Midpalatal Suture Density Evaluation after Rapid and Slow Maxillary Expansion with a Low-Dose CT Protocol: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
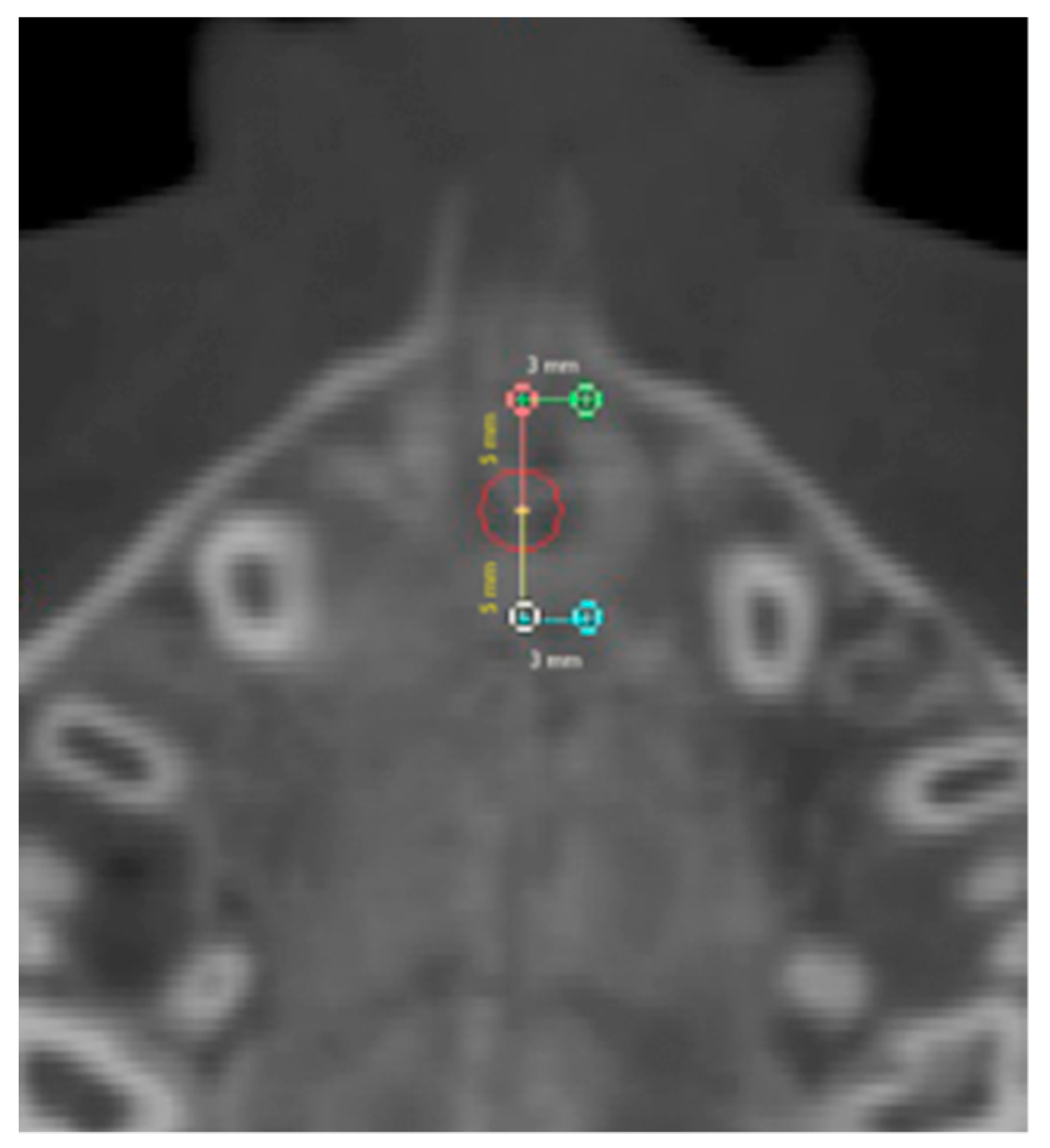
2.1. Round-Shaped ROIs
2.2. Rectangular-Shaped ROIs
3. Results
4. Discussion
5. Conclusions
- Bone density measured on the hard palate of prepubertal subjects did not show significant differences in values when compared with the density at the midpalatal suture before treatment;
- midpalatal suture density showed no significant changes when RME was performed;
- significant decreases in density were reported after the SME treatment in the whole suture area in the considered time interval, but no significant differences were detected between groups, suggesting similar rates of suture reorganization in spite of differing retention periods.
Author Contributions
Funding
Conflicts of Interest
References
- McNamara, J.A., Jr.; Lione, R.; Franchi, L.; Angelieri, F.; Cevidanes, L.H.; Darendeliler, M.A.; Cozza, P. The role of rapid maxillary expansion in the promotion of oral and general health. Prog. Orthod. 2015, 16, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucchese, A.; Sfondrini, M.F.; Manuelli, M.; Gangale, S. Fixed space maintainer for use with a rapid palatal expander. J. Clin. Orthod. 2005, 39, 557–558. [Google Scholar] [PubMed]
- Battikki, R. Rapid maxillary expansion: Review of literature. Saudi Dent. J. 2001, 13, 161–167. [Google Scholar]
- Militi, D.; Militi, A.; Cutrupi, M.C.; Portelli, M.; Rigoli, L.; Matarese, G.; Salpietro, D.C. Genetic basis of non syndromic hypodontia: A DNA investigation performed on three couples of monozygotic twins about PAX9 mutation. Eur. J. Paediatr. Dent. 2011, 12, 21–24. [Google Scholar] [PubMed]
- Palmieri, A.; Zollino, I.; Clauser, L.; Lucchese, A.; Girardi, A.; Farinella, F.; Carinci, F. Biological Effect of Resorbable Plates on Normal Osteoblasts and Osteoblasts Derived From Pfeiffer Syndrome. J. Craniofacial Surg. 2011, 22, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, A.; Lucchese, A.; Darnahal, A.; Zamali, Z.; Perillo, L. Cleft sidedness and congenitally missing teeth in patients with cleft lip and palatale patients. Prog. Orthod. 2016, 17, 14. [Google Scholar]
- Crupi, P.; Portelli, M.; Matarese, G.; Nucera, R.; Militi, A.; Mazza, M.; Cordasco, G. Correlations between cephalic posture and facial type in patients suffering from breathing obstructive syndrome. Eur. J. Paediatr. Dent. 2007, 8, 77–82. [Google Scholar]
- Biondi, K.; Lorusso, P.; Fastuca, R.; Mangano, A.; Zecca, P.A.; Bosco, M.; Caprioglio, A.; Levrini, L. Evaluation of masseter muscle in different vertical skeletal patterns in growing patients. Eur. J. Paediatr. Dent. 2016, 17, 47–52. [Google Scholar]
- Lo Giudice, A.; Fastuca, R.; Portelli, M.; Militi, A.; Bellocchio, M.; Spinuzza, P.; Briguglio, F.; Caprioglio, A.; Nucera, R. Effects of rapid vs slow maxillary expansion on nasal cavity dimensions in growing subjects: A methodological and reproducibility study. Eur. J. Paediatr. Dent. 2017, 18, 299–304. [Google Scholar]
- Martina, R.; Cioffi, I.; Farella, M.; Leone, P.; Manzo, P.; Matarese, G.; Portelli, M.; Nucera, R.; Cordasco, G. Transverse changes determined by rapid and slow maxillary expansion—A low-dose CT-based randomized controlled trial. Orthod. Craniofacial Res. 2012, 15, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Fastuca, R.; Zecca, P.; Caprioglio, A. Role of mandibular displacement and airway size in improving breathing after rapid maxillary expansion. Prog. Orthod. 2014, 15, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fastuca, R.; Perinetti, G.; Zecca, P.A.; Nucera, R.; Caprioglio, A. Airway compartments volume and oxygen saturation changes after rapid maxillary expansion: A longitudinal correlation study. Angle Orthod. 2015, 85, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Caprioglio, A.; Meneghel, M.; Fastuca, R.; Zecca, P.A.; Nucera, R.; Nosetti, L. Rapid maxillary expansion in growing patients: Correspondence between 3-dimensional airway changes and polysomnography. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Fastuca, R.; Meneghel, M.; Zecca, P.A.; Mangano, F.; Antonello, M.; Nucera, R.; Caprioglio, A. Multimodal airway evaluation in growing patients after rapid maxillary expansion. Eur. J. Paediatr. Dent. 2015, 16, 129–134. [Google Scholar] [PubMed]
- Isola, G.; Anastasi, G.; Matarese, G.; Williams, R.C.; Cutroneo, G.; Bracco, P.; Piancino, M.G. Functional and molecular outcomes of the human masticatory muscles. Oral Dis. 2018, 8, 1424–1441. [Google Scholar] [CrossRef]
- Caprioglio, A.; Fastuca, R.; Zecca, P.A.; Beretta, M.; Mangano, C.; Piattelli, A.; Macchi, A.; Iezzi, G. Cellular Midpalatal Suture Changes after Rapid Maxillary Expansion in Growing Subjects: A Case Report. Int. J. Mol. Sci. 2017, 18, 615. [Google Scholar] [CrossRef] [Green Version]
- Bucci, R.; D’Antò, V.; Rongo, R.; Valletta, R.; Martina, R.; Michelotti, A. Dental and skeletal effects of palatal expansion techniques: A systematic review of the current evidence from systematic reviews and meta-analyses. J. Oral. Rehabil. 2016, 43, 543–564. [Google Scholar] [CrossRef] [Green Version]
- Lucchese, A.; Manuelli, M.; Bassani, L.; Albertini, P.; Matarese, G.; Perillo, L.; Gastaldi, G.; Gherlone, E.F. Fiber reinforced composites orthodontic retainers. Minerva Stomatol. 2015, 64, 323–333. [Google Scholar]
- Isaacson, R.J.; Ingram, A.H. Forces produced by rapid maxillary expansion. II. Forces present during treatment. Angle Orthod. 1964, 34, 261–270. [Google Scholar]
- Da Silva Filho, O.G.; Montes, L.A.; Torelly, L.F. Rapid maxillary expansion in the deciduous and mixed dentition evaluated through posteroanterior cephalometric analysis. Am. J. Orthod. Dentofac. Orthop. 1995, 107, 268–275. [Google Scholar] [CrossRef]
- Inoue, H. Radiographic observation of rapid expansion of human maxilla. Bull. Tokyo Med. Dent. Univ. 1970, 17, 219–229. [Google Scholar]
- Schauseil, M.; Ludwig, B.; Zorkun, B.; Hellak, A.; Korbmacher-Steiner, H. Density of the midpalatal suture after RME treatment—A retrospective comparative low-dose CT-study. Head Face Med. 2014, 10, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lione, R.; Franchi, L.; Fanucci, E.; Laganà, G.; Cozza, P. Three-dimensional densitometric analysis of maxillary sutural changes induced by rapid maxillary expansion. Dentomaxillofacial Radiol. 2013, 42, 71798010. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Baccetti, T.; Lione, R.; Fanucci, E.; Cozza, P. Modifications of midpalatal sutural density induced by rapid maxillary expansion: A low-dose computed-tomography evaluation. Am. J. Orthod. Dentofacial Orthop. 2010, 137, 486–488. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Xu, T.; Zou, W. Effects of rapid maxillary expansion on the midpalatal suture: A systematic review. Eur. J. Orthod. 2015, 37, 651–655. [Google Scholar] [CrossRef] [Green Version]
- Cordasco, G.; Portelli, M.; Militi, A.; Nucera, R.; Lo Giudice, A.; Gatto, E.; Lucchese, A. Low-dose protocol of the spiral CT in orthodontics: Comparative evaluation of entrance skin dose with traditional X-ray techniques. Prog. Orthod. 2013, 14, 24. [Google Scholar] [CrossRef] [Green Version]
- Dahlberg, G. Statistical Methods for Medical and Biological Students; Interscience Publications: New York, NY, USA, 1940. [Google Scholar]
- Storey, E. Tissue response to the movement of bones. Am. J. Orthod. 1973, 64, 229–247. [Google Scholar] [CrossRef]
- Mossaz-Joëlson, K.; Mossaz, C.F. Slow maxillary expansion: A comparison between banded and bonded appliances. Eur. J. Orthod. 1989, 11, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Hicks, E.P. Slow maxillary expansion. A clinical study of the skeletal versus dental response to low-magnitude force. Am. J. Orthod. 1978, 73, 121–141. [Google Scholar] [CrossRef]
- Bell, R.A. The effects of maxillary expansion using a quad-helix appliance during the deciduous and mixed dentitions. Am. J. Orthod. 1981, 79, 152–161. [Google Scholar] [CrossRef]
- Cotton, L. A Slow maxillary expansion: Skeletal versus dental response to low magnitude force in Macaca mulatta. Am. J. Orthod. 1978, 73, 1–23. [Google Scholar] [CrossRef]
- Pinheiro, F.H.; Garib, D.G.; Janson, G.; Bombonatti, R.; de Freitas, M.R. Longitudinal stability of rapid and slow maxillary expansion. Dent. Press J. Orthod. 2014, 19, 70–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starnbach, H.K.; Bayne, D.I.; Cleall, J.F.; Subtelny, J.D. Facioskeletal and dental changes resulting from rapid maxillary expansion. Angle Orthod. 1966, 36, 152–164. [Google Scholar] [PubMed]
- Caprioglio, A.; Bergamini, C.; Franchi, L.; Vercellini, N.; Zecca, P.A.; Nucera, R.; Fastuca, R. Prediction of Class II improvement after rapid maxillary expansion in early mixed dentition. Prog. Orthod. 2017, 18, 9. [Google Scholar] [CrossRef] [Green Version]
- Fastuca, R.; Lorusso, P.; Lagravère, M.O.; Michelotti, A.; Portelli, M.; Zecca, P.; D’Antò, V.; Militi, A.; Nucera, R.; Caprioglio, A. Digital evaluation of nasal changes induced by rapid maxillary expansion with different anchorage and appliance design. BMC Oral. Health 2017, 17, 113. [Google Scholar] [CrossRef] [Green Version]
- Zecca, P.A.; Fastuca, R.; Beretta, M.; Caprioglio, A.; Macchi, A. Correlation Assessment between Three-Dimensional Facial Soft Tissue Scan and Lateral Cephalometric Radiography in Orthodontic Diagnosis. Int. J. Dent. 2016, 2016, 1473918. [Google Scholar] [CrossRef] [Green Version]
- Portelli, M.; Militi, A.; Cicciù, M.; Lo Giudice, A.; Cervino, G.; Fastuca, R.; Nucera, R. No compliance correction of class II malocclusion in growing patients with herbst appliance: A case report. Open Dent. J. 2018, 12, 605–613. [Google Scholar] [CrossRef]
- Giuliano Maino, B.; Pagin, P.; Di Blasio, A. Success of miniscrews used as anchorage for orthodontic treatment: Analysis of different factors. Prog. Orthod. 2012, 13, 202–209. [Google Scholar] [CrossRef]
- Isola, G.; Perillo, L.; Migliorati, M.; Matarese, M.; Dalessandri, D.; Grassia, V.; Alibrandi, A.; Matarese, G. The impact of temporomandibular joint arthritis on functional disability and global health in patients with juvenile idiopathic arthritis. Eur. J. Orthod. 2019, 41, 117–124. [Google Scholar] [CrossRef]
- Portelli, M.; Militi, A.; Lo Giudice, A.; Lo Giudice, R.; Fastuca, R.; Ielo, I.; Mongelli, V.; Lo Giudice, G.; Lucchese, A.; Nucera, R. Standard and Low-Dose cone beam computer tomography protocol for orthognatodonic diagnosis: A comparative evaluation. J. Biol. Regul. Homeost. Agents 2018, 32, 59–66. [Google Scholar]
- Lucchese, A.; Manuelli, M.; Albertini, P.; Asperio, P.; Gastaldi, G. Treatment of Severe Maxillary Hypoplasia with Combined Orthodontics and Distraction Osteogenesis. J. Craniofacial Surg. 2018, 29, 970–972. [Google Scholar] [CrossRef] [PubMed]
- Portelli, M.; Gatto, E.; Matarese, G.; Militi, A.; Catalfamo, L.; Gherlone, E.; Lucchese, A. Unilateral condylar hyperplasia: Diagnosis, clinical aspects and operative treatment. A case report. Eur. J. Paediatr. Dent. 2015, 16, 99–102. [Google Scholar] [PubMed]
- Portelli, M.; Nucera, R.; Militi, A.; Mazza, M.; Matarese, G. Valutazione dei diametri trasversi del mascellare e delle cavità nasali attraverso un protocollo TC a bassa dose. Mondo Ortod. 2007, 6, 353–358. [Google Scholar]
- Vitale, C.; Militi, A.; Portelli, M.; Cordasco, G.; Matarese, G. Maxillary Canine-First Premolar transposition in the permanent dentition. J. Clin. Orthod. 2009, XLIII, 517–524. [Google Scholar]
- Portelli, M.; Nucera, R.; Militi, A.; Matarese, G. Trattamento chirurgico-ortodontico di un primo molare mandibolareaffetto da cisti follicolare. Mondo Ortod. 2009, 4, 53–59. [Google Scholar]
- Militi, A.; Vitale, C.; Portelli, M.; Matarese, G.; Cordasco, G. Open bite anteriore con agenesia dei secondi premolari inferiori: Terapia estrattiva con utilizzo di attacchi auto leganti. Mondo Ortod. 2012, 37, 1–15. [Google Scholar] [CrossRef]



| Landmark | Definition |
|---|---|
| Posterior Nasal Spine (PNS) | the most posterior point of the posterior nasal spine |
| Anterior Nasal Spine (ANS) | the most anterior point of the anterior nasal spine |
| Right Palatal Foramen Point (RPFP) | the most posterior and external point of the right palatal foramen |
| Left Palatal Foramen Point (LPFP) | the most posterior and external point of the left palatal foramen |
| T0 | T1 | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Dependent t-Test | |
| AS ROI | 547.56 | 137.10 | 386.56 | 177.20 | 0.25 |
| PS ROI | 532.54 | 198.76 | 411.50 | 104.24 | 0.39 |
| AB ROI | 445.00 | 35.92 | 409.85 | 251.11 | 0.75 |
| PB ROI | 428.69 | 276.29 | 363.87 | 291.48 | 0.60 |
| ASD ROI | 519.58 | 123.61 | 391.03 | 59.87 | 0.18 |
| PSD ROI | 478.97 | 64.73 | 425.62 | 83.82 | 0.16 |
| T0 | T1 | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Dependent t-Test | |
| AS ROI | 390.79 | 141.37 | 295.98 | 162.55 | 0.12 |
| PS ROI | 560.05 | 162.27 | 387.81 | 146.91 | 0.01 * |
| AB ROI | 366.61 | 216.71 | 322.91 | 244.77 | 0.28 |
| PB ROI | 357.67 | 204.98 | 344.66 | 263.92 | 0.85 |
| ASD ROI | 380.39 | 140.84 | 212.41 | 127.37 | 0.04 * |
| PSD ROI | 326.91 | 163.92 | 193.70 | 142.60 | 0.04 * |
| RME | SME | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Independent t-Test | |
| ASC | −161.00 | 267.12 | −94.82 | 108.08 | 0.62 |
| PSC | −121.04 | 282.87 | −172.23 | 87.53 | 0.71 |
| ASL | −35.15 | 230.97 | −43.70 | 79.17 | 0.94 |
| PSL | −64.82 | 255.12 | −13.01 | 140.91 | 0.70 |
| ASD | −128.55 | 174.70 | −167.98 | 125.66 | 0.69 |
| PSD | −53.35 | 68.35 | −133.20 | 101.50 | 0.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fastuca, R.; Michelotti, A.; Nucera, R.; D’Antò, V.; Militi, A.; Logiudice, A.; Caprioglio, A.; Portelli, M. Midpalatal Suture Density Evaluation after Rapid and Slow Maxillary Expansion with a Low-Dose CT Protocol: A Retrospective Study. Medicina 2020, 56, 112. https://doi.org/10.3390/medicina56030112
Fastuca R, Michelotti A, Nucera R, D’Antò V, Militi A, Logiudice A, Caprioglio A, Portelli M. Midpalatal Suture Density Evaluation after Rapid and Slow Maxillary Expansion with a Low-Dose CT Protocol: A Retrospective Study. Medicina. 2020; 56(3):112. https://doi.org/10.3390/medicina56030112
Chicago/Turabian StyleFastuca, Rosamaria, Ambra Michelotti, Riccardo Nucera, Vincenzo D’Antò, Angela Militi, Antonino Logiudice, Alberto Caprioglio, and Marco Portelli. 2020. "Midpalatal Suture Density Evaluation after Rapid and Slow Maxillary Expansion with a Low-Dose CT Protocol: A Retrospective Study" Medicina 56, no. 3: 112. https://doi.org/10.3390/medicina56030112





