A Meta-Analysis Comparing the Efficacy and Safety of Peramivir with Other Neuraminidase Inhibitors for Influenza Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Search Strategy and Selection
2.2. Data Extraction and Outcome Assessment
2.3. Data Analysis and Study Quality Assessment
3. Results
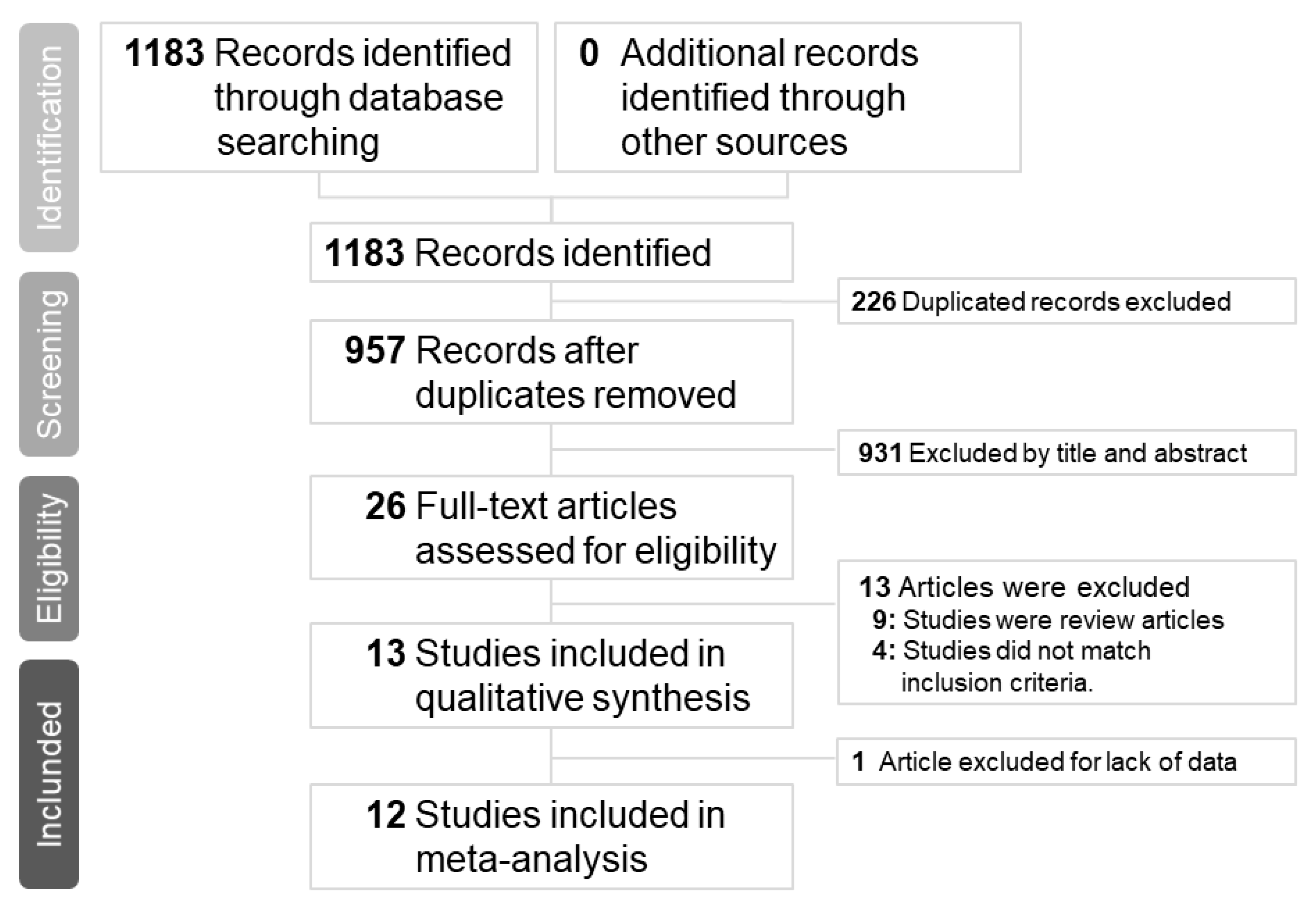
3.1. Study Search Outcomes and Included Patients
3.2. Meta-Analysis of Clinical Efficacy
3.3. Risk of Adverse Event
3.4. Heterogeneity and Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| NAI | neuraminidase inhibitors |
| RCTs | randomized controlled trials |
| OSs | observational studies |
| GRADE | Grading of Recommendations, Assessments, Development, and Evaluations |
| RoB 2.0 | cochrane risk-of-bias assessment tool 2.0 |
| ROBINS | risk of bias in non-randomized studies of interventionsg |
| MD | difference in meanss |
| RR | risk ratio |
| CI | confidence interval |
| AE | adverse events |
| SAE | serious adverse events |
Appendix A. List of Terms Used in the Search
References
- Centers for Disease Control and Prevention. Influenza Antiviral Medications: Summary for Clinicians. Available online: https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm (accessed on 30 December 2018).
- US Food and Drug Administration. FDA news release: FDA approves new drug to treat influenza. Available online: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm624226.htm (accessed on 24 October 2018).
- Weinstock, D.M.; Zuccotti, G. Adamantane resistance in influenza A. JAMA 2006, 295, 934–936. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.A. Baloxavir: First Global Approval. Drugs 2018, 78, 693–697. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.H.; Jwa, H.; Kim, Y.H. Comparison of Efficacy of Intravenous Peramivir and Oral Oseltamivir for the Treatment of Influenza: Systematic Review and Meta-Analysis. Yonsei Med. J. 2017, 58, 778–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, 14898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- Shankaran, S.; Elam, K.; Bearman, G.M.L. Treatment of severe cases of pandemic (H1N1) 2009 influenza: Review of antivirals and adjuvant therapy. Recent Pat. Antiinfect. Drug Discov. 2010, 5, 152–156. [Google Scholar] [CrossRef]
- Smith, S.M.; Gums, J.G. Antivirals for influenza: Strategies for use in pediatrics. Paediatr. Drugs 2010, 12, 285–299. [Google Scholar] [CrossRef]
- Thorlund, K.; Awad, T.; Boivin, G.; Thabane, L. Systematic review of influenza resistance to the neuraminidase inhibitors. BMC Infect Dis. 2011, 11, 134. [Google Scholar] [CrossRef] [Green Version]
- Farrukee, R.; Mosse, J.; Hurt, A.C. Review of the clinical effectiveness of the neuraminidase inhibitors against influenza B viruses. Expert Rev. Anti Infect. Ther. 2013, 11, 1135–1145. [Google Scholar] [CrossRef]
- Hata, A.; Akashi-Ueda, R.; Takamatsu, K.; Matsumura, T. Safety and efficacy of peramivir for influenza treatment. Drug Des. Dev. Ther. 2014, 8, 2017–2038. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, M.M.; Skoglund, E.W.; Ison, M.G. Peramivir: An intravenous neuraminidase inhibitor. Expert Opin. Pharmacother. 2015, 16, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Higashiguchi, M.; Matsumoto, T.; Fujii, T. A meta-analysis of laninamivir octanoate for treatment and prophylaxis of influenza. Antivir. Ther. 2018, 23, 157–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, L.J. Peramivir: A Review in Uncomplicated Influenza. Drugs 2018, 78, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.D.; Ison, M.G.; Monto, A.S.; Metev, H.; Clark, C.; O’Neil, B.; Elder, J.; McCullough, A.; Collis, P.; Sheridan, W.P. Evaluation of intravenous peramivir for treatment of influenza in hospitalized patients. Clin. Infect. Dis. 2014, 59, e172–e185. [Google Scholar] [CrossRef]
- Ison, M.G.; Fraiz, J.; Heller, B.; Jauregui, L.; Mills, G.; O’Riordan, W.; O’Neil, B.; Playford, E.G.; Rolf, J.D.; Sada-Diaz, E.; et al. Intravenous peramivir for treatment of influenza in hospitalized patients. Antivir. Ther. 2014, 19, 349–361. [Google Scholar] [CrossRef] [Green Version]
- Whitley, R.; Laughlin, A.; Carson, S.; Mitha, E.; Tellier, G.; Stich, M.; Elder, J.; Alexander, W.J.; Dobo, S.; Collis, P.; et al. Single dose peramivir for the treatment of acute seasonal influenza: Integrated analysis of efficacy and safety from two placebo-controlled trials. Antivir. Ther. 2015, 20, 709–719. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.W.; Choi, S.H.; Huh, J.W.; Lim, C.M.; Koh, Y.; Hong, S.B. Peramivir is as effective as oral oseltamivir in the treatment of severe seasonal influenza. J. Med. Virol. 2015, 87, 1649–1655. [Google Scholar] [CrossRef]
- Ishiguro, N.; Koseki, N.; Kaiho, M.; Ariga, T.; Kikuta, H.; Oba, K.; Togashi, T.; Morita, K.; Inagawa, A.; Okamura, A.; et al. Clinical effectiveness of four neuraminidase inhibitors (oseltamivir, zanamivir, laninamivir, and peramivir) for children with influenza A and B in the 2014-2015 to 2016-2017 influenza seasons in Japan. J. Infect. Chemother. 2018, 24, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Kohno, S.; Yen, M.Y.; Cheong, H.J.; Hirotsu, N.; Ishida, T.; Kadota, J.; Mizuguchi, M.; Kida, H.; Shimada, J. Phase III randomized, double-blind study comparing single-dose intravenous peramivir with oral oseltamivir in patients with seasonal influenza virus infection. Antimicrob. Agents Chemother. 2011, 55, 5267–5276. [Google Scholar] [CrossRef] [Green Version]
- Ison, M.G.; Hui, D.S.; Clezy, K.; O’Neil, B.J.; Flynt, A.; Collis, P.J.; Simon, T.J.; Alexander, W.J. A clinical trial of intravenous peramivir compared with oral oseltamivir for the treatment of seasonal influenza in hospitalized adults. Antivir. Ther. 2013, 18, 651–661. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, S.; Miyazaki, T.; Izumikawa, K.; Kakeya, H.; Saisho, Y.; Yanagihara, K.; Miyazaki, Y.; Mukae, H.; Kohno, S. Efficacy and Safety of Intravenous Peramivir Compared with Oseltamivir in High-Risk Patients Infected With Influenza A and B Viruses: A Multicenter Randomized Controlled Study. Open Forum Infect Dis. 2017, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sakata, H. Clinical efficiency in children treated with intravenous drip infusion of peramivir. Jpn. J. Antibiot. 2011, 64, 383–387. [Google Scholar] [PubMed]
- Yoshino, Y.; Seo, K.; Koga, I.; Kitazawa, T.; Ota, Y. Clinical efficacy of peramivir in adult patients with seasonal influenza during the winter of 2012 in Japan. Clin. Respir. J. 2015, 9, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, Y.; Seo, K.; Koga, I.; Kitazawa, T.; Ota, Y. Clinical efficacy of laninamivir and peramivir in patients with seasonal influenza: A randomized clinical trial. Infect. Dis. (Lond.) 2017, 49, 417–419. [Google Scholar] [CrossRef]
- Sugaya, N.; Sakai-Tagawa, Y.; Bamba, M.; Yasuhara, R.; Yamazaki, M.; Kawakami, C.; Yamaguchi, Y.; Ide, Y.; Ichikawa, M.; Mitamura, K.; et al. Comparison between virus shedding and fever duration after treating children with pandemic A H1N1/09 and children with A H3N2 with a neuraminidase inhibitor. Antivir. Ther. 2015, 20, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Hirotsu, N.; Saisho, Y.; Hasegawa, T.; Shishido, T. Clinical and virologic effects of four neuraminidase inhibitors in influenza A virus-infected children (aged 4–12 years): An open-label, randomized study in Japan. Expert Rev. Anti Infect. Ther. 2018, 16, 173–182. [Google Scholar] [CrossRef]
- Hikita, T.; Hikita, H.; Hikita, F.; Hikita, N.; Hikita, S. Clinical effectiveness of peramivir in comparison with other neuraminidase inhibitors in pediatric influenza patients. Int. J. Pediatr. 2012, 2012, 834181. [Google Scholar] [CrossRef]
- Shobugawa, Y.; Saito, R.; Sato, I.; Kawashima, T.; Dapat, C.; Dapat, I.C.; Kondo, H.; Suzuki, Y.; Saito, K.; Suzuki, H. Clinical effectiveness of neuraminidase inhibitors--oseltamivir, zanamivir, laninamivir, and peramivir--for treatment of influenza A(H3N2) and A(H1N1)pdm09 infection: An observational study in the 2010-2011 influenza season in Japan. J. Infect. Chemother. 2012, 18, 858–864. [Google Scholar] [CrossRef]
- Takemoto, Y.; Asai, T.; Ikezoe, I.; Yano, T.; Ichikawa, M.; Miyagawa, S.; Matsumoto, J. Clinical effects of oseltamivir, zanamivir, laninamivir and peramivir on seasonal influenza infection in outpatients in Japan during the winter of 2012–2013. Chemotherapy 2013, 59, 373–378. [Google Scholar] [CrossRef]
- Tochino, Y.; Fujioka, M.; Sakazaki, H.; Ikuno, Y.; Tochino, R.; Yoshii, N.; Shintaku, H.; Hirata, K. Current usage and effectiveness of influenza medications and factors regarding the time taken to alleviate fever based on postcard questionnaire survey. J. Gen. Fam. Med. 2017, 18, 386–392. [Google Scholar] [CrossRef]









| Randomized (RoB 2.0) | Domains 1 | Domains 2 | Domains 3 | Domains 4 | Domains 5 | Overall Bias |
|---|---|---|---|---|---|---|
| Kohno, S 2010 | Low | Low | Low | Low | Low | Low |
| Ison, MG 2013 | Low | Low | Low | Low | Low | Low |
| Nakamura, S 2017 | Low | Low | Some concerns * | Low | Some concerns + | Some concerns |
| Yoshino, Y 2017 | Some concerns+ | High * | Low | High* | Some concerns # | High |
| Hirotsu, N 2018 | Low | High # | Some concerns * | Low | Some concerns * | High |
| Non-Randomized (ROBINS-I) | Domain 1 | Domain 2 | Domain 3 | Domain 4 | Domain 5 | Domain 6 | Domain 7 | Overall |
|---|---|---|---|---|---|---|---|---|
| Sakata, H 2011 | Low | Low | Low | Low | Low | Low | Low | Low |
| Hikita, T 2012 | Low | Low | Low | Low | Low | Low | Low | Low |
| Shobugawa, Y 2012 | Low | Low | Low | Low | No information | Low | Low | Low |
| Takemoto, Y 2013 | Low | Low | Low | Low | Low | Low | Low | Low |
| Sugaya, N 2015 | Low | Low | Low | Moderate * | No information | Low | Low | Moderate * |
| Yoshino, Y 2015 | Low | Low | Low | No information | Low | Low | Low | Low |
| Tochino, Y 2017 | Low | Low | Low | No information | Serious ** | Low | Low | Serious ** |
| GRADE Assessment | |||||||
|---|---|---|---|---|---|---|---|
| Certainty Assessment | Certainty | ||||||
| No of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | |
| Time to Alleviation of Symptoms | |||||||
| 5 | randomized trials | serious a | not serious | not serious | not serious | none | ⨁⨁⨁◯ MODERATE |
| 7 | observational studies | serious b | serious b | not serious | very serious c | strong associationall plausible residual confounding would suggest spurious effect, while no effect was observed | ⨁⨁◯◯ LOW |
| Risk of Adverse Events | |||||||
| 3 | randomized trials | serious a | not serious | not serious | not serious | none | ⨁⨁⨁◯ MODERATE |
| 1 | observational studies | not serious | not serious | not serious | not serious | none | ⨁⨁⨁⨁ HIGH |
| Risk of Serious Adverse Event | |||||||
| 3 | randomized trials | serious a | not serious | not serious | not serious | none | ⨁⨁⨁◯ MODERATE |
| Author, Year | Influenza Virus Subtype | Patient Enrollment Criteria | Sample Number (Men/Women) | Age (Mean ± SD) | Age Group | Blind | Treatment Protocol | Outcome Measurement |
|---|---|---|---|---|---|---|---|---|
| Randomized controlled trials | ||||||||
| Kohno S et al., 2011 | A(H1) A(H3) B | Rapid test positive, body temperature ≧38.0 °C, two moderate to severe symptoms among seven symptoms: headache, muscle or joint pain, feverishness or chills, fatigue, cough, sore throat, and nasal stuffiness. | Peramivir: 726 (378/348) Oseltamivir: 365 (184/181) | Peramivir: 35.4 ± 11.6 Oseltamivir: 34.6 ± 11.7 | >18 | Yes, but no details of blind description | Intravenous with peramivir 300 or 600 mg once daily for 5 days or oral oseltamivir 75 mg twice daily for 5 days. | Time to alleviation of symptoms, change from the influenza virus titer, adverse events. |
| Ison MG et al., 2013 | A(H1N1) A(H3N2) B | Rapid test positive, influenza-like illness within the previous 72 h with documented fever or feverishness, ≥1 respiratory symptom (cough, sore throat or nasal congestion), ≥1 constitutional symptom (headache, myalgia, feverishness or malaise/fatigue). | Peramivir: 81 (38/43) Oseltamivir: 41 (19/22) | Peramivir: 58.0 ± 23.0 Oseltamivir: 62.2 ± 21.1 | >18 | Participant, care provider, investigator, outcomes assessor. | Intravenous with peramivir 200 or 400 mg once daily for 5 days or oral oseltamivir 75 mg twice daily for 5 days. | Time to clinical stability, time to alleviation of symptoms, time to hospital discharge, time to resumption of usual activities, change from the influenza virus titer, adverse events. |
| Nakamura S et al., 2017 | A(H3N2), A(H1N1) pdm09 B | Rapid test positive, body temperature ≥ 38.0 °C, treatment within 48 hours influenza illness as indicated by at least 1 symptom: headache, muscle or joint pain, feverishness or chills, and fatigue as general symptoms, and cough, sore throat, and nasal stuffiness as respiratory symptoms. | Peramivir: 46 (21/25) Oseltamivir: 46 (22/24) | Peramivir: 72.2 ± 14.1 Oseltamivir: 70.1 ± 11.1 | >18 | NO | Intravenous with peramivir 600 mg once daily (a second infusion at >2 days later, if necessary, was permitted) or oral oseltamivir 75 mg twice daily for 5 days. | Time to alleviation fever, time to alleviation of symptoms, change from the influenza virus titer, adverse events. |
| Yoshino Y et al., 2017 | A B | Rapid test positive, axillary temperature ≥37.0 °C, influenza-like illness, including fever, muscle pain, chills, sweating, headache, dry cough, fatigue, nasal congestion, and respiratory failure. | Peramivir: 13 (6/7) Oseltamivir: 9 (3/6) Laninamivir: 12 (3/9) | Peramivir: 43.6 ± 15.3 Oseltamivir: 40.4 ± 9.84 Laninamivir: 36.2 ± 10.0 | >18 | NO | Intravenous with peramivir 300 mg once daily or oseltamivir 75 mg twice daily for 5 days or inhaled laninamivir 40 mg once daily. | Time to alleviation fever, alleviation of other symptoms. |
| Hirotsu N et al., 2018 | A(H1N1) A(H3N2) A(H1N1) pdm09 B | Rapid test positive, axillary temperature ≥37.5 °C, influenza-like illness (cough, sore throat, headache, nasal discharge, muscle or joint pain, and fatigue). | Peramivir: 28 (15/13) Oseltamivir: 30 (22/8) Zanamivir: 26 (9/17) Laninamivir: 30 (13/17) | All groups between 4-12 | ≤18 | NO | Intravenous with peramivir 10 mg/kg once daily or oral oseltamivir 2 mg/kg twice daily, ≧37.5 kg or oral oseltamivir 75 mg twice daily for 5 days or inhaled zanamivir 10 mg twice daily for 5 days or inhaled laninamivir 40 mg (≥10 years) or 20 mg (<10 years) once daily. | Time to virus clearance, time to alleviation of fever, time to alleviation of symptoms, adverse events. |
| Author, Year | Influenza Virus Subtype | Patient Enrollment Criteria | Sample Number (Men/Women) | Age (Mean ± SD) | Age Group | Treatment Protocol | Outcome Measurement |
|---|---|---|---|---|---|---|---|
| Observational studies | |||||||
| Sakata H, 2011 | A B | Rapid test positive, influenza-like illness within the previous 48 h. | Peramivir: 30 (N/A) Oseltamivir: 30 (N/A) | Peramivir: 1.8 ± 4.9 Oseltamivir: 2.0 ± 3.9 | ≤18 | Intravenous with peramivir 10 mg/kg once daily or oral oseltamivir 4 mg/kg daily. | Time to alleviation fever, adverse events. |
| Hikita T et al., 2012 | A B | Rapid test positive, fever lasting for less than 48 h. | Peramivir: 63 (N/A) Oseltamivir: 124 (N/A) Zanamivir: 38 (N/A) Laninamivir: 14 (N/A) | Peramivir: 7.8 ± 42.4 Oseltamivir: 5.2 ± 34.2 Zanamivir: 10.5 ± 11.4 Laninamivir: 10.6 ± 5.8 | ≤18 | Intravenous with peramivir 10 mg/kg once daily or oral oseltamivir 2 mg/kg twice daily for 5 days or inhaled zanamivir 10 mg twice daily for 5 days or inhaled laninamivir 40 mg (≥10 years) or 20 mg (<10 years) once daily. | Time to alleviation fever, adverse events. |
| Shobugawa Y et al., 2012 | A(H3N2), A(H1N1) pdm09 | Rapid test positive, fever ≥ 37.5 °C with respiratory symptoms, headache, arthralgia, or myalgia | Peramivir: 4 (3/1) Oseltamivir: 119 (60/59) Zanamivir: 124 (78/46) Laninamivir: 9 (3/6) | Peramivir: 8.8 ± 3.9 Oseltamivir: 4.9 ± 2.3 Zanamivir: 9.4 ± 2.5 Laninamivir: 10.2 ± 2.3 | Mix | Intravenous with peramivir 300 or 600 mg with adult or 10 mg/kg for children once daily or oral oseltamivir 75 mg twice daily with adult or 2 mg/kg twice daily with children for 5 days or inhaled zanamivir 10 mg twice daily for 5 days or inhaled laninamivir 40 mg (≥10 years) or 20 mg (<10 years) once daily. | Time to alleviation fever. |
| Takemoto Y et al., 2013 | A B | Rapid test positive. | Peramivir: 53 (32/21) Oseltamivir: 51 (26/25) Zanamivir: 39 (28/11) Laninamivir: 44 (25/19) | Peramivir: 34.8 ± 23.2 Oseltamivir: 19.0 ± 27.0 Zanamivir: 17.8 ± 18.6 Laninamivir: 26.3 ± 23.2 | Mix | Intravenous with peramivir 300 mg with adult or 10 mg/kg for children once daily or oral oseltamivir 75 mg twice daily with adult or 2 mg/kg twice daily with children for 5 days or inhaled zanamivir 10 mg twice daily for 5 days or inhaled laninamivir 40 mg (adult) or 20 mg (children) once daily. | Time to alleviation fever. |
| Sugaya N et al., 2015 | A(H3N2), A(H1N1) pdm09 | Rapid test positive, fever >38 °C, upper respiratory symptoms such as cough or rhinorrhoea. | Peramivir: 17 (N/A) Oseltamivir: 163 (N/A) Laninamivir: 33 (N/A) | Peramivir: 7.6 ± 3.8 Oseltamivir: 6.3 ± 1.8 Laninamivir: 8.3 ± 2.0 | ≤18 | Intravenous with peramivir 10 mg/kg once daily or oral oseltamivir weight-based dose twice daily for 5 days or inhaled laninamivir 20 mg once daily. | Viral shedding patterns, time to alleviation fever |
| Yoshino Y et al., 2015 | Not mentioned | Rapid test positive, oral temperature ≥37.2 °C, influenza-like illness. | Peramivir: 23 (14/9) Oseltamivir: 9 (4/5) | Peramivir: 77.6 ± 14.4 Oseltamivir: 70.3 ± 13.8 | >18 | Intravenous with peramivir 300 mg once daily or oral oseltamivir 75 mg twice daily for 5 days. | Time to defervescence, survival rate, side effects |
| Tochino Y et al., 2017 | A B | Rapid test positive, fever ≥ 37 °C influenza-like illness. | Peramivir: 10 (4/6) Oseltamivir: 108 (55/53) Zanamivir: 28 (14/14) Laninamivir: 95 (43/52) | Peramivir: 44.0 ± 53.2 Oseltamivir: 25.3 ± 343.3 Zanamivir: 15.8 ± 70.9 Laninamivir: 33.0 ± 273.5 | Mix | Not mentioned | Time to alleviation fever, time to alleviation of symptoms, adverse events. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-Y.; Wei, S.-K.; Lai, C.-C.; Weng, T.-S.; Wang, H.-H. A Meta-Analysis Comparing the Efficacy and Safety of Peramivir with Other Neuraminidase Inhibitors for Influenza Treatment. Medicina 2020, 56, 63. https://doi.org/10.3390/medicina56020063
Chen J-Y, Wei S-K, Lai C-C, Weng T-S, Wang H-H. A Meta-Analysis Comparing the Efficacy and Safety of Peramivir with Other Neuraminidase Inhibitors for Influenza Treatment. Medicina. 2020; 56(2):63. https://doi.org/10.3390/medicina56020063
Chicago/Turabian StyleChen, Jui-Yi, Shih-Kai Wei, Chih-Cheng Lai, Teng-Song Weng, and Hsin-Hua Wang. 2020. "A Meta-Analysis Comparing the Efficacy and Safety of Peramivir with Other Neuraminidase Inhibitors for Influenza Treatment" Medicina 56, no. 2: 63. https://doi.org/10.3390/medicina56020063
APA StyleChen, J.-Y., Wei, S.-K., Lai, C.-C., Weng, T.-S., & Wang, H.-H. (2020). A Meta-Analysis Comparing the Efficacy and Safety of Peramivir with Other Neuraminidase Inhibitors for Influenza Treatment. Medicina, 56(2), 63. https://doi.org/10.3390/medicina56020063





