Circulating Irisin in Healthy Adults: Changes after Acute Exercise, Correlation with Body Composition, and Energy Expenditure Parameters in Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Body Composition and Metabolism Measurements
2.3. Irisin Measurement
2.4. Statistical Analysis
3. Results
4. Discussion
Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1α-dependent myokine that drives browning of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Lee, P.; Linderman, J.D.; Smith, S.; Brychta, R.J.; Wang, J.; Idelson, C.; Perron, R.M.; Werner, C.D.; Phan, G.Q.; Kammula, U.S.; et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014, 19, 302–309. [Google Scholar] [CrossRef]
- Vaughan, R.A.; Gannon, N.P.; Barberena, M.A.; Garcia-Smith, R.; Bisoffi, M.; Mermier, C.M.; Conn, C.A.; Trujillo, K.A. Characterization of the metabolic effects of irisin on skeletal muscle in vitro. Diabetes Obes. Metab. 2014, 16, 711–718. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Duan, Y.; Hu, C.-A.A.; Tang, Y.; Yin, Y. Myokines and adipokines: Involvement in the crosstalk between skeletal muscle and adipose tissue. Cytokine Growth Factor Rev. 2017, 33, 73–82. [Google Scholar] [CrossRef]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.-J.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell 2018, 175, 1756–1768.e1717. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Guo, P.; Li, X.; Ke, J.; Wang, Y.; Wu, H. Neuroprotective effects of irisin against cerebral ischemia/ reperfusion injury via Notch signaling pathway. Biomed. Pharmacother. 2019, 120, 109452. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.T.; Wang, J.; Yano, N.; Zhang, L.X.; Wang, H.; Zhang, S.; Qin, G.; Dubielecka, P.M.; Zhuang, S.; Liu, P.Y.; et al. Irisin promotes cardiac progenitor cell-induced myocardial repair and functional improvement in infarcted heart. J. Cell. Physiol. 2019, 234, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Canivet, C.M.; Bonnafous, S.; Rousseau, D.; Leclere, P.S.; Lacas-Gervais, S.; Patouraux, S.; Sans, A.; Luci, C.; Bailly-Maitre, B.; Iannelli, A.; et al. Hepatic FNDC5 is a potential local protective factor against Non-Alcoholic Fatty Liver. Biochim. Et Biophys. Acta (Bba) Mol. Basis Dis. 2020, 1866, 165705. [Google Scholar] [CrossRef]
- Chang, C.L.; Huang, S.Y.; Hsu, Y.C.; Chin, T.H.; Soong, Y.K. The serum level of irisin, but not asprosin, is abnormal in polycystic ovary syndrome patients. Sci. Rep. 2019, 9, 6447. [Google Scholar] [CrossRef] [PubMed]
- Natalicchio, A.; Marrano, N.; Biondi, G.; Spagnuolo, R.; Labarbuta, R.; Porreca, I.; Cignarelli, A.; Bugliani, M.; Marchetti, P.; Perrini, S.; et al. The Myokine Irisin Is Released in Response to Saturated Fatty Acids and Promotes Pancreatic β-Cell Survival and Insulin Secretion. Diabetes 2017, 66, 2849–2856. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Di Comite, M.; Mori, G.; et al. The myokine irisin increases cortical bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef] [PubMed]
- Raschke, S.; Elsen, M.; Gassenhuber, H.; Sommerfeld, M.; Schwahn, U.; Brockmann, B.; Jung, R.; Wisloff, U.; Tjonna, A.E.; Raastad, T.; et al. Evidence against a beneficial effect of irisin in humans. PLoS ONE 2013, 8, e73680. [Google Scholar] [CrossRef] [PubMed]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Polyzos, S.A.; Saridakis, Z.G.; Kynigopoulos, G.; Skouvaklidou, E.C.; Molyvas, D.; Vasiloglou, M.F.; Apostolou, A.; Karagiozoglou-Lampoudi, T.; Siopi, A.; et al. Circulating irisin in healthy, young individuals: Day-night rhythm, effects of food intake and exercise, and associations with gender, physical activity, diet, and body composition. J. Clin. Endocrinol. Metab. 2014, 99, 3247–3255. [Google Scholar] [CrossRef]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef]
- Hecksteden, A.; Wegmann, M.; Steffen, A.; Kraushaar, J.; Morsch, A.; Ruppenthal, S.; Kaestner, L.; Meyer, T. Irisin and exercise training in humans - results from a randomized controlled training trial. BMC Med. 2013, 11, 235. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Ijichi, T.; Goto, K. Effect of sprint training on resting serum irisin concentration - Sprint training once daily vs. twice every other day. Metabolism 2016, 65, 492–495. [Google Scholar] [CrossRef]
- Fox, J.; Rioux, B.V.; Goulet, E.D.B.; Johanssen, N.M.; Swift, D.L.; Bouchard, D.R.; Loewen, H.; Senechal, M. Effect of an acute exercise bout on immediate post-exercise irisin concentration in adults: A meta-analysis. Scand. J. Med. Sci. Sports 2018, 28, 16–28. [Google Scholar] [CrossRef]
- Pekkala, S.; Wiklund, P.K.; Hulmi, J.J.; Ahtiainen, J.P.; Horttanainen, M.; Pollanen, E.; Makela, K.A.; Kainulainen, H.; Hakkinen, K.; Nyman, K.; et al. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J. Physiol. 2013, 591, 5393–5400. [Google Scholar] [CrossRef]
- Nygaard, H.; Slettalokken, G.; Vegge, G.; Hollan, I.; Whist, J.E.; Strand, T.; Ronnestad, B.R.; Ellefsen, S. Irisin in blood increases transiently after single sessions of intense endurance exercise and heavy strength training. PLoS ONE 2015, 10, e0121367. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Ando, D.; Goto, K.; Kiuchi, M.; Yamakita, M.; Koyama, K. High-intensity exercise causes greater irisin response compared with low-intensity exercise under similar energy consumption. Tohoku J. Exp. Med. 2014, 233, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Daskalopoulou, S.S.; Cooke, A.B.; Gomez, Y.H.; Mutter, A.F.; Filippaios, A.; Mesfum, E.T.; Mantzoros, C.S. Plasma irisin levels progressively increase in response to increasing exercise workloads in young, healthy, active subjects. Eur. J. Endocrinol. 2014, 171, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Bosnyak, E.; Treff, G.; Steinacker, J.M.; Niess, A.M.; Kruger, K.; Mooren, F.C.; Zugel, M.; Schumann, U. Acute exercise-induced irisin release in healthy adults: Associations with training status and exercise mode. Eur. J. Sport Sci. 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Zaichenko, L.; Brinkoetter, M.; Thakkar, B.; Sahin-Efe, A.; Joung, K.E.; Tsoukas, M.A.; Geladari, E.V.; Huh, J.Y.; Dincer, F.; et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 4899–4907. [Google Scholar] [CrossRef]
- Stengel, A.; Hofmann, T.; Goebel-Stengel, M.; Elbelt, U.; Kobelt, P.; Klapp, B.F. Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity--correlation with body mass index. Peptides 2013, 39, 125–130. [Google Scholar] [CrossRef]
- Pardo, M.; Crujeiras, A.B.; Amil, M.; Aguera, Z.; Jimenez-Murcia, S.; Banos, R.; Botella, C.; de la Torre, R.; Estivill, X.; Fagundo, A.B.; et al. Association of irisin with fat mass, resting energy expenditure, and daily activity in conditions of extreme body mass index. Int. J. Endocrinol. 2014, 2014, 857270. [Google Scholar] [CrossRef]
- Mehrabian, S.; Taheri, E.; Karkhaneh, M.; Qorbani, M.; Hosseini, S. Association of circulating irisin levels with normal weight obesity, glycemic and lipid profile. J. Diabetes Metab. Disord. 2016, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Ortega, F.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernandez-Real, J.M. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778. [Google Scholar] [CrossRef] [PubMed]
- Swick, A.G.; Orena, S.; O’Connor, A. Irisin levels correlate with energy expenditure in a subgroup of humans with energy expenditure greater than predicted by fat free mass. Metabolism 2013, 62, 1070–1073. [Google Scholar] [CrossRef]
- Compher, C.; Frankenfield, D.; Keim, N.; Roth-Yousey, L. Best Practice Methods to Apply to Measurement of Resting Metabolic Rate in Adults: A Systematic Review. J. Am. Diet. Assoc. 2006, 106, 881–903. [Google Scholar] [CrossRef]
- Perakakis, N.; Triantafyllou, G.A.; Fernandez-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef]
- Biniaminov, N.; Bandt, S.; Roth, A.; Haertel, S.; Neumann, R.; Bub, A. Irisin, physical activity and fitness status in healthy humans: No association under resting conditions in a cross-sectional study. PLoS ONE 2018, 13, e0189254. [Google Scholar] [CrossRef] [PubMed]
- Winn, N.C.; Grunewald, Z.I.; Liu, Y.; Heden, T.D.; Nyhoff, L.M.; Kanaley, J.A. Plasma Irisin Modestly Increases during Moderate and High-Intensity Afternoon Exercise in Obese Females. PLoS ONE 2017, 12, e0170690. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Shi, L.; Peng, N.; Zhang, Q.; Li, H. Higher Baseline Serum Irisin Decreases Risk for Body Mass Index Increment in Chinese Populations: A 3.2-Year Cohort Study. Diabetes 2019, 10, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Szumilewicz, A.; Worska, A.; Piernicka, M.; Kuchta, A.; Jastrzębski, Z.; Radzimiński, Ł.; Kozłowska, M.; Micielska, K.; Ziemann, E. Acute Postexercise Change in Circulating Irisin Is Related to More Favorable Lipid Profile in Pregnant Women Attending a Structured Exercise Program and to Less Favorable Lipid Profile in Controls: An Experimental Study with Two Groups. Int. J. Endocrinol. 2019, 2019, 1932503. [Google Scholar] [CrossRef] [PubMed]
- Blizzard LeBlanc, D.R.; Rioux, B.V.; Pelech, C.; Moffatt, T.L.; Kimber, D.E.; Duhamel, T.A.; Dolinsky, V.W.; McGavock, J.M.; Sénéchal, M. Exercise-induced irisin release as a determinant of the metabolic response to exercise training in obese youth: The EXIT trial. Physiol. Rep. 2017, 5, e13539. [Google Scholar] [CrossRef]
- Hwang, Y.C.; Jeon, W.S.; Park, C.Y.; Youn, B.S. The ratio of skeletal muscle mass to visceral fat area is a main determinant linking circulating irisin to metabolic phenotype. Cardiovasc. Diabetol. 2016, 15, 9. [Google Scholar] [CrossRef]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Belen Crujeiras, A.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef]
- Hew-Butler, T.; Landis-Piwowar, K.; Byrd, G.; Seimer, M.; Seigneurie, N.; Byrd, B.; Muzik, O. Plasma irisin in runners and nonrunners: No favorable metabolic associations in humans. Physiol. Rep. 2015, 3. [Google Scholar] [CrossRef]
- Arhire, L.I.; Mihalache, L.; Covasa, M. Irisin: A Hope in Understanding and Managing Obesity and Metabolic Syndrome. Front. Endocrinol. 2019, 10, 524. [Google Scholar] [CrossRef]
- Kerstholt, N.; Ewert, R.; Nauck, M.; Spielhagen, T.; Bollmann, T.; Stubbe, B.; Felix, S.B.; Wallaschofski, H.; Gläser, S.; Friedrich, N. Association of circulating irisin and cardiopulmonary exercise capacity in healthy volunteers: Results of the Study of Health in Pomerania. BMC Pulm. Med. 2015, 15, 41. [Google Scholar] [CrossRef] [PubMed]

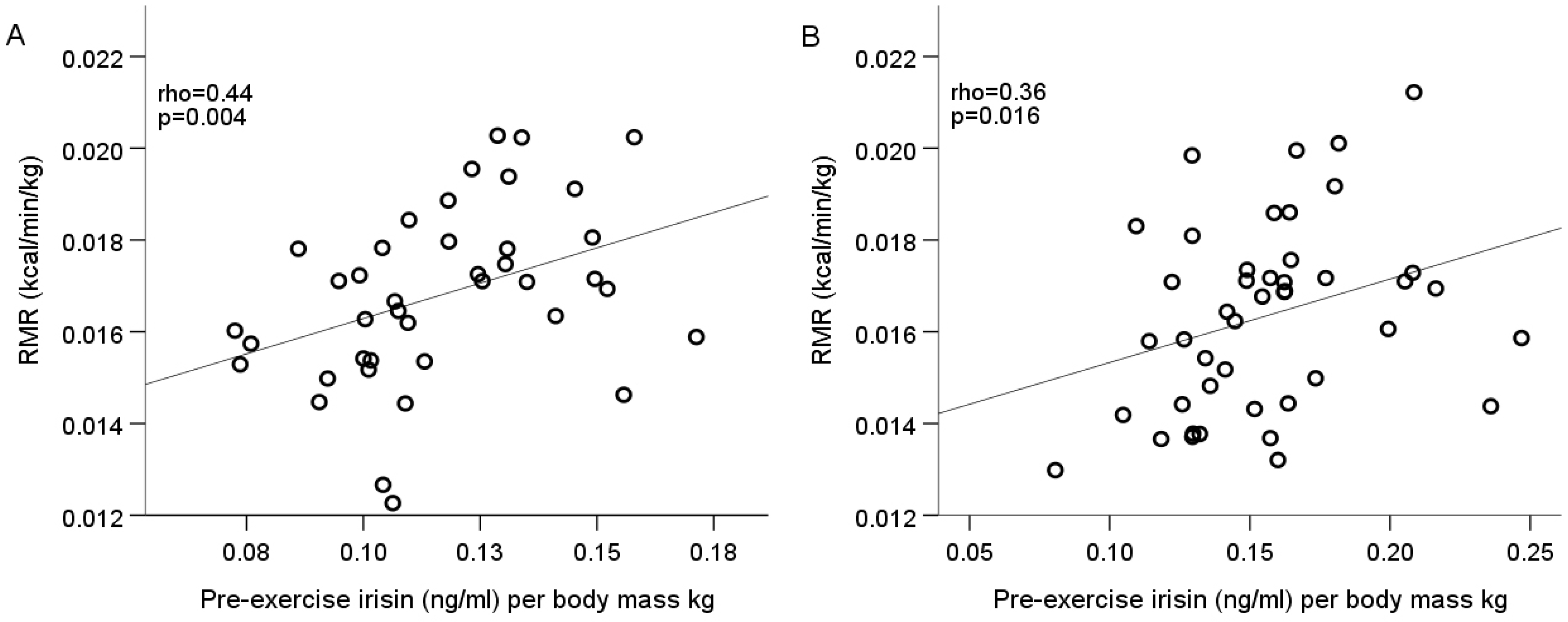
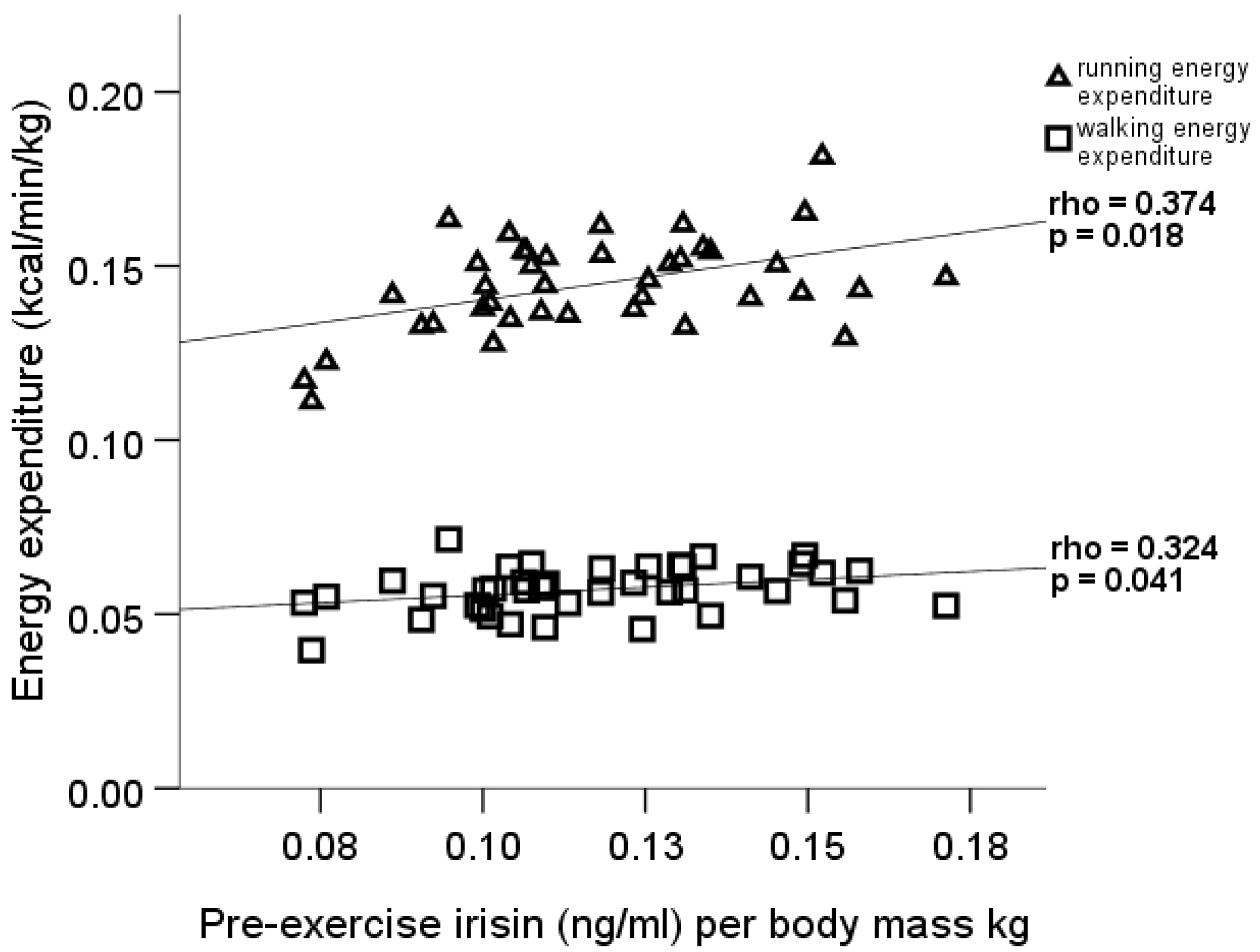

| Variable (units) | Male Mean ± SD (min–max) n = 40 | Female Mean ± SD (min–max) n = 44 |
|---|---|---|
| Age (years) | 31.2 ± 5.3 (21–46) | 29.9 ± 6.4 (21–49) |
| Height (cm) | 184 ± 7 (170–198) | 169 ± 5 (158–188) |
| Body mass (kg) | 81.5 ± 9.7 (63.7–99.4) | 64.3 ± 7.6 (50.1–79.0) |
| BMI (kg/m2) | 24.2 ± 2.5 (19.6–31.9) | 22.3 ± 2.7 (17.6–29.7) |
| Total fat tissue (kg) | 12.8 ± 5.2 (3.5–24.3) | 15.6 ± 4.9 (8.5–27.9) |
| Body fat (% from body mass) | 15.3 ± 5.1 (5.2–26.4) | 23.9 ± 5.3 (15.0–35.7) |
| Visceral fat (rating) 1 | 3.0 (2.3–6.0) | 2.0 (1.0–3.0) |
| Lean body mass (kg) | 68.8 ± 6.7 (53.9–83.0) | 48.7 ± 4.4 (39.9–60.9) |
| Total muscle tissue (kg) | 65.3 ± 6.4 (51.2–79.0) | 46.2 ± 4.2 (37.8–57.85) |
| Variable (units) | Male Mean ± SD n = 40 | Female Mean ± SD n = 44 | p-Value 1 |
|---|---|---|---|
| Pre-exercise irisin (ng/mL) | 9.5 ± 1.9 | 9.9 ± 1.9 | 0.400 |
| Post-exercise irisin (ng/mL) | 9.6 ± 2.1 | 9.7 ± 2.0 | 0.943 |
| Pre-exercise irisin per body mass kg (ng/mL) | 0.12 ± 0.02 | 0.16 ± 0.03 | <0.001 |
| Post-exercise irisin per body mass kg (ng/mL) | 0.12 ± 0.03 | 0.15 ± 0.03 | <0.001 |
| Variable (units) | Male Pre-Exercise Irisin (ng/mL) Per Body Mass kg | Female Pre-Exercise Irisin (ng/mL) Per Body Mass kg |
|---|---|---|
| LBMI (kg/m2) | −0.15 | −0.59 ** |
| Lean tissue (% from body mass) | 0.41 * | 0.28 |
| Visceral fat (rating) | −0.52 ** | −0.26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagzdina, R.; Rumaka, M.; Gersone, G.; Tretjakovs, P. Circulating Irisin in Healthy Adults: Changes after Acute Exercise, Correlation with Body Composition, and Energy Expenditure Parameters in Cross-Sectional Study. Medicina 2020, 56, 274. https://doi.org/10.3390/medicina56060274
Lagzdina R, Rumaka M, Gersone G, Tretjakovs P. Circulating Irisin in Healthy Adults: Changes after Acute Exercise, Correlation with Body Composition, and Energy Expenditure Parameters in Cross-Sectional Study. Medicina. 2020; 56(6):274. https://doi.org/10.3390/medicina56060274
Chicago/Turabian StyleLagzdina, Rudite, Maija Rumaka, Gita Gersone, and Peteris Tretjakovs. 2020. "Circulating Irisin in Healthy Adults: Changes after Acute Exercise, Correlation with Body Composition, and Energy Expenditure Parameters in Cross-Sectional Study" Medicina 56, no. 6: 274. https://doi.org/10.3390/medicina56060274
APA StyleLagzdina, R., Rumaka, M., Gersone, G., & Tretjakovs, P. (2020). Circulating Irisin in Healthy Adults: Changes after Acute Exercise, Correlation with Body Composition, and Energy Expenditure Parameters in Cross-Sectional Study. Medicina, 56(6), 274. https://doi.org/10.3390/medicina56060274






