Electrocardiographic Changes in Liver Cirrhosis—Clues for Cirrhotic Cardiomyopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
- history of cardiovascular disease;
- antiarrhythmic treatment in the previous 6 months, including beta-blockers, or use of any other medication that can alter ECG patterns;
- diuretic treatment in the previous month;
- presence of pulmonary diseases (evaluated by history, clinical examination and chest radiography);
- presence of stage 3 or above chronic kidney disease or acute chronic kidney disease;
- presence of arrhythmias on ECG on admission;
- history of alcohol abuse;
- history of autoimmune liver disease;
- presence of hepatocellular carcinoma or other malignancies;
- anemia with hemoglobin levels under 10 g/dL;
- presence of thyroid disease (hypo or hyperthyroidism, autoimmune thyroiditis).
2.2. Electrocardiography Analysis
- QRS amplitude in all limb (DI, DII, DIII, aVL, aVF and aVR) and precordial (V1-V6) leads. Criteria for low-voltage QRS was amplitude of less than 0.5 mV in one of the limb leads and less than 1 mV in one of the precordial leads [23]. Mean values for QRS voltage in the limb leads and QRS voltage in the precordial leads were also calculated;
- QT interval, measured in leads DII and V6, from the beginning of the QRS interval to the end of the T wave. The lead with the longest QT interval was then considered, and the average QT interval from three consecutive heartbeats was recorded. Corrected QT (QTc) was calculated using Bazzet’s formula:QTc= QT/√RR.QT prolongation was defined as a corrected length interval of over 440 ms in male patients and over 460 ms in female patients;
- Tpe was calculated in all the leads using the tangent method, and a mean value was recorded. The peak was measured at the highest amplitude of the T wave relative to the isoelectric line. The T-end was defined as the intersection of the downslope of the T wave with the isoelectric line [24]. If there was a U wave present, the end of the T wave was considered at the lowest point between the T and the U waves.
2.3. Echocardiography Confirmation
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. ECG Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kowalski, H.J.; Abelmann, W.H. The cardiac output at rest in Laennec’s cirrhosis. J. Clin. Investig. 1953, 32, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.V.H.; Kroll, P.C.; Kroll, R.T.M.; Carvalho, V.N. Cirrhotic cardiomyopathy: The liver affects the heart. Braz. J. Med. Biol. Res. 2019, 52, e7809. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.; Maggioli, C.; Dibra, V.; Zaccherini, G. QT interval prolongation in the liver cirrhosis: Innocent by stander or serious threat? Expert Rev. Gastroenterol. Hepatol. 2012, 6, 57–66. [Google Scholar] [CrossRef]
- Henriksen, J.H.; Fuglsang, S.; Bendtsen, F.; Christensen, E.; Moller, S. Dyssynchronous electrical and mechanical systole in patients with cirrhosis. J. Hepatol. 2002, 36, 513–520. [Google Scholar] [CrossRef]
- Gorgis, N.M.; Kennedy, C.; Lam, F.; Thompson, K.; Coss-Bu, J.; Arikan, A.A.; Nguyen, T.; Hosek, K.; Miloh, T.; Karpen, S.J.; et al. Clinical Consequences of Cardiomyopathy in Children With Biliary Atresia Requiring Liver Transplantation. Hepatology 2019, 69, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Arikan, C.; Kilic, M.; Nart, D.; Ozgenc, F.; Ozkan, T.; Tokat, Y.; Yagci, R.V.; Aydogdu, S. Hepatocellular carcinoma in children and effect of living-donor liver transplantation on outcome. Pediatr. Transpl. 2006, 10, 42–47. [Google Scholar] [CrossRef]
- Rainer, P.P.; Primessnig, U.; Harenkamp, S.; Doleschal, B.; Wallner, M.; Fauler, G.; Stojakovic, T.; Wachter, R.; Yates, A.; Groschner, K.; et al. Bile acids induce arrhythmias in human atrial myocardium--implications for altered serum bile acid composition in patients with atrial fibrillation. Heart 2013, 99, 1685–1692. [Google Scholar] [CrossRef]
- Zardi, E.M.; Abbate, A.; Zardi, D.M.; Dobrina, A.; Margiotta, D.; Van Tassel, B.W.; Afeltra, A.; Sanyal, A.J. Cirrhotic Cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 539–549. [Google Scholar] [CrossRef] [Green Version]
- Izzy, M.; VanWagner, L.B.; Lin, G.; Altieri, M.; Findlay, J.Y.; Oh, J.K.; Watt, K.D.; Lee, S.S. Cirrhotic Cardiomyopathy Consortium. Redefining Cirrhotic Cardiomyopathy for the Modern Era. Hepatology 2019, 71, 334–345. [Google Scholar] [CrossRef]
- Wiese, S.; Hove, J.D.; Bendtsen, F.; Moller, S. Cirrhotic cardiomyopathy: Pathogenesis and clinical relevance. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 177–186. [Google Scholar] [CrossRef]
- Chen, Y.; Chan, A.C.; Chan, S.C.; Chok, S.H.; Sharr, W.; Fung, J.; Liu, J.H.; Zhen, Z.; Sin, W.C.; Lo, C.M.; et al. A detailed evaluation of cardiac function in cirrhotic patients and its alteration with or without liver transplantation. J. Cardiol. 2016, 67, 140–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimbas, R.C.; Baldea, S.M.; Guerra, R.D.G.A.; Visoiu, S.I.; Rimbas, M.; Pop, C.S.; Vinereanu, D. New definition criteria of myocardial dysfunction in patients with liver cirrhosis: A speckle tracking and tissue Doppler imaging study. Ultrasound Med Biol. 2018, 44, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Farr, M.; Schulze, P.C. Recent advances in the diagnosis and management of cirrhosis-associated cardiomyopathy in liver transplant candidates: Advanced echo imaging cardiac biomarkers, and advanced heart failures therapies. Clin. Med. Insights Cardiol. 2015, 8, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P.; James, O.F.; Butler, T.J.; Campbell, R.W. QT prolongations and sudden cardiac death in patients with alcoholic liver disease. Lancet 1993, 341, 1423–1428. [Google Scholar] [PubMed]
- Ma, Z.; Meddiings, J.B.; Lee, S.S. Membrane physical properties determine cardiac beta-adrenergic receptor function in cirrhotic rats. Am. J. Physiol. 1994, 267, G87–G93. [Google Scholar] [CrossRef] [PubMed]
- Usoro, A.O.; Bradford, N.; Shah, A.J.; Soliman, E.Z. Risk of mortality in individuals with low QRS voltage and free of cardiovascular disease. Am. J. Cardiol. 2014, 113, 1514–1517. [Google Scholar] [CrossRef]
- Mozos, I. Arrhythmia risk in liver cirrhosis. World J. Hepatol. 2015, 7, 662–672. [Google Scholar] [CrossRef]
- Janahi, E.M.; Ilyas, Z.; Al-Othman, S.; Darwish, A.; Sanad, S.J.; Almusaifer, B.; Al-Mannai, M.; Golbahar, J.; Perna, S. Hepatitis B Virus Genotypes in the Kingdom of Bahrain: Prevalence, Gender Distribution and Impact on Hepatic Biomarkers. Medicina 2019, 55, 622. [Google Scholar] [CrossRef] [Green Version]
- Mansberg, K.; Kull, K.; Salupere, R.; Prükk, T.; Margus, B.; Kariis, T.; Remmel, T.; Suurmaa, K.; Ott, K.; Jaago, K.; et al. A Population-Based Surveillance Study on the Epidemiology of Hepatitis C in Estonia. Medicina 2018, 54, 9. [Google Scholar] [CrossRef] [Green Version]
- Iliescu, L.; Mercan-Stanciu, A.; Toma, L.; Dodot, M.; Isac, T.; Grumeza, M. All Oral Antiviral Treatment with Paritaprevir/Ombitasvir/Ritonavir and Dasabuvir in Chronic HCV Infection—Real Life Experience. Proc. 35th Balk. Med. Week 2018, 138–143. [Google Scholar]
- European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J. Hepatol. 2018, 69, 461–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EASL. 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madias, J.E. Low QRS voltage and its causes. J. Electrocardiol. 2008, 41, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, T.M.; Masvidal, D.; Abi Samra, F.M.; Bernard, M.L.; Khatib, S.; Polin, G.M.; Rogers, P.A.; Xue, J.Q.; Morin, D.P. Optimal method of measuring the T-peak to T-end interval for risk stratification in primary prevention. EP Eur. 2018, 20, 698–705. [Google Scholar] [CrossRef]
- Bernardi, M.; Rubboli, A.; Trevisani, F.; Cancellieri, C.; Ligabue, A.; Baraldini, M.; Gasbarrini, G. Reduced cardiovascular responsiveness to exercise-induced sympathoadrenergic stimulation in patients with cirrhosis. J. Hepatol. 1991, 12, 207–216. [Google Scholar] [CrossRef]
- Pourafkari, L.; Ghaffari, S.; Nazeri, L.; Lee, J.B.; Masnadi-Shirazi, K.; Tajlil, A.; Nader, N.D. Electrocardiographic findings in hepatic cirrhosis and their association with the severity of disease. Cor et Vasa 2017, 59, e105–e113. [Google Scholar] [CrossRef]
- Moaref, A.; Zamirian, M.; Yazdani, M.; Salehi, O.; Sayadi, M.; Aghasadeghi, K. The correlation between echocardiographic findings and QT interval in cirrhotic patients. Int. Cardiovasc. Res. J. 2014, 8, 39–43. [Google Scholar]
- Genovesi, S.; Prata Pizzala, D.M.; Pozzi, M.; Ratti, L.; Milanese, M.; Pieruzzi, F.; Vincenti, A.; Stella, A.; Mancia, G.; Stramba-Badiale, M. QT interval prolongation and decreased heart rate variability in cirrhotic patients: Relevance of hepatic venous pressure gradient and serum calcium. Clin. Sci. 2009, 116, 851–859. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, M.; Calandra, S.; Colantoni, A.; Trevisani, F.; Raimondo, M.L.; Sica, G.; Schepis, F.; Mandini, M.; Simoni, P.; Contin, M.; et al. Q-T interval prolongation in cirrhosis: Prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepatology 1998, 27, 28–34. [Google Scholar] [CrossRef]
- Kupari, M.; Koskinen, P. Alcohol, cardiac arrhythmias and sudden death. Novartis Found Symp. 1998, 216, 68–79. [Google Scholar]
- Testino, G.; Leone, S.; Pellicano, R. Atrial fibrillation and alcoholic beverages. Minerva Med. 2019, 110, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Kempler, P.; Szalay, F.; Váradi, A.; Keresztes, K.; Kádár, E.; Tánczos, E.; Petrik, J. Prolongation of the QTc-interval reflects the severity of autonomic neuropathy in primary biliary cirrhosis and in other non-alcoholic liver diseases. Z. Gastroenterol. 1993, 31, 96–98. [Google Scholar] [PubMed]
- Tsiompanidis, E.; Siakavellas, S.I.; Tentolouris, A.; Eleftheriadou, I.; Chorepsima, S.; Manolakis, A.; Oikonomou, K.; Tentolouris, N. Liver cirrhosis-effect on QT interval and cardiac autonomic nervous system activity. World J. Gastrointest Pathophysiol. 2018, 9, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Geraldino-Pardilla, L.; Gartshteyn, Y.; Piña, P.; Cerrone, M.; Giles, J.T.; Zartoshti, A.; Bathon, J.M.; Askanase, A.D. ECG non-specific ST-T and QTc abnormalities in patients with systemic lupus erythematosus compared with rheumatoid arthritis. Lupus Sci. Med. 2016, 3, e000168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panoulas, V.F.; Toms, T.E.; Douglas, K.M.; Sandoo, A.; Metsios, G.S.; Stavropoulos-Kalinoglou, A.; Kitas, G.D. Prolonged QTc interval predicts all-cause mortality in patients with rheumatoid arthritis: An association driven by high inflammatory burden. Rheumatology 2014, 53, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Lazzerini, P.E.; Capecchi, P.L.; Guideri, F.; Bellisai, F.; Selvi, E.; Acampa, M.; Costa, A.; Maggio, R.; Garcia-Gonzalez, E.; Bisogno, S.; et al. Comparison of frequency of complex ventricular arrhythmias in patients with positive versus negative anti-Ro/SSA and connective tissue disease. Am. J. Cardiol. 2007, 100, 1029–1034. [Google Scholar] [CrossRef]
- Wong, F. Cirrhotic cardiomyopathy. Hepatol. Int. 2009, 3, 294–304. [Google Scholar] [CrossRef] [Green Version]
- Ytting, H.; Henriksen, J.H.; Fuglsang, S.; Bendtsen, F.; Møller, S. Prolonged Q-T(c) interval in mild portal hypertensive cirrhosis. J. Hepatol. 2005, 43, 637–644. [Google Scholar] [CrossRef]
- Arques, S.; Ambrosi, P. Human Serum Albumin in the Clinical Syndrome of Heart Failure. J. Card. Fail. 2011, 17, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Marconi, V.C.; Duncan, M.S.; So-Armah, K.; Re, V.L., 3rd; Lim, J.K.; Butt, A.A.; Goetz, M.B.; Rodriguez-Barradas, M.C.; Alcorn, C.W.; Lennox, J.; et al. Bilirubin Is Inversely Associated With Cardiovascular Disease Among HIV-Positive and HIV-Negative Individuals in VACS (Veterans Aging Cohort Study). J. Am. Heart Assoc. 2018, 7, e007792. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, S.; Mookerjee, R.P.; Rackayova, V.; Rangroo Thrane, V.; Vairappan, B.; Ott, P.; Rose, C.F. Ammonia toxicity: From head to toe? Metab. Brain Dis. 2017, 32, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Cichoż-Lach, H.; Tomaszewski, M.; Kowalik, A.; Lis, E.; Tomaszewski, A.; Lach, T.; Boczkowska, S.; Celinski, K. QT Interval Prolongation and QRS Voltage Reduction in Patients with Liver Cirrhosis. Adv. Clin. Exp. Med. 2015, 24, 615–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuculi, F.; Jamshidi, P.; Kobza, R.; Rohacek, M.; Erne, P. Precordial low voltage in patients with ascites. Europace 2008, 10, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Arteyeva, N.V.; Goshka, S.L.; Sedova, K.A.; Bernikova, O.G.; Azarov, J.E. What does the T(peak)-T(end) interval reflect? An experimental and model study. J. Electrocardiol. 2013, 46, 296.e1–296.e8. [Google Scholar] [CrossRef]
- Kilicaslan, F.; Tokatli, A.; Ozdag, F.; Uzun, M.; Uz, O.; Isilak, Z.; Yiginer, O.; Yalcin, M.; Guney, M.S.; Cebeci, B.S. Tp-e interval, Tp-e/QT ratio, and Tp-e/QTc ratio are prolonged in patients with moderate and severe obstructive sleep apnea. Pacing Clin. Electrophysiol. 2012, 35, 966–972. [Google Scholar] [CrossRef]
- Demir, C.; Demir, M. Evaluation of Tp-e interval and Tp-e/QT ratio in patients with chronic hepatitis B. Prague Med. Rep. 2013, 114, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Salgado, A.A.; Barbosa, P.R.B.; Ferreira, A.G.; Reis, C.A.; Terra, C. Prognostic Value of a New Marker of Ventricular Repolarization in Cirrhotic Patients. Arq. Bras. Cardiol. 2016, 107, 523–531. [Google Scholar] [CrossRef]
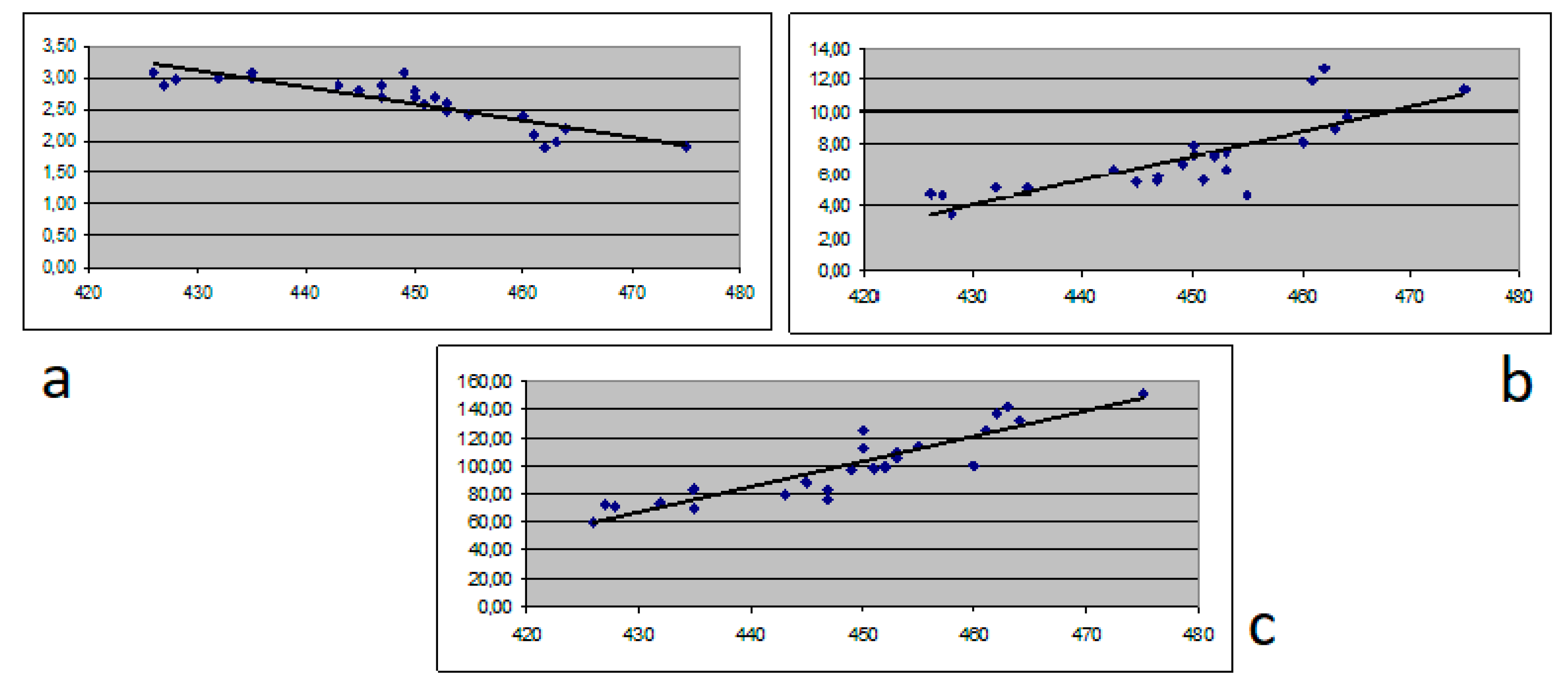

| Parameter | Chronic hepatitis (N = 54) | Cirrhosis Child A (N = 18) | Cirrhosis Child B (N = 20) | Cirrhosis Child C (N = 25) | p Value |
|---|---|---|---|---|---|
| Age (y) | 48.4 +/− 5.3 | 50.4 +/− 10.5 | 55.7 +/− 13.2 | 60.8 +/− 14.3 | 0.0043 |
| Gender (M/F) | 25/29 | 9/9 | 11/9 | 11/14 | 0.67 |
| Etiology (HBV/HCV) | 28/26 | 8/10 | 7/13 | 9/14 | 0.589 |
| ALT (IU/mL) | 46 +/− 28 | 62+/− 23 | 75 +/− 39 | 77 +/− 45 | 0.52 |
| AST (IU/mL) | 78 +/− 45 | 104+/− 67 | 112 +/− 53 | 101 +/− 62 | 0.25 |
| Total bilirubin (mg/dL) | 0.5 +/− 0.4 | 1.2 +/− 0.7 | 2.5 +/− 1.3 | 7.1 +/− 3.8 | 0.035 |
| Albumin (g/dL) | 4.2 +/− 0.6 | 3.3 +/− 0.3 | 3.1 +/− 0.7 | 2.8 +/− 0.9 | 0.028 |
| Total cholesterol (mg/dL) | 212 +/− 39 | 177 +/− 48 | 152 +/− 46 | 134 +/− 72 | 0.045 |
| Ammonia (μg/dL) | 32 +/− 17 | 47 +/− 12 | 71 +/− 23 | 89 +/− 21 | 0.021 |
| Creatinine (mg/dL) | 0.9 +/− 0.3 | 0.9 +/− 0.4 | 1.0 +/− 0.3 | 0.9 +/− 0.4 | 0.626 |
| Sodium (mEq/L) | 143 +/− 3 | 140 +/− 4 | 136 +/− 4 | 131 +/− 6 | 0.05 |
| Potassium (mEq/L) | 4.4 +/− 0.5 | 4.2+/− 0.6 | 4.3 +/ 0.5 | 4.1 +/− 0.7 | 0.274 |
| Total calcium (mg/dL) | 9.4 +/− 0.8 | 9.2 +/− 0.7 | 9.3+/− 0.6 | 9.0 +/− 0.7 | 0.053 |
| Magnesium (mg/dL) | 2.1 +/− 0.5 | 2.1+/− 0.7 | 2.0 +/− 0.6 | 1.9 +/− 1.7 | 0.62 |
| Phosphorus (mg/dL) | 3.6 +/− 1.2 | 3.2 +/− 0.7 | 3.3 +/− 0.4 | 3.0 +/− 0.8 | 0.09 |
| Bicarbonate (mmol/L) | 24 +/− 3 | 25 +/− 4 | 23 +/− 4 | 23 +/− 5 | 0.13 |
| INR | 0.9 +/− 0.1 | 1.1 +/0.3 | 1.3 +/− 0.2 | 2.2 +/− 1,5 | 0.0012 |
| LVEF (%) | 62 +/− 9 | 55 +/− 6 | 52 +/− 7 | 52 +/− 3 | 0.07 |
| Septal e’ velocity (cm/s) | 12 +/− 3 | 11 +/− 3 | 8 +/− 2 | 6 +/− 2 | 0.01 |
| E/e’ ratio | 19 +/− 3 | 16 +/− 4 | 13 +/− 4 | 11 +/− 2 | 0.001 |
| Parameter | Chronic Hepatitis (N = 54) | Cirrhosis Child A (N = 18) | Cirrhosis Child B (N = 20) | Cirrhosis Child C (N = 25) | p Value | |
|---|---|---|---|---|---|---|
| Mean QRS voltage | Precordial Leads | 10.2 +/− 3.5 | 9.8 +/− 2.5 | 9.1 +/− 3.1 | 8.9 +/− 2.9 | 0.043 |
| Limb Leads | 5.1 +/− 1.9 | 4.9 +/− 1.4 | 4.8 +/− 2.1 | 4.5 +/− 1.7 | 0.032 | |
| Criteria for QRS Hypovoltage | 3 (5.5%) | 6 (33.33%) | 12 (60%) | 16 (64%) | 0.041 | |
| Heart Rate (bpm) | 81 +/− 19 | 82 +/− 21 | 88 +/− 16 | 92 +/− 15 | 0.039 | |
| QRS Duration (ms) | 85 +/ 16 | 85 +/− 14 | 83 +/− 12 | 84 +/− 17 | 0.134 | |
| QTc (ms) | 418 +/− 1 | 445 +/− 27 | 451 +/− 23 | 459 +/− 31 | 0.045 | |
| Criteria for Prolonged QT | 2 (3,7%) | 6 (33.33%) | 10 (50%) | 13 (52%) | 0.021 | |
| Mean Tpe (ms) | 73 +/− 18 | 71 +/− 17 | 70 +/− 23 | 64 +/− 15 | 0.023 | |
| Criteria for Shortened Tpe (<50ms) | 1 (1.8%) | 3 (16.66%) | 9 (45%) | 15 (60%) | 0.034 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toma, L.; Stanciu, A.M.; Zgura, A.; Bacalbasa, N.; Diaconu, C.; Iliescu, L. Electrocardiographic Changes in Liver Cirrhosis—Clues for Cirrhotic Cardiomyopathy. Medicina 2020, 56, 68. https://doi.org/10.3390/medicina56020068
Toma L, Stanciu AM, Zgura A, Bacalbasa N, Diaconu C, Iliescu L. Electrocardiographic Changes in Liver Cirrhosis—Clues for Cirrhotic Cardiomyopathy. Medicina. 2020; 56(2):68. https://doi.org/10.3390/medicina56020068
Chicago/Turabian StyleToma, Letitia, Adriana Mercan Stanciu, Anca Zgura, Nicolae Bacalbasa, Camelia Diaconu, and Laura Iliescu. 2020. "Electrocardiographic Changes in Liver Cirrhosis—Clues for Cirrhotic Cardiomyopathy" Medicina 56, no. 2: 68. https://doi.org/10.3390/medicina56020068






