. Am. Acad. Dermatol. 1987, 16 Pt 1, 1015–1021. [Google Scholar] [CrossRef]
- Saeedi, P.; Shavandi, A.; Meredith-Jones, K. Nail properties and bone health: A review. J. Funct. Biomater. 2018, 9, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brzozka, P.; Kolodziejskia, W. Sex-related chemical differences in keratin from fingernail plates: A solid–state carbon-13 NMR study. RSC Adv. 2017, 7, 28213–28223. [Google Scholar] [CrossRef] [Green Version]
- Ohgitani, S.; Fujita, T.; Fujii, Y.; Hayashi, C.; Nishio, H. Nail calcium and magnesium content in relation to age and bone mineral density. J. Bone Miner Metab. 2005, 23, 318–322. [Google Scholar] [CrossRef]
- de Berker, D.A.; André, J.; Baran, R. Nail biology and nail science. Int. J. Cosmet. Sci. 2007, 29, 241–275. [Google Scholar] [CrossRef]
- de Berker, D.A. Nail anatomy. Clin. Dermatol. 2013, 31, 509–515. [Google Scholar] [CrossRef]
- Rice, R.H.; Xia, Y.; Alvarado, R.J.; Phinney, B.S. Proteomic analysis of human nail plate. J. Proteome. Res. 2010, 9, 6752–6758. [Google Scholar] [CrossRef] [Green Version]
- Cashman, M.W.; Sloan, S.B. Nutrition and nail disease. Clin. Dermatol. 2010, 28, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.E.; Little, R.R.; Lorenz, R.A.; Malone, J.I.; Nathan, D.; Peterson, C.M.; Sacks, D.B. Tests of glycemia in diabetes. Diabetes Care 2004, 27, 1761–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Academy of Ophthalmology Retina-Vitreous Panel. Preferred Practice Pattern® Guidelines. Diabetic Retinopathy; American Academy of Ophthalmology: San Francisco, CA, USA, 2014. [Google Scholar]
- Coopman, R.; Van de Vyver, T.; Kishabongo, A.S.; Katchunga, P.; Van Aken, E.H.; Cikomola, J.; Monteyne, T.; Speeckaert, M.M.; Delanghe, J.R. Glycation in human fingernail clippings using ATR–FTIR spectrometry, a new marker for the diagnosis and monitoring of diabetes mellitus. Clin. Biochem. 2017, 50, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.A.; Renaud, D.E.; Krishnasamy, S.; Meriç, P.; Buduneli, N.; Çetinkalp, Ş.; Liu, K.Z. Diabetes-related molecular signatures in infrared spectra of human saliva. Diabetol. Metab. Syndr. 2010, 2, 48. [Google Scholar] [CrossRef] [Green Version]
- Cikomola, J.C.; Kishabongo, A.S.; Speeckaert, M.M.; Delanghe, J.R. Diabetes mellitus and laboratory medicine in sub-Saharan Africa: Challenges and perspectives. Acta Clin. Belg. 2019, 74, 137–142. [Google Scholar] [CrossRef]
- Welsh, K.J.; Kirkman, M.S.; Sacks, D.B. Role of glycated proteins in the diagnosis and management of diabetes: Research gaps and future directions. Diabetes Care 2016, 39, 1299–1306. [Google Scholar] [CrossRef] [Green Version]
- Farhan, K.M.; Sastry, T.P.; Mandal, A.B. Comparative study on secondary structural changes in diabetic and non-diabetic human finger nail specimen by using FTIR spectra. Clin. Chim. Acta 2011, 412, 386–389. [Google Scholar] [CrossRef]
- Katchunga, P.B.; Mirindi, P.N.; Kishabongo, A.S.; Cikomola, J.C.; Bwanamdogo, S.; Philippé, J.; Speeckaert, M.M.; Delanghe, J.R. Glycated nail proteins as a new biomarker in management of the South Kivu Congolese diabetics. Biochem. Med. 2015, 25, 469–473. [Google Scholar] [CrossRef] [Green Version]
- Kishabongo, A.S.; Katchunga, P.; Van Aken, E.H.; Speeckaert, M.M.; Lagniau, S.; Husein, D.; Taes, Y.E.; Delanghe, J.R. Glycated nail proteins: A new approach for detecting diabetes in developing countries. Trop. Med. Int. Health 2014, 19, 58–64. [Google Scholar] [CrossRef]
- Monteyne, T.; Coopman, R.; Kishabongo, A.S.; Himpe, J.; Lapauw, B.; Shadid, S.; Van Aken, E.H.; Berenson, D.; Speeckaert, M.M.; De Beer, T.; et al. Analysis of protein glycation in human fingernail clippings with near-infrared (NIR) spectroscopy as an alternative technique for the diagnosis of diabetes mellitus. Clin. Chem. Lab. Med. 2018, 56, 1551–1558. [Google Scholar] [CrossRef]
- George, J.A.; Erasmus, R.T. Haemoglobin A1c or glycated albumin for diagnosis and monitoring diabetes: An African perspective. Indian J. Clin. Biochem. 2018, 33, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Tantisiriwat, N.; Janchai, S. Common foot problems in diabetic foot clinic. J. Med. Assoc. Thail. 2008, 91, 1097–1101. [Google Scholar]





| Solution of | Peaks in Region of Interest | |
|---|---|---|
| Automatically Detected | Manually Detected | |
| 10% glucose D(+)–glucose monohydrate | 10 | 1 |
| 0.9% sodium chloride | 5 | 6 |
| 5% glucose D(+)–glucose monohydrate | 1 | 10 |
| Process | Absorbance at | DM Group (n = 32) | Control Group (n = 28) | p Value |
|---|---|---|---|---|
| In vitro glycation | 970 and 1140 cm−1 | 88.50 ± 4.42 | 82.07 ± 6.86 | <0.001 * |
| 530 nm | 0.025 ± 0.008 | 0.017 ± 0.008 | 0.002 * | |
| In vitro | 970 and 1140 cm−1 | 77.34 ± 2.05 | 77.24 ± 1.68 | 0.847 |
| deglycation | 530 nm | 0.059 ± 0.039 | 0.025 ± 0.016 | <0.001 * |
| Process | Absorption Spectra and Cut–Off Value | AUC | Specificity/Sensitivity | DM group Control Group (n, %) | p Value | OR [% 95 CI] |
|---|---|---|---|---|---|---|
| In vitro glycation | 970 and 1140 cm−1; >83% | 0.778 ± 0.065 | 0.893/0.656 | 11(34.4)/25(89.3) | <0.001 * | 15.909 [3.914–64.660] |
| 530 nm, >0.016 | 0.751 ± 0.068 | 0.880/0.548 | 14(45.2)/22(88.0) | 0.001 * | 8.905 [2.199 36.052] | |
| In vitro deglycation | 530 nm, >0.038 | 0.779 ± 0.067 | 0.667/0.871 | 4(12.9)/16(66.7) | <0.001 * | 13.500 [3.499–52.083] |
| Process | Absorbance at | Good Glycemic Control Group (n = 18) | Poor Glycemic Control Group (n = 14) | p Value |
|---|---|---|---|---|
| In vitro glycation | 970 and 1140 cm−1 | 87.99 ± 4.69 | 87.38 ± 4.23 | 0.517 |
| 530 nm | 0.024 ± 0.009 | 0.026 ± 0.008 | 0.412 | |
| In vitro | 970 and 1140 cm−1 | 77.56 ± 2.03 | 76.86 ± 1.94 | 0.604 |
| deglycation | 530 nm | 0.024 ± 0.00168 | 0.0446 ± 0.036 | 0.114 |
| Process | Absorbance at | DM Group (n = 32) | p Value | |
|---|---|---|---|---|
| Mild/Moderate DR (n = 11) | Severe/Proliferative DR (n = 21) | |||
| In vitro glycation | 970 and 1140 cm−1 | 89.37 ± 4.267 | 87.45 ± 4.72 | 0.427 |
| 530 nm | 0.026 ± 0.011 | 0.024 ± 0.008 | 0.702 | |
| In vitro deglycation | 970 and 1140 cm−1 | 79.210 ± 2.946 | 76.870 ± 2.084 | 0.049 * |
| 530 nm | 0.057 ± 0.050 | 0.052 ± 0.032 | 0.741 | |
| Process | Absorbance at | DM Group (n = 32) | p Value | |
|---|---|---|---|---|
| DME (n = 20) | No DME (n = 12) | |||
| In vitro glycation | 970 and 1140 cm−1 | 87.49 ± 5.16 | 89.15 ± 3.41 | 0.259 |
| 530 nm | 0.024 ± 0.008 | 0.025 ± 0.011 | 0.409 | |
| In vitro deglycation | 970 and 1140 cm−1 | 77.21 ± 1.98 | 78.74 ± 3.61 | 0.345 |
| 530 nm | 0.053 ± 0.032 | 0.054 ± 0.046 | 0.886 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurgeleviciene, I.; Stanislovaitiene, D.; Tatarunas, V.; Jurgelevicius, M.; Zaliuniene, D. Assessment of Absorption of Glycated Nail Proteins in Patients with Diabetes Mellitus and Diabetic Retinopathy. Medicina 2020, 56, 658. https://doi.org/10.3390/medicina56120658
Jurgeleviciene I, Stanislovaitiene D, Tatarunas V, Jurgelevicius M, Zaliuniene D. Assessment of Absorption of Glycated Nail Proteins in Patients with Diabetes Mellitus and Diabetic Retinopathy. Medicina. 2020; 56(12):658. https://doi.org/10.3390/medicina56120658
Chicago/Turabian StyleJurgeleviciene, Ieva, Daiva Stanislovaitiene, Vacis Tatarunas, Marius Jurgelevicius, and Dalia Zaliuniene. 2020. "Assessment of Absorption of Glycated Nail Proteins in Patients with Diabetes Mellitus and Diabetic Retinopathy" Medicina 56, no. 12: 658. https://doi.org/10.3390/medicina56120658
APA StyleJurgeleviciene, I., Stanislovaitiene, D., Tatarunas, V., Jurgelevicius, M., & Zaliuniene, D. (2020). Assessment of Absorption of Glycated Nail Proteins in Patients with Diabetes Mellitus and Diabetic Retinopathy. Medicina, 56(12), 658. https://doi.org/10.3390/medicina56120658





