Immunohistochemical Femoral Nerve Study Following Bisphosphonates Administration
Abstract
:1. Introduction
1.1. Femoral Nerve
1.1.1. Anatomy and Physiology of the Femoral Nerve
1.1.2. Femoral Nerve Damage
1.2. Bisphosphonates
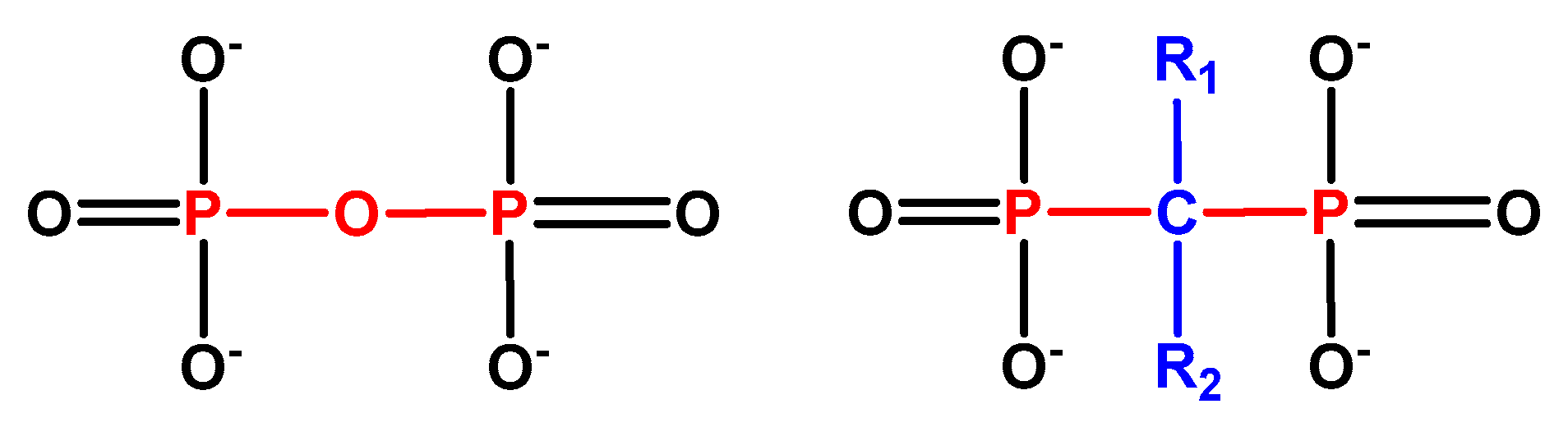
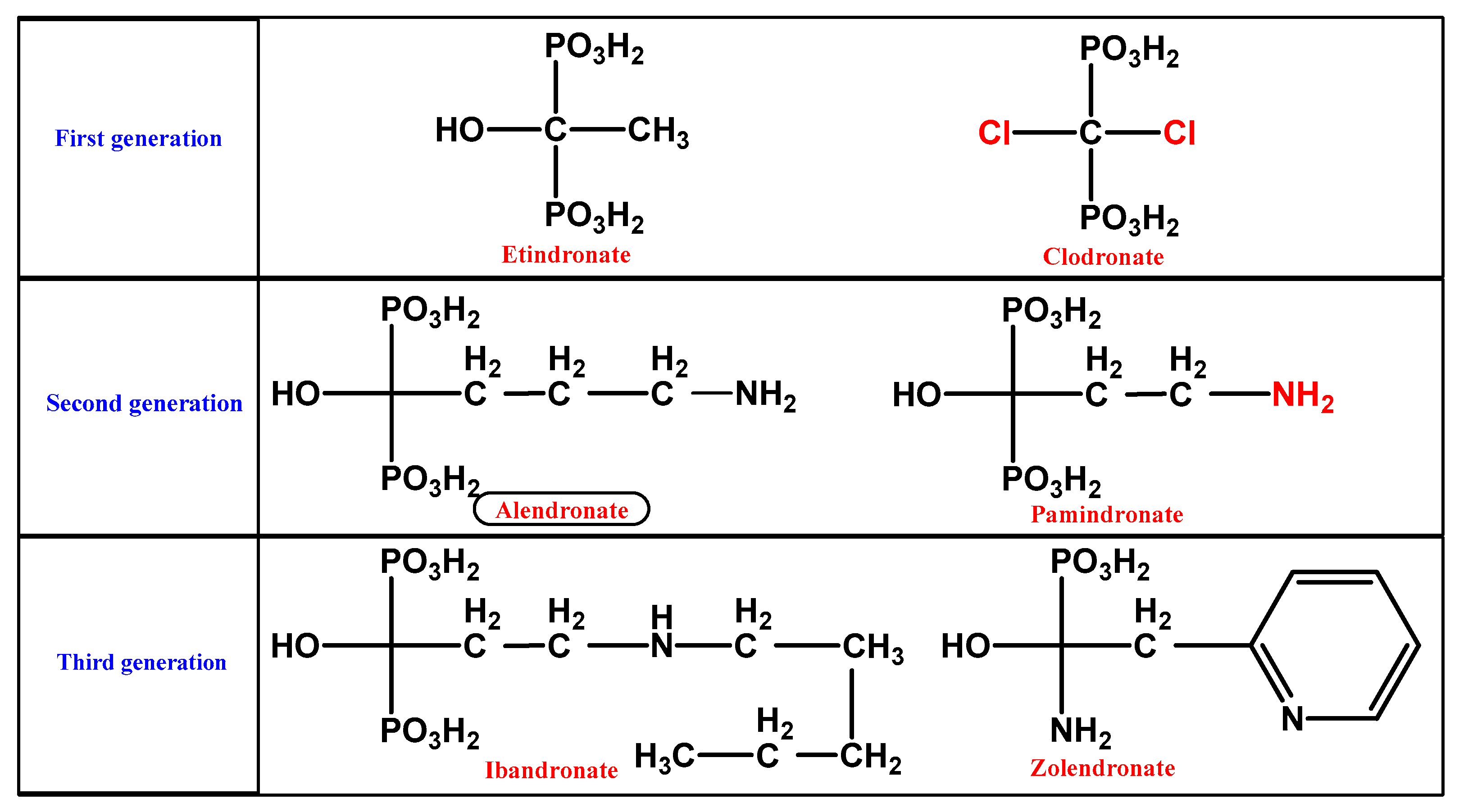
1.2.1. BP Chemistry
1.2.2. BP Pharmacodynamics
1.2.3. BP Distribution
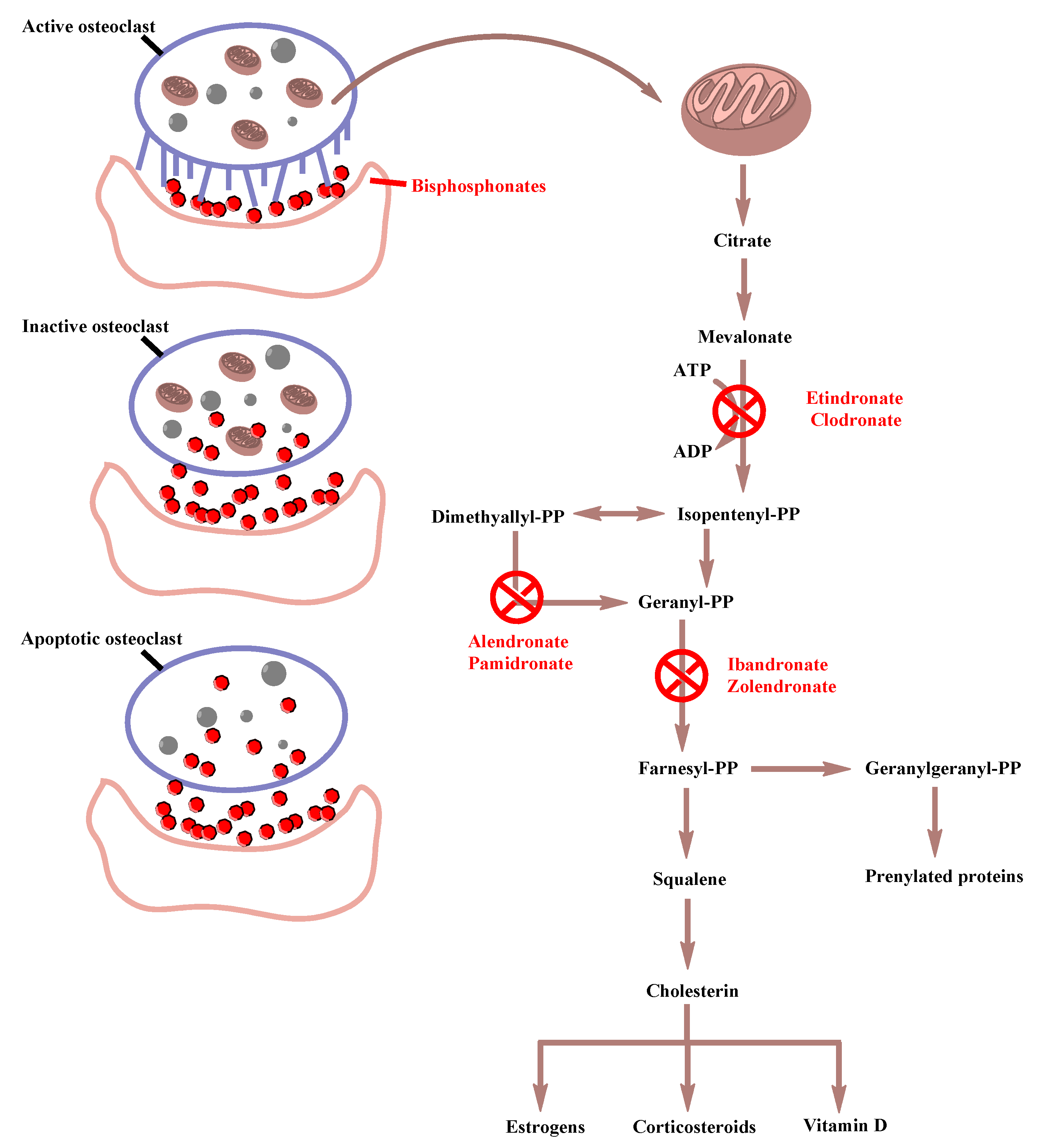
1.2.4. BP Mechanism of Action
1.2.5. BP Adverse and Side Effects
1.2.5.1. Short-Term Side Effects
1.2.5.2. Long-Term Side Effects
Osteonecrosis of the Jaw (ONJ)
Atrial Fibrillation
Bone Turnover Suppression and Atypical Femoral Fractures (AFFs)
2. Materials and Methods
2.1. Animals
2.2. Dose
2.3. Hematoxylin-Eosin staining
2.4. Immunohistochemistry
2.5. Immunohistochemical Markers
2.5.1. NeuN
2.5.2. Sox10
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sven, O. Medication-Related Osteonecrosis of the Jaws, 1st ed.; Sven, O., Ed.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Ebetino, F.H.; Hogan, A.M.; Sun, S.; Tsoumpra, M.K.; Duan, X.; Triffitt, J.T.; Kwaasi, A.A.; Dunford, J.E.; Barnett, B.L.; Oppermann, U.; et al. The relationship between the chemistry and biological activity of the bisphosphonates. Bone 2011, 49, 20–33. [Google Scholar] [CrossRef]
- Zameer, S.; Najmi, A.K.; Vahora, D.; Akhtar, M. Bisphosphonates: Future perspective for neurological disorders. Pharmacol. Rep. 2018, 70, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cheong, Y.K.; Wang, H.; Ren, G.; Yang, Z. Neuroprotective effects of Etindronate and 2,3,3-Triphosphonate against Glutamate-induced toxicity in PC12 cells. Neurochem. Res. 2016, 41, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Mhaskar, R.; Djulbegovic, B. Bisphosphonates for Patients Diagnosed With Multiple Myeloma. JAMA 2018, 320, 1483–1484. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.K.; Zhang, Y.M.; Qin, S.Q.; Wang, X.; Ma, T.; Guo, J.B.; Zhu, C.; Luo, Z.J. Effects of alendronate for treatment of glucocorticoid-induced osteoporosis: A meta-analysis of randomized controlled trials. Medicine 2018, 97, e12691. [Google Scholar] [CrossRef]
- Inoue, Y.; Mitsunaga, K.; Yamamoto, T.; Chiba, K.; Yamaide, F.; Nakano, T.; Morita, Y.; Yamaide, A.; Suzuki, S.; Arima, T.; et al. Early use of alendronate as a protective factor against the development of glucocorticoid-induced bone loss in childhood-onset rheumatic diseases: A cross-sectional study. Pediatr. Rheumatol. 2018, 16, 36. [Google Scholar] [CrossRef]
- Deng, J.; Feng, Z.; Li, Y.; Pan, T.; Li, Q.; Zhao, C. Efficacy and safety of recombinant human parathyroid hormone (1-34) are similar to those of alendronate in the treatment of postmenopausal osteoporosis. Medicine 2018, 97, e13341. [Google Scholar] [CrossRef]
- Gustafson, K.J.; Pinault, G.C.; Neville, J.J.; Syed, I.; Davis, J.A., Jr.; Jean-Claude, J.; Triolo, R.J. Fascicular anatomy of human femoral nerve: Implications for neural prostheses using nerve cuff electrodes. J. Rehabil. Res. Dev. 2009, 46, 973–984. [Google Scholar] [CrossRef]
- Hung, C.Y.; Hsiao, M.Y.; Ozcakar, L.; Chang, K.V.; Wu, C.H.; Wang, T.G.; Chen, W.S. Sonographic tracking of the lower limb peripheral nerves: A pictorial essay and video demonstration. Am. J. Phys. Med. Rehabil. 2016, 95, 698–708. [Google Scholar] [CrossRef]
- Chang, K.V.; Mezian, K.; Nanka, O.; Wu, W.T.; Lou, Y.M.; Wang, J.C.; Martinoli, C.; Ozcakar, L. Ultrasound imaging for the Cutaneous nerves of the extremities and relevant entrapment syndromes: From anatomy to clinical implications. J. Clin. Med. 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Schiefer, M.A.; Polasek, K.H.; Triolo, R.J.; Pinault, G.C.; Tyler, D.J. Selective stimulation of the human femoral nerve with a flat interface nerve electrode. J. Neural. Eng. 2010, 7, 26006. [Google Scholar] [CrossRef] [PubMed]
- Lalkhen, A.G. Perioperative Peripheral Nerve Injuries. Contin. Educ. Anaesth. Crit. Care Pain 2012, 12, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Ohishi, T.; Matsuyama, Y. Minodronate for the treatment of osteoporosis. Ther. Clin. Risk Manag. 2018, 14, 729–739. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, E.M.; Papamitsou, T.; Sioga, A.; Koimtzis, G.; Neloum, E. Ultrastructural alterations of the inferior alveolar nerve in wistar rats after alendronate administration per os: Hypothesis for the generation of the “numb chin syndrome”. Histol. Histopathol. 2015, 2. [Google Scholar] [CrossRef] [Green Version]
- Jachewicz, T.K.; Jakiel, J. Bone necrosis associated with the use of bisphosphonates: Case report. Dent. Med Probl. 2017, 54, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Diniz, R.F.; Maciel, F.J.L.; Maciel, A.A.B.; Araujo, M.A.R. Osteonecrosis of the Jaw associated with the Use of Bisphosphonates. World J. Dent. 2015, 6, 116–122. [Google Scholar]
- Pavone, V.; Testa, G.; Giardina, S.M.C.; Vescio, A.; Restivo, D.A.; Sessa, G. Pharmacological Therapy of Osteoporosis: A Systematic Current Review of Literature. Front. Pharmacol. 2017, 8, 803. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Di Bella, G.; Belvedere, M.; Barbagallo, M. Physiology of the aging bone and mechanisms of action of bisphosphonates. Biogerontology 2011, 12, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Matsumoto, T. Recent advances in the management of osteoporosis. F1000Research 2017, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennel, K.A.; Drake, M.T. Adverse effects of bisphosphonates: Implications for osteoporosis management. Mayo Clin. Proc. 2009, 84, 632–637. [Google Scholar] [CrossRef] [Green Version]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of Action and Role in Clinical Practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharwadkar, N.; Mayne, B.; Lawrence, J.E.; Khanduja, V. Bisphosphonates and atypical subtrochanteric fractures of the femur. Bone Jt. Res. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Saita, Y.; Ishijima, M.; Kaneko, K. Atypical femoral fractures and bisphosphonate use: Current evidence and clinical implications. Ther Adv. Chronic Dis. 2015, 6, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehman, R.A., Jr.; Kuklo, T.R.; Freedman, B.A.; Cowart, J.R.; Mense, M.G.; Riew, K.D. The effect of alendronate sodium on spinal fusion: A rabbit model. Spine J. 2004, 4, 36–43. [Google Scholar] [CrossRef]
- Gusel’nikova, V.V.; Korzhevskiy, D.E. NeuN as a Neuronal Nuclear Antigen and Neuron Differentiation Marker. Acta Nat. 2015, 7, 42–47. [Google Scholar] [CrossRef]
- Srinivasan, R.; Sun, G.; Keles, S.; Jones, E.A.; Jang, S.W.; Krueger, C.; Moran, J.J.; Svaren, J. Genome-wide analysis of EGR2/SOX10 binding in myelinating peripheral nerve. Nucleic Acids Res. 2012, 40, 6449–6460. [Google Scholar] [CrossRef] [Green Version]
- Hung, H.A.; Sun, G.; Keles, S.; Svaren, J. Dynamic regulation of Schwann cell enhancers after peripheral nerve injury. J. Biol. Chem. 2015, 290, 6937–6950. [Google Scholar] [CrossRef] [Green Version]
- Hornig, J.; Fröb, F.; Vogl, M.R.; Hermans-Borgmeyer, I.; Tamm, E.R.; Wegner, M. The transcription factors Sox10 and Myrf define an essential regulatory network module in differentiating oligodendrocytes. PLoS Genet. 2013, 9, e1003907. [Google Scholar] [CrossRef] [Green Version]
- Miettinen, M.; McCue, P.A.; Sarlomo-Rikala, M.; Biernat, W.; Czapiewski, P.; Kopczynski, J.; Thompson, L.D.; Lasota, J.; Wang, Z.; Fetsch, J.F. Sox10—A marker for not only schwannian and melanocytic neoplasms but also myoepithelial cell tumors of soft tissue: A systematic analysis of 5134 tumors. Am. J. Surg. Pathol. 2015, 39, 826–835. [Google Scholar] [CrossRef] [Green Version]
- Turnescu, T.; Arter, J.; Reiprich, S.; Tamm, E.R.; Waisman, A.; Wegner, M. Sox8 and Sox10 jointly maintain myelin gene expression in oligodendrocytes. Glia 2018, 66, 279–294. [Google Scholar] [CrossRef]
- Van Raamsdonk, C.D.; Deo, M. Links between Schwann cells and melanocytes in development and disease. Pigment Cell Melanoma Res. 2013, 26, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, A.; Stykel, M.G.; Touahri, Y.; Stratton, J.A.; Biernaskie, J.; Biernaskie, J.; Schuurmans, C. Temporal Analysis of Gene Expression in the Murine Schwann Cell Lineage and the Acutely Injured Postnatal Nerve. PLoS ONE 2016, 11, e0153256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nonaka, D.; Chiriboga, L.; Rubin, B.P. Sox10: A pan-schwannian and melanocytic marker. Am. J. Surg. Pathol. 2008, 33, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Salzer, J.L. Schwann cell myelination. Cold Spring Harb. Perspect. Biol. 2015, 7, a020529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, T.D. Charcot-Marie-Tooth (CMT) Hereditary Neuropathy Overview. In GeneReviews; University of Washington: Seattle Washington, DC, USA, 2019; Volume 1. [Google Scholar]
- Cassarino, D.S.; Su, A.; Robbins, B.A.; Altree-tacha, D.; Ra, S. SOX10 immunohistochemistry in sweat ductal/glandular neoplasms. J. Cutan. Pathol. 2017, 44, 544–547. [Google Scholar] [CrossRef]
- Papamitsou, T.; Papakoulas, A.; Papaliagkas, V.; Karachrysafi, S.; Dietrich, E.M.; Sioga, A. Histologic evaluation of femoral nerve demyelinating and axonal neuropathy in Wistar rats due to alendronate intake: A randomized study. J. Biol. Res. 2020, 2. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Tang, Z.; Wang, D.; Buckley, M.J. Changes in the inferior alveolar nerve after mandibular lengthening with different rates of distraction. J. Oral Maxillofac. Surg. 2001, 59, 1041–1045. [Google Scholar] [CrossRef]
- Fercakova, A.; Orendacova, J.; Marsala, J.; Marossy, A. Changes in spinal ganglia satellite and Schwann cells after aortic occlusion. J. Hirnforsch. 1983, 24, 627–632. [Google Scholar]
- Landesberg, R.; Cozin, M.; Cremers, S.; Woo, V.; Kousteni, S.; Sinha, S.; Garrett-Sinha, L.; Raghavan, S. Inhibition of oral mucosal cell wound healing by bisphosphonates. J. Oral Maxillofac. Surg. 2008, 66, 839–847. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yi, Q.; Liu, G.; Shen, X.; Xuan, L.; Tian, Y. Cerebral white matter injury and damage to myelin sheath following whole-brain ischemia. Brain Res. 2013, 7. [Google Scholar] [CrossRef]
- Iida, H.; Schmeichel, A.M.; Wang, Y.; Schmelzer, J.D.; Low, P.A. Schwann cell is a target in ischemia-reperfusion injury to peripheral nerve. Muscle Nerve 2004, 30, 761–766. [Google Scholar] [CrossRef]
- Sladky, J.T.; Tschoepe, R.L.; Greenberg, J.H.; Brown, M.J. Peripheral neuropathy after chronic endoneurial ischemia. Ann. Neurol. 1991, 29, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Slater, B.J.; Vilson, F.L.; Guo, Y.; Weinreich, D.; Hwang, S.; Bernstein, S.L. Optic nerve inflammation and demyelination in a rodent model of nonarteritic anterior ischemic optic neuropathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7952–7961. [Google Scholar] [CrossRef] [PubMed]
- Doyle, S.; Hansen, D.B.; Vella, J.; Bond, P.; Harper, G.; Zammit, C.; Valentino, M.; Fern, R. Vesicular glutamate release from central axons contributes to myelin damage. Nat. Commun. 2018, 9, 1032. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakousis, V.A.; Liouliou, D.; Loula, A.; Kagianni, N.; Dietrich, E.-M.; Meditskou, S.; Sioga, A.; Papamitsou, T. Immunohistochemical Femoral Nerve Study Following Bisphosphonates Administration. Medicina 2020, 56, 140. https://doi.org/10.3390/medicina56030140
Karakousis VA, Liouliou D, Loula A, Kagianni N, Dietrich E-M, Meditskou S, Sioga A, Papamitsou T. Immunohistochemical Femoral Nerve Study Following Bisphosphonates Administration. Medicina. 2020; 56(3):140. https://doi.org/10.3390/medicina56030140
Chicago/Turabian StyleKarakousis, Vasileios Alexandros, Danai Liouliou, Aikaterini Loula, Nikoleta Kagianni, Eva-Maria Dietrich, Soultana Meditskou, Antonia Sioga, and Theodora Papamitsou. 2020. "Immunohistochemical Femoral Nerve Study Following Bisphosphonates Administration" Medicina 56, no. 3: 140. https://doi.org/10.3390/medicina56030140




