Relationship between Night Shifts and Risk of Breast Cancer among Nurses: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Databases and Documentary Sources Consulted
- Cochrane Plus Library
- PubMed
- CINAHL (Cumulative Index to Nursing and Allied Health Literature)
- Web of Science
- ScienceDirect
- Scopus
- Dialnet
2.3. Keywords
2.4. Inclusion and Exclusion Criteria
- Inclusion criteria:
- Studies carried out over the last 10 years (2010–2020).
- In Spanish and English.
- Peer-reviewed articles.
- Typology: original articles and clinical trials, systematic meta-analysis and reviews, short/brief communication, and case reporting.
- They should analyze at least one of the following characteristics on a nursing sample (population): risk factors related to shift work among nursing professionals and breast cancer, associated hormonal changes and/or alterations after blood sample, light levels to which nurses are exposed at night work, and circadian gene expression.
- Exclusion criteria:
- Records of low scientific evidence.
- Articles that have no relation to the purpose of the review.
- Typology: opinion articles, editorials, and letters to the director/editor.
2.5. Critical Appraisal and Level of Evidence
- Author.
- Date.
- Study design, objective, location, and period of completion.
- Study population, intervention/comparison, analyzed results, and follow-up time.
- Number of participants, intervention in experimental and control groups, masking method, and post-randomization losses.
- Results, beneficial and adverse clinical effects.
- Conclusions.
- Study quality.
2.6. Reverse Search
3. Results
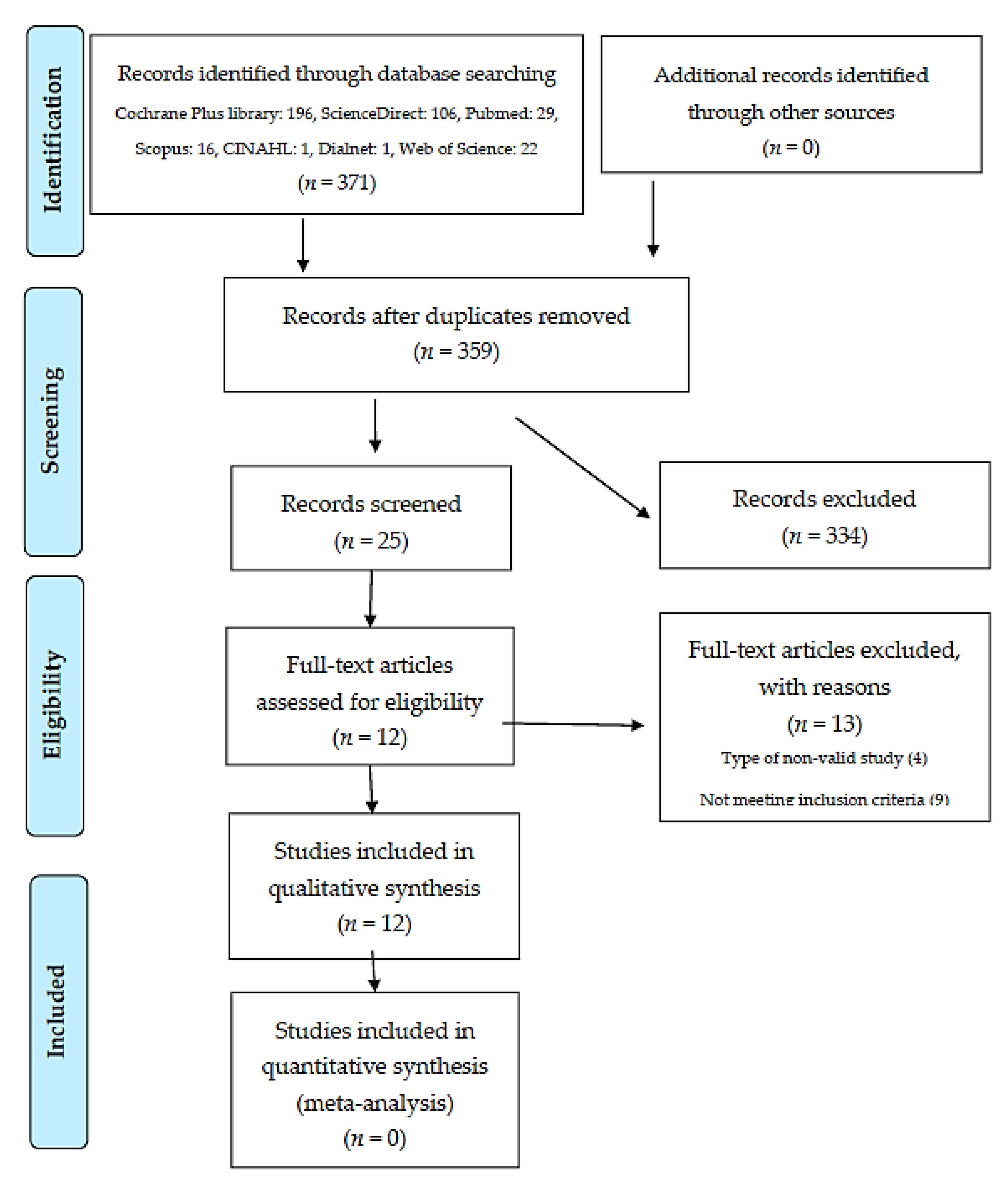
3.1. Records’ Selection
3.2. Summary of the Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Angulo, M.M.; Yustos, M.A.; León, M.V.; Soto, M.; Álvarez, D.M. Cáncer de mama. Med. Programa Formación Médica Contin. Acreditado 2013, 11, 1629–1640. [Google Scholar] [CrossRef]
- Dickerman, B.; Liu, J. Does current scientific evidence support a link between light at night and breast cancer among female night-shift nurses? Review of evidence and implications for occupational and environmental health nurses. Work. Health Saf. 2012, 60, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Weiderpass, E.; Meo, M.; Vainio, H. Risk Factors for Breast Cancer, Including Occupational Exposures. Saf. Health Work 2011, 2, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, J.; Stevens, R.G. Case–control study of shift-work and breast cancer risk in Danish nurses: Impact of shift systems. Eur. J. Cancer 2012, 48, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Reszka, E.; Peplonska, B.; Wieczorek, E.; Sobala, W.; Bukowska, A.; Gromadzińska, J.; Lie, J.-A.; Kjuus, H.; Wąsowicz, W. Circadian gene expression in peripheral blood leukocytes of rotating night shift nurses. Scand. J. Work. Environ. Health 2013, 39, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Lie, J.-A.S.; Kjuus, H.; Zienolddiny, S.; Haugen, A.; Stevens, R.G.; Kjaerheim, K. Night Work and Breast Cancer Risk Among Norwegian Nurses: Assessment by Different Exposure Metrics. Am. J. Epidemiol. 2011, 173, 1272–1279. [Google Scholar] [CrossRef] [Green Version]
- Erdem, J.S.; Notø, H.Ø.; Skare, Ø.; Lie, J.S.; Petersen-Øverleir, M.; Reszka, E.; Pepłońska, B.; Zienolddiny, S. Mechanisms of breast cancer risk in shift workers: Association of telomere shortening with the duration and intensity of night work. Cancer Med. 2017, 6, 1988–1997. [Google Scholar] [CrossRef] [Green Version]
- Lie, J.-A.S.; Kjuus, H.; Zienolddiny, S.; Haugen, A.; Kjærheim, K. Breast Cancer Among Nurses: Is the Intensity of Night Work Related to Hormone Receptor Status? Am. J. Epidemiol. 2013, 178, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Peplonska, B.; Bukowska, A.; Lie, J.A.; Gromadzinska, J.; Zienolddiny, S. Night shift work and other determinants of estradiol, testosterone, and dehydroepiandrosterone sulfate among middle-aged nurses and midwives. Scand. J. Work Environ. Health 2016, 42, 435–446. [Google Scholar] [CrossRef]
- Salamanca-Fernández, E.; Rodríguez-Barranco, M.; Guevara, M.; Ardanaz, E.; Lima, A.O.D.L.; Sánchez, M. Night-shift work and breast and prostate cancer risk: Updating the evidence from epidemiological studies. An. Sist. Sanit. Navar. 2018, 41. [Google Scholar] [CrossRef] [Green Version]
- Kamdar, B.B.; Tergas, A.I.; Mateen, F.J.; Bhayani, N.H.; Oh, J. Night-shift work and risk of breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2013, 138, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Peplonska, B.; Bukowska, A.; Sobala, W.; Reszka, E.; Gromadzińska, J.; Wąsowicz, W.; Lie, J.A.; Kjuus, H.; Ursin, G.; Santen, R.J.; et al. Rotating Night Shift Work and Mammographic Density. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1028–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reszka, E.; Peplonska, B.; Wieczorek, E.; Sobala, W.; Bukowska, A.; Gromadzinska, J.; Lie, J.-A.; Kjuus, H.; Wasowicz, W. Rotating night shift work and polymorphism of genes important for the regulation of circadian rhythm. Scand. J. Work Environ. Health 2012, 39, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Wegrzyn, L.R.; Tamimi, R.M.; Rosner, B.A.; Brown, S.B.; Stevens, R.G.; Eliassen, A.H.; Laden, F.; Willett, W.C.; Hankinson, S.E.; Schernhammer, E.S. Rotating Night-Shift Work and the Risk of Breast Cancer in the Nurses’ Health Studies. Am. J. Epidemiol. 2017, 186, 532–540. [Google Scholar] [CrossRef] [Green Version]
- Rosa, D.; Terzoni, S.; Dellafiore, F.; Destrebecq, A. Systematic review of shift work and nurses’ health. Occup. Med. 2019, 69, 237–243. [Google Scholar] [CrossRef]
- Touitou, Y.; Reinberg, A.; Touitou, D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017, 173, 94–106. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [Green Version]
- Calero, M.; Ángel, R.; Gomila, C.J.V.; Fullana, P.S. Advanced practice nurses and evidence-based practice. An opportunity for change. Enfermería Clínica (Engl. Ed.) 2019, 29, 119–124. [Google Scholar] [CrossRef]
- López de Argumedo, M.; Reviriego, E.; Gutiérrez, A.; Bayón, J.C. Actualización del Sistema de Trabajo Compartido para Revisiones Sistemáticas de la Evidencia Científica y Lectura Crítica (Plataforma FLC 3.0). Ministerio de Sanidad, Servicios Sociales e Igualdad. Servicio de Evaluación de Tecnologías Sanitarias del País Vasco. 2017. Available online: http://www.lecturacritica.com/es/acerca.php (accessed on 3 December 2020).
- Jia, Y.; Lu, Y.; Wu, K.; Lin, Q.; Shen, W.; Zhu, M.; Huang, S.; Chen, J. Does night work increase the risk of breast cancer? A systematic review and meta-analysis of epidemiological studies. Cancer Epidemiol. 2013, 37, 197–206. [Google Scholar] [CrossRef]
- Bracci, M.; Manzella, N.; Copertaro, A.; Staffolani, S.; Strafella, E.; Barbaresi, M.; Copertaro, B.; Rapisarda, V.; Valentino, M.; Santarelli, L. Rotating-shift nurses after a day off: Peripheral clock gene expression, urinary melatonin, and serum 17-β-estradiol levels. Scand. J. Work Environ. Health 2014, 40, 295–304. [Google Scholar] [CrossRef]
- Haus, E.; Smolensky, M.H. Shift work and cancer risk: Potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med. Rev. 2013, 17, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Yong, M.; Nasterlack, M. Shift Work and Cancer: State of Science and Practical Consequences. Arch. Ind. Hyg. Toxicol. 2012, 63, 153–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundy, A.; Schuetz, J.M.; Lai, A.S.; Janoo-Gilani, R.; Leach, S.; Burstyn, I.; Richardson, H.; Brooks-Wilson, A.; Spinelli, J.J.; Aronson, K.J. Shift work, circadian gene variants and risk of breast cancer. Cancer Epidemiol. 2013, 37, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.Z.; Ohman-Strickland, P.; Kelly-McNeil, K.; Kipen, H.; Crabtree, B.F.; Lew, J.P.; Zarbl, H. Sleep interruption associated with house staff work schedules alters circadian gene expression. Sleep Med. 2015, 16, 1388–1394. [Google Scholar] [CrossRef] [Green Version]
- Grundy, A.; Tranmer, J.; Richardson, H.; Graham, C.H.; Aronson, K.J. The Influence of Light at Night Exposure on Melatonin Levels among Canadian Rotating Shift Nurses. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2404–2412. [Google Scholar] [CrossRef] [Green Version]
- Gu, F.; Han, J.; Laden, F.; Pan, A.; Caporaso, N.E.; Stampfer, M.J.; Kawachi, I.; Rexrode, K.M.; Willett, W.C.; Hankinson, S.E.; et al. Total and Cause-Specific Mortality of U.S. Nurses Working Rotating Night Shifts. Am. J. Prev. Med. 2015, 48, 241–252. [Google Scholar] [CrossRef] [Green Version]
- Nagata, C.; Tamura, T.; Wada, K.; Konishi, K.; Goto, Y.; Nagao, Y.; Ishihara, K.; Yamamoto, S. Sleep duration, nightshift work, and the timing of meals and urinary levels of 8-isoprostane and 6-sulfatoxymelatonin in Japanese women. Chronobiol. Int. 2017, 34, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Carugno, M.; Maggioni, C.; Crespi, E.; Bonzini, M.; Cuocina, S.; Dioni, L.; Tarantini, L.; Consonni, D.; Ferrari, L.; Pesatori, A.C. Night Shift Work, DNA Methylation and Telomere Length: An Investigation on Hospital Female Nurses. Int. J. Environ. Res. Public Health 2019, 16, 2292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zienolddiny, S.; Haugen, A.; Lie, J.-A.S.; Kjuus, H.; Anmarkrud, K.H.; Kjærheim, K. Analysis of polymorphisms in the circadian-related genes and breast cancer risk in Norwegian nurses working night shifts. Breast Cancer Res. 2013, 15, R53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bracci, M.; Ciarapica, V.; Zabaleta, M.E.; Tartaglione, M.F.; Pirozzi, S.; Giuliani, L.; Piva, F.; Valentino, M.; Ledda, C.; Rapisarda, V.; et al. BRCA1 and BRCA2 Gene Expression: Diurnal Variability and Influence of Shift Work. Cancers 2019, 11, 1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega-Escaño, J.; Porcel-Gálvez, A.M.; Barrientos-Trigo, S.; Romero-Sánchez, J.M.; De Diego-Cordero, R. La turnicidad como factor determinante en la aparición de insomnio en población laboral: Revisión sistemática. Rev. Esp. Salud Pública. 2020, 94, e202007047. [Google Scholar] [PubMed]
| P (Population) | Nurses |
| I (Intervention) | Work exposure: shift work hours |
| C (Comparison) | Breast cancer risk factors |
| O (Outcomes) | Level of association between risk factors (shift work schedule and breast cancer) |
| DeCS Term | MeSH Term |
|---|---|
| Horario de Trabajo por Turnos | Shift work schedule |
| Trastorno por trabajo a turnos | Shift work disorder |
| Neoplasia de mama | Breast neoplasms |
| Cáncer de mama | Breast cancer |
| Lactancia | Breast feeding |
| Enfermeras | Nurses |
| Enfermería | Nursing |
| Title | Date | Language | Study Type | Abstract | Meets Objective | Result |
|---|---|---|---|---|---|---|
| Does current scientific evidence support a link between light at night and breast cancer among female night-shift nurses? Review of evidence and implications for occupational and environmental health nurses [2]. | YES | YES | YES | YES | YES | ☺ |
| Risk Factors for Breast Cancer, Including Occupational Exposures [3]. | YES | YES | NO | YES | YES | ☹ |
| Case–control study of shift-work and breast cancer risk in Danish nurses, Impact of shift systems [4]. | YES | YES | YES | YES | YES | ☺ |
| Circadian gene expression in peripheral blood leukocytes of rotating night shift nurses [5]. | YES | YES | YES | YES | YES | ☺ |
| Night Work and Breast Cancer Risk Among Norwegian Nurses: Assessment by Different Exposure Metrics [6]. | YES | YES | YES | YES | YES | ☺ |
| Mechanisms of breast cancer risk in shift workers, Association of telomere shortening with the duration and intensity of night work [7]. | YES | YES | YES | YES | YES | ☺ |
| Breast Cancer Among Nurses Is the Intensity of Night Work Related to Hormone Receptor Status? [8] | YES | YES | YES | YES | YES | ☺ |
| Night shift work and other determinants of oestradiol, testosterone, and dehydroepiandrosterone sulphate among middle-aged nurses and midwives [9] | YES | YES | YES | YES | YES | ☺ |
| Night-shift work and breast and prostate cancer risk: updating the evidence from epidemiological studies [10] | YES | YES | YES | YES | NO | ☹ |
| Night-shift work and risk of breast cancer: a systematic review and meta-analysis [11] | YES | YES | YES | YES | NO | ☹ |
| Rotating Night Shift Work and Mammographic Density [12] | YES | YES | YES | YES | NO | ☹ |
| Rotating night shift work and polymorphism of genes important for the regulation of circadian rhythm [13] | YES | YES | YES | YES | YES | ☺ |
| Rotating night shift work and risk of breast cancer in the nurses’ health studies [14] | YES | YES | YES | YES | YES | ☺ |
| Systematic review of shift work and nurses’ health [15] | YES | YES | YES | YES | YES | ☺ |
| Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption [16] | YES | YES | NO | YES | YES | ☹ |
| Does night work increase the risk of breast cancer? A systematic review and meta-analysis of epidemiological studies [20] | YES | YES | YES | YES | NO | ☹ |
| Rotating-shift nurses after a day off: peripheral clock genes’ expression, urinary melatonin, and serum 17-β-oestradiol levels [21]. | YES | YES | YES | YES | YES | ☺ |
| Shift work and cancer risk: Potential mechanistic roles of circadian disruption, light at night, and sleep deprivation [22] | YES | YES | NO | YES | YES | ☹ |
| Shift work and cancer: State of Science and Practical Consequences [23] | YES | YES | YES | YES | NO | ☹ |
| Shift work, circadian gene variants and risk of breast cancer [24] | YES | YES | YES | YES | NO | ☹ |
| Sleep interruption associated with house staff work schedules alters circadian gene expression [25] | YES | YES | NO | YES | YES | ☹ |
| The Influence of Light at Night Exposure on Melatonin Levels among Canadian Rotating Shift Nurses [26] | YES | YES | YES | YES | NO | ☹ |
| Total and Cause-Specific Mortality of U.S. Nurses Working Rotating Night Shifts [27] | YES | YES | YES | YES | NO | ☹ |
| Sleep duration, nightshift work, and the timing of meals and urinary levels of 8-isoprostane and 6-sulfatoxymelatonin in Japanese women [28] | YES | YES | YES | YES | NO | ☹ |
| Night Shift Work, DNA Methylation and Telomere Length: An Investigation on Hospital Female Nurses [29] | YES | YES | YES | YES | YES | ☺ |
| TOTAL EXCLUDED RECORDS: 13 | ||||||
| TOTAL SELECTED ARTICLES: 12 | ||||||
| STUDY | Research Question: Is the Study Based on a Clearly Defined Research Question? | Method: Has the Study Method Allowed Minimizing Bias? | Results: Have the Outcomes Been Correctly Synthetized and Described? | Conclusions: Are the Conclusions Justified? | Conflict of Interests: Is the Existence or Absence of Conflict of Interests Well Described? | External Validity: Are the Study Outcomes Generalizable to the Population and Context of Interest? | Study Quality |
|---|---|---|---|---|---|---|---|
| Dickerman et al., 2012 [2] | Yes | Partially | Partially | Partially | Yes | Yes | INTERMEDIATE |
| Hansen et al., 2012 [4] | Yes | Partially | Yes | Partially | Yes | Partially | INTERMEDIATE |
| Reszka et al., 2013 [5] | Yes | Partially | Yes | Yes | Yes | Yes | INTERMEDIATE |
| Lie et al., 2011 [6] | Yes | Yes | Yes | Yes | Yes | Yes | HIGH |
| Erdem et al., 2017 [7] | Yes | Yes | Yes | Yes | Yes | Yes | HIGH |
| Lie et al., 2013 [8] | Yes | Partially | Yes | Yes | No information | Partially | INTERMEDIATE |
| Peplonska et al., 2016 [9] | Yes | Partially | Yes | Yes | Yes | Yes | INTERMEDIATE |
| Reszka et al., 2012 [13] | Yes | Partially | Yes | Yes | Yes | Yes | INTERMEDIATE |
| Wegrzyn et al., 2017 [14] | Yes | Partially | Yes | Yes | Yes | Yes | INTERMEDIATE |
| Rosa et al., 2019 [15] | Yes | Partially | Partially | Yes | Yes | Yes | INTERMEDIATE |
| Bracci et al., 2014 [21] | Yes | Partially | Yes | Yes | Yes | Yes | INTERMEDIATE |
| Carugno et al., 2019 [29] | Yes | Partially | Yes | Partially | Yes | Partially | INTERMEDIATE |
| F.L.C 3.0 Platform Suggestions for Assessment. | |||||||
| “Method” Area: Yes | “Method” Area: Partially | “Method” Area: No | |||||
| Majority rest of areas: Yes | High quality | Intermediate quality | Low quality | ||||
| Majority rest of areas: Partially | Intermediate quality | Intermediate quality | Low quality | ||||
| Majority rest of areas: No | Low quality | Low quality | Low quality | ||||
| Not assessable: Having answered ‘No information’ in the “Method” area or in “Majority of areas”, so assessing study quality is not possible | |||||||
| Author, Year, and Reference | Main Study Characteristics | Aim of the Study | Intervention and Instrument | Main Findings and Conclusions | Quality |
|---|---|---|---|---|---|
| Dickerman and Liu, 2012 [2] | Narrative review. Search terms: light at night, shift work, night shift, and breast cancer. Limits: English, human studies, publication after 2001. Critical appraisal tools are not specified. | To examine the impact of light at night exposure on breast cancer risk among female night-shift nurses, discuss possible mechanisms of action, and recommend future research and implications for practice. | Literature search in three databases. Reference lists at the end of found articles were also reviewed. | 11 studies were found. Duration of Shift Work: studies reported a relationship between increased cancer risk and increased years and hours-per-week of night-shift work, compared with those who never worked at night. Rotating shifts: The risk of breast cancer may be proportional to the number of consecutive night shifts worked. Risk increases in permanent night shifts and long-term day-night rotating shifts. Light at night and melatonin levels have not been significantly associated in this study. Although the number of epidemiological studies is somewhat limited and further research is needed, evidence suggests that exposure to light during night shift work may increase the risk of breast cancer. Several potential mechanisms of action have been proposed: melatonin suppression, clock gene expression, sleep disruption, lifestyle factors, and lower vitamin D levels. | INTERMEDIATE |
| Hansen and Stevens, 2012 [4] | Case–control study: questionnaire. Sample: cohort of 58,091 female nurses (267 cases and 1035 controls) | To explore whether shiftwork causes breast cancer and which aspects of shiftwork are most problematic. | Telephone interview. Sociodemographic data were obtained. Questionnaire: years of schooling, occupational history and work schedule on cumulative years (day–evening, day–night, day–evening-night), tobacco, alcohol consumption, physical activity, reproductive history, use of hormone replacement therapy, occurrence of breast cancer in mother and/or sister. | Nurses who worked at night had a significantly longer working life, were younger at menarche and menopause, had fewer children, were older at the birth of their first child, spent fewer hours weekly on sport, and had slept fewer hours per night. Day–evening–night shiftwork is the most frequent rotating shift system. There is a tendency to increasing odds ratios for breast cancer by cumulative years of shiftwork and shift systems that disrupt circadian rhythms (i.e., night shifts and rotating day-night shifts). | INTERMEDIATE |
| Reszka et al., 2013 [5] | Cross-sectional study: questionnaire and gene expression analysis. Sample: 354 nurses and midwives currently working rotating night shifts and 370 nurses who work only during the day, all female. | To determine the effect of rotating night shift work on the expression of selected core circadian genes as indicators of peripheral clock. | Questionnaire: age, menopausal status, current job history (total years, years working at night and years without working at night [both from 0 to 15 years or more]), smoking, physical activity, quality of sleep, alcohol and antidepressants intake. Biological samples: Gene expression analysis was conducted among 92 pairs of nurses and midwives in the morning (6 a.m.–10 a.m.). | All the sample of this study had worked rotating night shifts in the past or during the study. An elevated circadian gene expression was observed among rotating night shift compared with day workers, influenced by the time of blood sampling. There was no association of the selected core genes of this study with the years working at night. The highest expression of a selected gene (Period1 - PER1) was found in nurses with longest lifetime duration of night shift. | INTERMEDIATE |
| Lie et al., 2011 [6] | Nested case and control study: telephonic structured questionnaire. Cohort: 49.402 Norwegian nurses. Cases: 699. Controls: 895. | To examine the relationship between shift work and breast cancer risk, including detailed evaluation of different exposure metrics of night-shift work. | Questionnaire: potential breast cancer risk factors (age, body mass index, menarche, menopause, hormonal therapy, alcohol and tobacco, breast cancer in mother/sister) and work-related factors (years of starting and ending employment, type of work site, radiographic procedures, type of work schedule [only days, only nights, both days and nights], years working at least 3 nights per month or rotating shifts, cumulative lifetime night shifts). Night shift: 12 p.m. until 6 a.m. | Previously identified risk factors for breast cancer are confirmed, for example, early menarche, lower number of childbirths, breast cancer in mother or sister, and hormonal treatment. Risk of breast cancer significantly increased among nurses who had worked for 5 years with ≥ 6 consecutive night shifts. | HIGH |
| Erdem et al., 2017 [7] | Nested case and control study: telephonic structured questionnaire and saliva samples for DNA extraction. Cohort: 49.402 Norwegian nurses. Cases: 699. Controls: 895. | To investigate telomere length variation as a potential mechanism of the association between long duration of night shift with consecutive nights and the increased risk of breast cancer. | Questionnaire: information on potential breast cancer risk factors and lifetime occupational history. Saliva samples were received from 563 cases and 619 controls. Telomere length was measured by polymerase chain reaction. Night shift: 12 p.m. until 6 a.m. | Telomere lengths were not significantly different in nurses that had worked night shifts compared with those that had worked only days. The shortening of telomeres is affected by intensive night work schedules and is associated with an increased risk of breast cancer among workers with long periods of consecutive night shifts., i.e., six consecutive nights over a period of more than 5 years. | HIGH |
| Lie et al., 2013 [8] | Nested case and control study: telephonic structured questionnaire. Cohort: 49.402 Norwegian nurses. Cases: 590. Controls: 757 | To examine the relation between night work and hormone-receptor breast cancer subtypes (estrogen and progesterone). | Questionnaire: information on potential breast cancer risk factors and lifetime occupational history. Night shift: 12 p.m. until 6 a.m. Exposure measure: duration of work with a minimum of 6 consecutive night shifts. Information on the hormone receptor status of breast cancer cases was taken from the pathology reports submitted to the Cancer Registry for each cancer diagnosis. | A long duration (≥5 years) of night work with ≥6 consecutive night shifts was significantly associated with estrogen and progesterone positive tumors. The observed association between consecutive night shifts and positive progesterone receptor cancers suggests that progesterone could play an important role in the detrimental effects of night work. | INTERMEDIATE |
| Peplonska et al., 2016 [9] | Cross-sectional study: questionnaire and blood/urine collection. Sample: 594 female nurses and midwives; 345 premenopausal and 187 postmenopausal. Of them, 263 rotating night shifts and 269 day shifts. | To examine night shift work and body concentrations of sex hormones among pre- and postmenopausal women. | Questionnaire: information on potential breast cancer risk factors and lifetime occupational history (characteristics of night work: frequency of night shifts per month, duration of night shift work in years). Blood samples were collected in the morning (6 a.m.–10 a.m.). Night shift: 12 h of duration, from 7 p.m. to 7 a.m. | The most frequent working schedule was 6–7-night duties per month. There was no significant difference in the circulating sex hormone concentrations between current night shift workers and day workers. There was significant association between total duration of night work (>15 years) and higher estradiol levels among postmenopausal women. No significant associations were found with night work among premenopausal women, although the mean concentration of hormones is higher among women with longer night shift duration. | INTERMEDIATE |
| Reszka et al., 2012 [13] | Cross-sectional study: questionnaire and blood collection. Sample: 709 nurses and midwives; 348 in rotating shifts and 361 in non-rotating shifts. | To investigate the association between circadian genes polymorphisms and rotating night work adaptative mechanism. | Questionnaire: information on potential breast cancer risk factors and lifetime occupational history (characteristics of night work: frequency of night shifts per month, lifetime duration of night shift work in years). Blood samples (n = 709) were collected in the morning (6 a.m.–10 a.m.). Night shift: 12 h of duration, from 7 p.m. to 7 a.m. | There were no differences in clock genes (circadian) between nurses and midwives working on night and day rotating shifts. Differences were found in a specific genotype (cryptochrome 1) among nurses working long night shifts, as compared to those on the day shift, being more frequent in association with >8 night per month and >3 nights per week. | INTERMEDIATE |
| Wegrzyn et al., 2017 [14] | Case and control: questionnaires and medical records. Sample: 2 cohorts; NHS 78.516 women; NHS-2 114.559 women. | To examine the association between working on rotating night shifts and the risk of breast cancer on two prospective cohorts. | Questionnaire: lifestyle, occupational and environmental exposure, medication use, and medical condition. Rotating night shift work was defined as “3 or more night-shifts in one month”. Rotating shift work duration in prior years was assessed in a range from 0 years to >20 years. Medical records were consulted to confirm cancer diagnosis among nurses of the study. | Long-term rotating night work (>20 years) was associated with an increased risk of breast cancer among young women (ages 25–42) who accumulated night shifts in their early career. The median time to a first breast cancer event was of 13–14 years. Breast cancer risk by hormone receptor status was determined: associations with estrogen and progesterone positive tumors were significant for >20 years of cumulative shift work. | INTERMEDIATE |
| Rosa et al., 2019 [15] | Systematic review. Keywords: nurses, circadian rhythm, breast neoplasm, work schedule, among others. Dates: 2005–2016. Included: randomized control trials, observational studies and reviews. Limited to English language. | To describe the effects of shift work and desynchronization of circadian rhythms on nurse’s health. | Literature review in 5 databases. Quality assessment was performed. | 24 articles were assessed. Shift work schedule causes physiological and psychological disturbances, also excessive fatigue, and interrupted sleep. Duration of shifts and number of consecutive nights are the main factors influencing sleep disorders. Rotating night shift work, stress and disruptions in circadian rhythms may lead to overweight and type 2 diabetes. A link between oestrogen, circulating melatonin, and breast cancer values is suggested. Risk of breast cancer was significantly higher in nursing staff working for >5 years with six consecutive night shifts. | INTERMEDIATE |
| Bracci et al., 2014 [21] | Cross-sectional study: questionnaire and blood/urine sampling. Sample: 60 female nurses with ≥ 2 years of rotating shifts and 56 female nurses with permanent day shifts | To compare levels of selected core clock genes expression, 6-sulfatoxymelatonine (aMT6s), and 17-β-oestradiol among workers in rotation shifts and day shifts after a day off. | Questionnaire: lifestyles, occupational and environmental exposures, medication use and chronotype (Morningness–Eveningness Questionnaire). Blood/urine samples: collected at 7 a.m. Gene expression, aMT6s, and estradiol levels were measured. Night shift: 10 p.m. to 7 a.m. | Significant expression of circadian genes was observed in shift workers. The influence of long-term shift work on circadian rhythm regulation is suggested, altering the expression of peripheral clock genes. Rotating shift participants did not show a significant difference in aMT6 levels but did show a significant difference in 17-β-oestradiol levels, as compared to day shift nurses. | INTERMEDIATE |
| Carugno et al., 2019 [29] | Cross-sectional study: questionnaire and blood sampling. Sample: 46 female nurses on night shift and 51 nurses working on morning shifts. | To analyze the association between night shift work (>2 years) and molecular alterations potentially related to increased carcinogenic risk. | Questionnaire: information on potential breast cancer risk factors and lifetime occupational history (focusing on shift work schedule and duration). Blood sample: extracted between 7:15 a.m. and 7:45 a.m. The analysis focused on DNA methylation of estrogen receptor genes, tumor suppressor genes, and telomere length. | DNA methylation of oestrogen receptor genes (ESR1, ESR2) play a significant role in the proliferation of breast tissue stimulated by estrogens, which is a known as breast cancer risk factor. Reduced telomere length is found in nurses with at least 12 years of night shifts. | INTERMEDIATE |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fagundo-Rivera, J.; Gómez-Salgado, J.; García-Iglesias, J.J.; Gómez-Salgado, C.; Camacho-Martín, S.; Ruiz-Frutos, C. Relationship between Night Shifts and Risk of Breast Cancer among Nurses: A Systematic Review. Medicina 2020, 56, 680. https://doi.org/10.3390/medicina56120680
Fagundo-Rivera J, Gómez-Salgado J, García-Iglesias JJ, Gómez-Salgado C, Camacho-Martín S, Ruiz-Frutos C. Relationship between Night Shifts and Risk of Breast Cancer among Nurses: A Systematic Review. Medicina. 2020; 56(12):680. https://doi.org/10.3390/medicina56120680
Chicago/Turabian StyleFagundo-Rivera, Javier, Juan Gómez-Salgado, Juan Jesús García-Iglesias, Carlos Gómez-Salgado, Selena Camacho-Martín, and Carlos Ruiz-Frutos. 2020. "Relationship between Night Shifts and Risk of Breast Cancer among Nurses: A Systematic Review" Medicina 56, no. 12: 680. https://doi.org/10.3390/medicina56120680







