Fracture Resistance of New Metal-Free Materials Used for CAD-CAM Fabrication of Partial Posterior Restorations
Abstract
1. Introduction
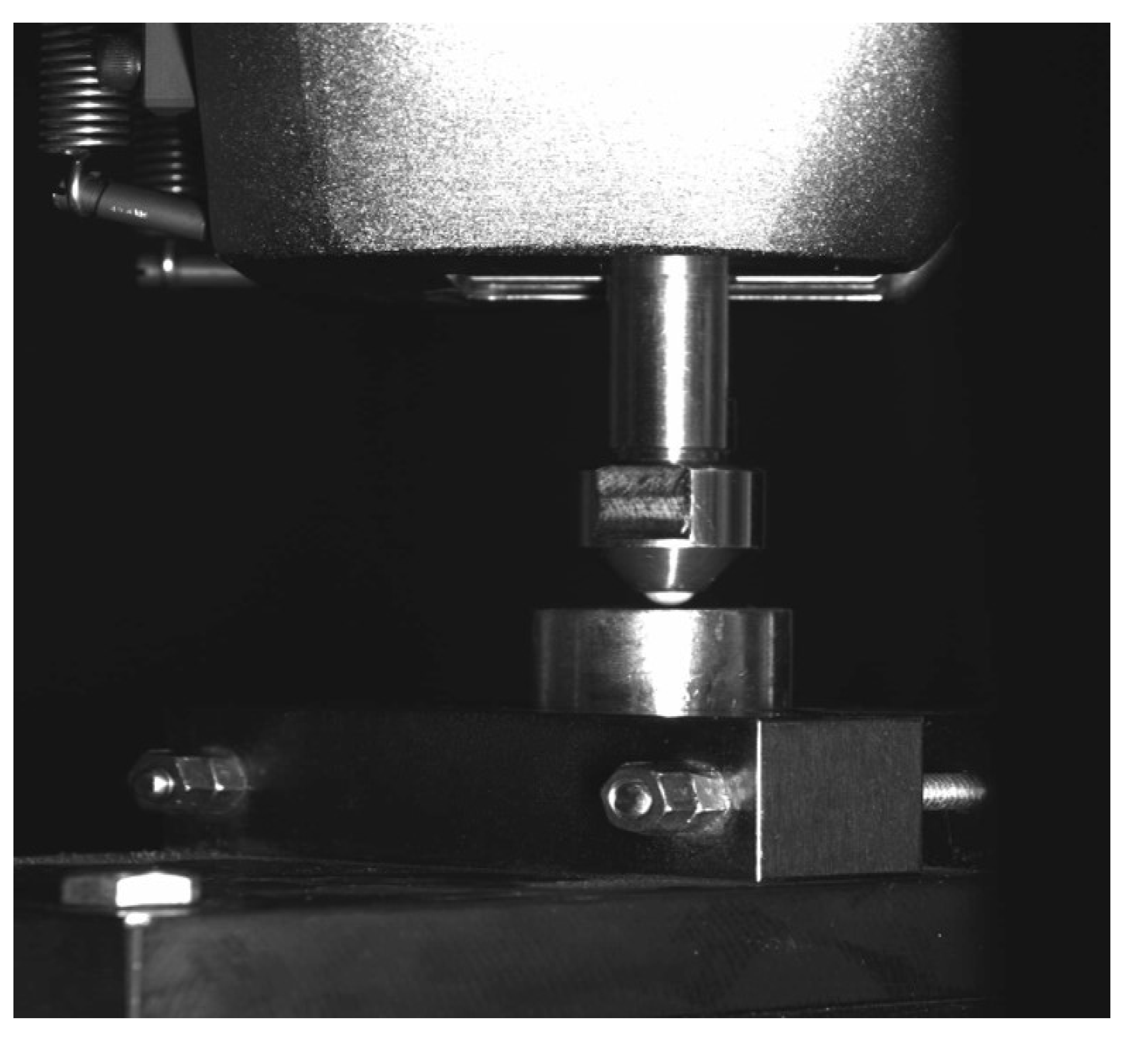
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
- The CAD-CAM restoration materials analyzed showed high fracture resistance values that were adequate for use in partial coverage dental restorations in the posterior region.
- IPS e.max CAD® ceramic obtained the highest fracture resistance, although Weibull distribution showed that it had less predictable behavior than the other materials tested.
- Hybrid materials presented lower fracture resistance than ceramic materials due to their internal composition.
- The resilient behavior exhibited by hybrid materials generated surface wear patterns prior to fracture, which implied more conservative behavior than ceramic materials.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Edelhoff, D.; Sorensen, J.A. Tooth structure removal associated with various preparation designs for anterior teeth. J. Prosthet. Dent. 2002, 87, 503–509. [Google Scholar] [CrossRef]
- Cheung, G.; Lai, S.; Ng, R. Fate of vital pulps beneath a metal-ceramic crown or a bridge retainer. Int. Endod. J. 2005, 38, 521–530. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Brägger, U.; Lang, N.P.; Zwahlen, M. Comparison of survival and complication rates of tooth-supported fixed dental prostheses (FDPs) and implant-supported FDPs and single crowns (SCs). Clin. Oral Implants Res. 2007, 18, 97–113. [Google Scholar] [CrossRef]
- Ercoli, C.; Caton, J.G.J. Dental prostheses and tooth-related factors. Clin. Periodontol. 2018, 45, S207–S218. [Google Scholar] [CrossRef]
- Abduo, J.; Sambrook, R.J. Longevity of ceramic onlays: A systematic review. J. Esthet. Restor. Dent. 2018, 30, 193–215. [Google Scholar] [CrossRef]
- Edelhoff, D.; Ahlers, O. Occlusal onlays as a modern treatment concept for the reconstruction of severely worn occlusal surface. Quintessence Int. 2018, 49, 521–533. [Google Scholar]
- Anadioti, E.; Aquilino, S.A.; Gratton, D.; Holloway, J.; Denry, I.; Thomas, G.; Quian, F. Internal fit of pressed and computer-aided design/computed-aided manufacturing ceramic crowns made from digital and conventional impressions. J. Prosthet. Dent. 2015, 113, 304–309. [Google Scholar] [CrossRef]
- Lauvahutanon, S.; Takahashi, H.; Shiozawa, M.; Iwasaki, N.; Asakawa, Y.; Oki, M.; Finger, W.J.; Arksornnukit, M. Mechanical properties of composite resin blocks for cad/cam. Dent. Mater. 2014, 33, 705–710. [Google Scholar] [CrossRef]
- Gracis, S.; Thompson, V.P.; Ferencz, J.L.; Silva, N.; Bonfante, E. A new classification system for all-ceramic and ceramic -like restorative materials. Int. J. Prosthodont. 2015, 28, 227–235. [Google Scholar] [CrossRef]
- Phark, J.; Sartori, N.; Duarte, S., Jr. Bonding to silica-Based Glass-ceramic: A Review of current techniques and novel Self-Etching ceramic primers. QDT 2016, 2016, 27–36. [Google Scholar]
- Tekçe, N.; Tuncer, S.; Demirci, M. The effect of sandblasting duration on the bond durability of dual-cure adhesive cement to CAD/CAM resin restoratives. J. Adv. Prosthodont. 2018, 10, 211–217. [Google Scholar] [CrossRef]
- Driscoll, C.F.; Freilich, M.A.; Guckes, A.D.; Knoernschild, K.L.; Mcgarry, T.J.; Goldstein, G.; Goodacre, C. The Glossary of Prosthodontic Terms. J. Prosthet. Dent. 2017, 1, e1–e105. [Google Scholar]
- Goujat, A.; Abouelleil, H.; Colon, P.; Jeannin, C.; Pradelle, N.; Seux, D.; Grosgogeat, B. Mechanical properties and internal fit of 4 CAD-CAM block materials. J. Prosthet. Dent. 2018, 119, 384–389. [Google Scholar] [CrossRef]
- Elsaka, S.; Elnaghy, A. Mechanical properties of zirconia reinforced lithium silicate glass-ceramic. Dent. Mater. 2016, 32, 908–914. [Google Scholar] [CrossRef]
- Della Bona, A.; Nogueira, A.; Pecho, O. Optical properties of CAD-CAM ceramic systems. J. Dent. 2014, 42, 1202–1209. [Google Scholar] [CrossRef]
- Sillas-Duarte, J.R.; Sartori, N.; Phark, J. Ceramic-reinforced polymers: CAD/CAM Hybrid Restorative Materials. Curr. Oral Health 2016, 3, 198–202. [Google Scholar] [CrossRef]
- Lawson, N.; Bansal, R.; Burges, J.O. Wear, Strength, modulus and hardness of CAD/CAM restorative materials. J. Dent. Mater. 2016, 32, e275–e283. [Google Scholar] [CrossRef]
- Ramos, N.C.; Campos, T.M.; Paz, I.S.; Machado, J.P.; Bottino, M.A.; Cesar, P.F. Microstructure characterization and SCG of newly engineered dental ceramics. Dent. Mater. 2016, 32, 870–878. [Google Scholar] [CrossRef]
- Zimmermann, M.; Egli, G.; Zaruba, M.; Mehl, A. Influence of material thickness on fractural strength of CAD/CAM fabricated ceramic crowns. Dent. Mater. J. 2017, 36, 778–783. [Google Scholar] [CrossRef]
- ISO 6872. Dentistry–Ceramic Materials; International Organization for Standards: Geneva, Switzerland, 2008. [Google Scholar]
- IPS e.max CAD Product Data Sheet. Available online: https://www.ivoclarvivadent.es/es-es/ (accessed on 1 January 2020).
- Vita Surpinity and Vita Enamic Product Data Sheet. Available online: https://www.vita-zahnfabrik.com/ (accessed on 1 January 2020).
- Lava™ Ultimate Product Data Sheet. Available online: https://www.3m.com.es/3M/es_ES/empresa-es/ (accessed on 1 January 2020).
- Ren, L.; Li, M.; Pan, Y.; Meng, X. Influence of polishing methods on the bonding effectiveness and durability of different resin cements to dentin. BioMed. Res. Int. 2018, 2018, 9189354. [Google Scholar] [CrossRef]
- Bueno, R.P.; Salomone, P.; Villetti, M.A.; Pozzobon, R.T. Effet of bleaching agents on the fluorescence of composite resins. Eur. J. Esthet. Dent. 2013, 8, 582–591. [Google Scholar]
- Langer, A.; Llie, N. Dentin infiltration ability of different classes of adhesive systems. Clin. Oral Investig. 2012, 17, 205–216. [Google Scholar] [CrossRef]
- Loguercio, A.D.; Stanislawczuk, R.; Mena-Serrano, A.; Reis, A. Effect of 3-year wáter storage on the performance of one-step self-etch adhesives applied actively on dentine. J. Dent. 2011, 39, 578–587. [Google Scholar] [CrossRef]
- Van Meerbeeck, B.; Inokoshi, S.; Braem, M. Morphological aspects of the resin-dentin interdiffusion zone with different dentin adhesive systems. J. Dent. Res. 1992, 71, 1530–1540. [Google Scholar] [CrossRef]
- Agustín-Panadero, R.; León-Martínez, R.; Sola-Ruiz, M.F.; Fons-Font, A.; García-Engra, G.; Fernández-Estevan, L. Are metal-free monolithic Crowns the present of prothesis? Study of mechanical Behaviour. Materials 2019, 12, 3663. [Google Scholar] [CrossRef]
- Farga-niñoles, I.; Aguntín-Panadero, R.; Román-Rodríguez, J.L.; Solá-Ruíz, M.F.; Granell-Ruíz, M.; Fons-Font, A. Fractographic study of the behavior of different Ceramic veneres on full coverage crowns in relation to supporting core materials. J. Clin. Exp. Dent. 2013, 5, e260–e266. [Google Scholar] [CrossRef]
- Guess, P.C.; Schultheis, S.; Wolkewitz, M.; Zhang, Y.; Strub, J.R. Influence of preparation design and ceramic thicknesses on fracture resistance and failure modes of premolar partial coverage restorations. J. Prosthet. Dent. 2013, 110, 264–273. [Google Scholar] [CrossRef]
- ISO 20501. Weibull Statistics for Strength Data; International Organization for Standards: Geneva, Switzerland, 2003. [Google Scholar]
- Fabbri, G.; Zarone, F.; Dellificorelli, G.; Cannistraro, G.; De Lorenzi, M.; Mosca, A. Clinical evaluation of 860 anterior and posterior lithium disilicate restorations: Retrospective study with a mean follow-up of 3 years and a maximum observational period of 6 years. Int. J. Periodont. Restor. Dent. 2014, 34, 1–15. [Google Scholar] [CrossRef]
- Guess, P.C.; Strub, J.R.; Steinhart, N.; Wolkewitz, M.; Stappert, C.F.J. All-ceramic partial coverage restorations—Midterm results of a 5-year prospective clinical splitmouth study. J. Dent. 2009, 37, 627–637. [Google Scholar] [CrossRef]
- Yildiz, C.; Akoglu-Vanlioglu, B.; Evren, B.; Uludamar, A.; Kulak-Ozkan, Y. Fracture resistance of manually and CAD/CAM manufactured ceramic onlays. J. Prosthodont. 2013, 22, 537–542. [Google Scholar] [CrossRef]
- Suk-Ho Kang, J.; Chang, H.; Son, H.H. Flexural strength and microstructure of two lithium disilicato glass ceramics for CAD/CAM restoration in the dental clinic. Rest. Dent. Endod. 2013, 38, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Bruzi, G.; Giannini, M.; Magne, P. Fatigue resistance of CAD/CAM complete crowns with a simplified cementation process. J. Prosthet. Dent. 2014, 111, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Schlichting, L.H.; Maia, H.P.; Baratieri, L.N.; Magne, P. Novel-design ultra-thin CAD/CAM composite resin and Ceramic oclusal veneres for the treatment of severe dental erosion. J. Prosthet. Dent. 2011, 105, 217–226. [Google Scholar] [CrossRef]
- Wendler, M.; Belli, R.; Valladares, D.; Petschelt, A.; Lohbauer, U. Chairside CAD/CAM materials. Part 3: Cyclic fatigue parameters and lifetime predictions. Dent. Mater. 2018, 34, 910–921. [Google Scholar] [CrossRef]
- Sieper, K.; Wille, S.; Kern, M. Fracture strength of lithium disilicate crowns compared to polymer-infiltrated ceramic-network and zirconia reinforced lithium silicate crowns. J. Mech. Behav. Biomed. 2017, 74, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.A.; Sophr, A.M.; De Goes, M.F.; Sobrinho, L.C.; Chan, D.C. Effect of etching and airborne particle abrasion on the microstructure of different dental ceramics. J. Prosthet. Dent. 2003, 89, 479–488. [Google Scholar] [CrossRef]
- Lung, C.Y.; Matinlinna, J.P. Aspects of silane coupling agents and surface conditioning in dentistry: An overview. Dent. Mater. 2012, 28, 467–477. [Google Scholar] [CrossRef]
- Özcan, M.; Barbosa, S.H.; Melo, R.M.; Galhano, G.A.; Bottino, M.A. Effect of surface conditioning methods on the microtensile bond strength of resin composite to composite after aging conditions. Dent. Mater. 2007, 23, 1276–1282. [Google Scholar] [CrossRef]
- Yoshihara, K.; Nagaoka, N.; Sonoda, A.; Maruo, Y.; Makita, Y.; Okihara, T.; Irie, M.; Yoshida, Y.; Van Meerbeek, B. Effectiveness and stability of silane coupling agent incorporated in ‘universal’ adhesives. Dent. Mater. 2016, 32, 1218–1225. [Google Scholar] [CrossRef]
- Yoshihara, K.; Nagaoka, N.; Maruo, Y.; Nishigawa, G.; Irie, M.; Yoshida, Y.; Van Meerbeek, B. Sandblasting may damage the Surface of composite CAD-CAM blocks. Dent. Mater. 2017, 33, 124–135. [Google Scholar] [CrossRef]
- Van den Breemer, C.; Özcan, M.; Cune, M.; Van Der Giezen, R.; Kerdijk, W.; Gresnigth, M. Effect of immediate dentin sealing on the fracgture strength of lithium disilicate and multiphase resin composite inlay restorations. J. Mech. Behav. Biomed. 2017, 72, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Magne, P.; Schlichting, L.H.; Maia, H.P.; Baratieri, L.N. In vitro fatigue resistance of CAD/CAM composite resin and ceramic posterior occlusal veneers. J. Prosthet. Dent. 2010, 104, 149–157. [Google Scholar] [CrossRef]
- Waltimo, A.; Nystrom, M.; Kononen, M. Bite force and dentofacial morphology in men with severe dental attrition. Scand. J. Dent. Res. 1994, 102, 92–96. [Google Scholar]
- Quinn, J.; Quinn, G. A practical and systematic review of Weibull statistics for reporting stregths of dental materials. Dent. Mater. 2010, 26, 135–147. [Google Scholar] [CrossRef]
- Kittl, P.; Diaz, G. Weibull’s fracture statistics, or probabilistic strength of materials: State of the art. Res. Mech. 1988, 24, 99–207. [Google Scholar]
- Huysmans, M.; Van Der Varst, P.; Peters, M.; Plasschaert, A. The Weibull distribution applied to post and core failure. Dent. Mater. 1992, 3, 283–288. [Google Scholar] [CrossRef]
- Yin, R.; Kim, Y.; Jang, Y.; Lee, J.; Lee, M.; Bae, T. Comparative evaluation of the mechanical properties of CAD/CAM dental blocks. Odontology 2019, 107, 360–367. [Google Scholar] [CrossRef]
- Al-Akhali, M.; Sad Chaar, M.; Elsayed, A.; Samran, A.; Kern, M. Fracture resistance of ceramic and polymer-based occlusal veneer restorations. J. Mech. Behav. Biomed. 2017, 74, 245–250. [Google Scholar] [CrossRef]
- Bakeman, E.M.; Rego, N.; Chaiyabutr, Y.; Kois, J.C. Influence of ceramic thickness and ceramic materials on fracture resistance of posterior partial coverage restorations. Oper. Dent. 2015, 40, 211–217. [Google Scholar] [CrossRef]
- Monteiro, J.B.; Riquieri, H.; Prochnow, C.; Guilardi, L.F.; Pereira, G.K.R.; Borges, A.L.S.; De Melo, R.M.; Valandro, L.F. Fatigue failure load of two resin-bonded zirconia-reinforced lithium silicate glass-ceramics: Effect of ceramic thickness. Dent. Mater. 2018, 34, 891–900. [Google Scholar] [CrossRef]
- Rammelsberg, P.; Eickemeyer, G.; Erdelt, K.; Pospiech, P. Fracture resistance of posterior metal-free polymer crowns. J. Prosthet. Dent. 2000, 84, 303–308. [Google Scholar] [CrossRef]
- Zhang, Y.; Chai, H.; Lee, J.J.; Lawn, B.R. Chipping resistance of graded zirconia ceramics for dental crowns. J. Dent. Res. 2012, 91, 311–315. [Google Scholar] [CrossRef]
- Wendler, M.; Belli, R.; Petschelt, A.; Mevec, D.; Harrer, W.; Lube, T.; Danzer, R.; Lohbauer, U. Chairside CAD/CAM materials. Part 2. Flexural strength testing. Dent. Mater. 2017, 33, 99–109. [Google Scholar] [CrossRef]



| Group | Material | Type | Manufacturing Data | Chemical Composition | Properties | Characteristics/ Lot nº |
|---|---|---|---|---|---|---|
| LDS group (GC) | IPS e.max CAD® | High-strength ceramic | Ivoclar Vivadent, Schaan, Liechtenstein | Crystalline phase: 70% lithium disilicate | Fracture resistance 360 MPa Elasticity modulus (95 GPa) Poisson modulus 0.25 Marginal fit 0.06 mm Acid-sensitive | U40015 U40016 MT BL4/C14 |
| ZRLS group | VITA SUPRINITY® | High-strength ceramic | VITA Zahnfabrik, Bad Zäckingen, Germany | Crystalline phase: 64% lithium silicate 15% lithium disilicate 10% zirconium dioxide Glass-ceramic matrix | Fracture resistance 420 MPa Elasticity modulus (70 GPa) Poisson modulus 0.23 Marginal fit 0.06 mm Acid-sensitive | 74740 74742 A2-HT PC-14 |
| PICN group | VITA ENAMIC® | Hybrid material: PICN | VITA Zahnfabrik, Bad Zäckingen, Germany | Glass-ceramic matrix: 86% conventional feldspathic ceramic (leucite and zirconia) Organic phase: 14% UDMA and TEGDMA | Fracture resistance 160 MPa Elasticity modulus (30 GPa) Poisson modulus 0.23 Acid-sensitive | 56171 82120 1M1-HT EMC-14 |
| RNC group | LAVA™ ULTIMATE | Hybrid material: RNC | 3M ESPE, St Paul, Minn, USA | Crystalline phase: 80% Nanoceramic (silica and zirconia) Organic matrix: 20% organic filling | Fracture resistance 250 MPa Elasticity modulus (12.77 GPa) Poisson modulus 0.30 Marginal fit 0.01 mm Acid-resistant | N429938 N429987 A3-LT/14 |
| Group | Material and Cementation Procedure | Type | Chemical Composition | Duration | Manufacturer | Lot No. |
|---|---|---|---|---|---|---|
| LDS group (CG) | 1. IPS Ceramic Etching Gel® | Acid etching for ceramic | 4.9% hydrofluoric acid | 20’ | Ivoclar Vivadent | T76221 |
| 2. IPS Ceramic Neutralizing Powder® | Neutralizing powder | Sodium carbonate 25%–50%, calcium carbonate 25%–50%. | 20’ | Ivoclar Vivadent | V47224 | |
| 3. Monobond Plus® | Silane | Adhesive monomers 4%, ethanol 96% | 60’ | Ivoclar Vivadent | X43365 | |
| 1. Excite DSC® a | Adhesive agent | Phosphonic acid acrylate, dimethacrylates, hydroxyethyl methacrylate, highly dispersed silicon dioxide, ethanol, catalysts, stabilizers, and fluoride | 20’ rub 20’ light-cure | Ivoclar Vivadent | Z33289 | |
| 2. Variolink Esthetic DC Neutral® | Dual-cure resin cement | Barium glass filling, mixture of oxide 52.2%, dimethacrylate 22%, high dispersión silica, ytterbium trifluoride 25%, initiators and stabilizers 0.8%, pigments <0.1% | 20’ light-cure each face | Ivoclar Vivadent | W95564 W95566 | |
| 3. Liquid Strip® | Glycerine | Glycerine gel | 20’ | Ivoclar Vivadent | K44713 | |
| ZRLS group / PICN group | 1. Vita Ceramics Etch® | Acid etching for ceramic | 5% hydrofluoric acid | 60’ | VITA Zahnfabrik | G32613 |
| 2. VITASIL® | Silane | 3-methacryloxypropyltrimethoxysilane, ethanol, and water | 60’ | VITA Zahnfabrik | I18532 | |
| 3. Vita A.R.T Bond® b | Adhesive agent | Bond: methacrylate 97%–99% and polyalkenoate 1%–3% Primer A: water 96%–98%, sodium fluoride <0.1%, organic substances 2%–4% Primer B: methacrylate 89%–91%, polyalkeonate 6%–8%, water 2%–4% | 20’ primer 20’ light-cure adhesive | VITA Zahnfabrik | H15866 | |
| 4. Vita DUO CEMENT® | Resin cement dual | Methyl methacrylate 28%–32%, inorganic components 63%–77% | 20’ light-cure each face | VITA Zahnfabrik | F72605 | |
| RNC group | 1. Cojet Prep® | Sandblasting | Aluminium particles, particle size: 30 μm, pressure 2.0 bars | 30’ | 3M ESPE | |
| 2. Scotchbond™ Universal Adhesive c | Universal adhesive agent | BisGMA, HEMA, decamethylene dimethacrylate, ethanol, water, silane-treated silica, 2-propenoic acid, methacrylated phosphoric acid, copolymer of acrylic and itaconic acid, ethyl-4-dimethylaminobenzoat, camphorquinone, (dimethylamino) ethyl methacrylate, methyl ethyl ketone | 20’ rub 20’ light-cure | 3M ESPE | 4636134 | |
| 3. RelyX ™ Ultimate | Dual-cure resin cement | Base paste: silane-treated glass powder, 2-propenoic acid, 2-methyl, reaction products with 2-hydroxy-1,3-propanedyl dimethacrylate and phosphorus oxide, TEGDMA, silane-treated silica, oxide glass chemicals, sodium persulfate, tertbutyl peroxy-3,5,5- trimethylhexanoate, copper acetate monohydrate Catalyst paste: silane-treated glass powder, substituted dimethacrylate, 1,12-dodecane dimethacrylate, silane-treated silica, 1-benzyl-5-phentyl-barbic-acid, calcium salt, sodium p-toluenesulfinate, 2-propenic acid, 2-methyl-, di-2,1-ethanediyl ester, calcium hydroxide, titanium dioxide | 20’ light-cure each face | 3M ESPE | 4751433 | |
| Tooth | 1. Scotchbond™ Universal Etchand | Acid etching agent Tooth | 3 mL 37.5% orthophosphoric acid | 15’ dentin 30’ enamel | 3M ESPE | 4638524 |
| 2. Adhesive recommended for each material a,b,c | ||||||
| Groups | ||||
|---|---|---|---|---|
| LDS Group (Control Group) | ZRLS Group | PICN Group | RNC Group | |
| N | 20 | 20 | 20 | 20 |
| σ (MPa) | 4588.6 | 4476.3 | 4014.2 | 3110.0 |
| σf ± SD | 1843.5 | 762.6 | 681.1 | 169.0 |
| 95% CI of mean | 3725.8–5451.4 | 4119.4–4833.2 | 3695.5–4333.0 | 3030.9–3189.1 |
| Minimum (MPa) | 1784.1 | 2919.4 | 2775.7 | 2864.1 |
| Maximum (MPa) | 7277.9 | 5913.2 | 5344.3 | 3537.5 |
| Median (MPa) | 4508.2 | 4659.4 | 3925.1 | 3058.3 |
| m | 2.36 | 6.32 | 6.11 | 16 |
| R2 | 0.97 | 0.942 | 0.971 | 0.636 |
| Fracture Type | LDS Group (CG) | ZRLS Group | PICN Group | RNC Group | |
|---|---|---|---|---|---|
| Adhesive | 0 | 0 | 0 | 0 | |
| Cohesive | Indentation | 0 | 0 | 16 (80%) | 15 (75%) |
| Radial | 19 (95%) | 20 (100%) | 16 (80%) | 15 (75%) | |
| Complete | 1(5%) | 0 | 4 (20%) | 5 (25%) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Engra, G.; Fernandez-Estevan, L.; Casas-Terrón, J.; Fons-Font, A.; Castelo-Baz, P.; Agustín-Panadero, R.; Román-Rodriguez, J.L. Fracture Resistance of New Metal-Free Materials Used for CAD-CAM Fabrication of Partial Posterior Restorations. Medicina 2020, 56, 132. https://doi.org/10.3390/medicina56030132
García-Engra G, Fernandez-Estevan L, Casas-Terrón J, Fons-Font A, Castelo-Baz P, Agustín-Panadero R, Román-Rodriguez JL. Fracture Resistance of New Metal-Free Materials Used for CAD-CAM Fabrication of Partial Posterior Restorations. Medicina. 2020; 56(3):132. https://doi.org/10.3390/medicina56030132
Chicago/Turabian StyleGarcía-Engra, Georgina, Lucia Fernandez-Estevan, Javier Casas-Terrón, Antonio Fons-Font, Pablo Castelo-Baz, Rubén Agustín-Panadero, and Juan Luis Román-Rodriguez. 2020. "Fracture Resistance of New Metal-Free Materials Used for CAD-CAM Fabrication of Partial Posterior Restorations" Medicina 56, no. 3: 132. https://doi.org/10.3390/medicina56030132
APA StyleGarcía-Engra, G., Fernandez-Estevan, L., Casas-Terrón, J., Fons-Font, A., Castelo-Baz, P., Agustín-Panadero, R., & Román-Rodriguez, J. L. (2020). Fracture Resistance of New Metal-Free Materials Used for CAD-CAM Fabrication of Partial Posterior Restorations. Medicina, 56(3), 132. https://doi.org/10.3390/medicina56030132






