Evaluation of Surgical Treatment of Oroantral Fistulae in Smokers Versus Non-Smokers
Abstract
1. Introduction
2. Materials and Methods
- OAFs secondary to excision of pathology, other than odontogenic cyst or granuloma
- OAFs s/p sequestrectomy in patients with Medication Related Osteonecrosis of the Jaws (MRONJ)
- History of radiation therapy to the maxilla
- Cases with insufficient data, or no follow-up visit after surgery
- Former smokers (10 patients) were excluded due to insufficient data regarding smoking cessation period [18].
- Age
- Gender
- Medical status based on the American Society of Anesthesiologists (ASA) physical status classification [24]
- OAFs etiology—extraction, odontogenic infection, pathology, preprosthetic surgery (insertion of dental implants and sinus augmentation)
- OAFs size—measured clinically, in millimeters, as maximum diameter of soft tissue fistula
- Size of bony defect underlying OAFs—measured in millimeters as the maximum diameter of bony defect, either clinically during surgery or radiographically on a preoperative computed tomography (CT) scan, Cone beam computed tomography (CBCT), panoramic or Water’s view. Whenever a CT was used the measurement was conducted on the coronal reconstruction, and when a CBCT was used the panoramic reconstruction was used.
- Soft tissue fistula surface area (soft tissue deficit)—calculated as π*(0.5*soft tissue fistula diameter) 2.
- Bone defect surface area—calculated as π*(0.5*bony defect diameter) 2
- Soft tissue deficit relative to underlying bone defect—calculated as the ratio between the soft tissue fistula surface area relative to the bone defect surface area.
- History of previous FESS.
- Preoperative radiographic appearance of the antral cavity was determined based on either a CT scan, CBCT, panoramic view, or water’s view, and categorized into clear, thickened mucosal lining (>2 mm) or occluded sinus. Presence and type of foreign bodies inside the antral cavity were also recorded [25].
- Operative time in minutes.
- Type of flap used for fistula repair—Palatal flap, buccal advancement flap, buccal fat pad, or combinations.
- Caldwell-Luc operation (yes/no), either with or without inferior meatal antrostomy.
- Postoperative follow up time (months).
- Duration of hospitalization (days).
- Analgesic consumption during hospitalization (mean analgesic dose/day).
- Postoperative pain level during hospitalization was categorized into no pain, mild, moderate, and severe pain based on the type of analgesics consumed and according to the world health organization (WHO) analgesic ladder [27]
- Postoperative complications included:
- a.
- Bleeding
- b.
- Infection of surgical site
- c.
- Postoperative pain > four weeks
- d.
- Delayed wound healing—defined as incomplete soft tissue healing of the flap or incomplete soft tissue coverage of the denuded palate observed eight weeks postoperatively [3].
- e.
- Infraorbital sensory disturbance (paresthesia/hypoesthesia) lasting longer than eight weeks postoperatively [28]
- f.
- Epiphora
- g.
- Persistent sino nasal symptoms of chronic rhinitis, nasal congestion, or sinusitis.
- h.
- Failure was defined as residual or recurrent OAF observed 12 weeks postoperatively, requiring further surgical intervention [3].
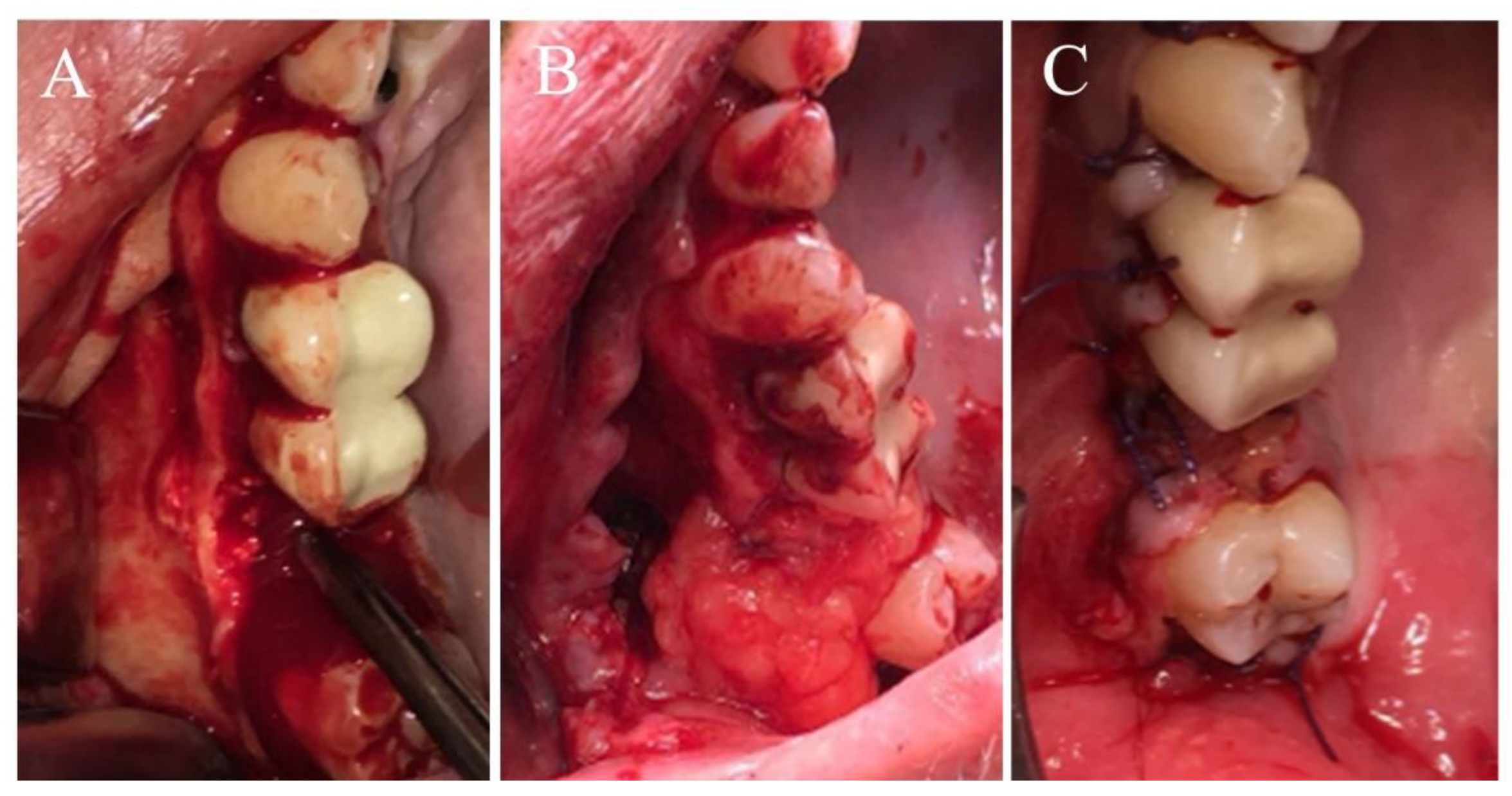
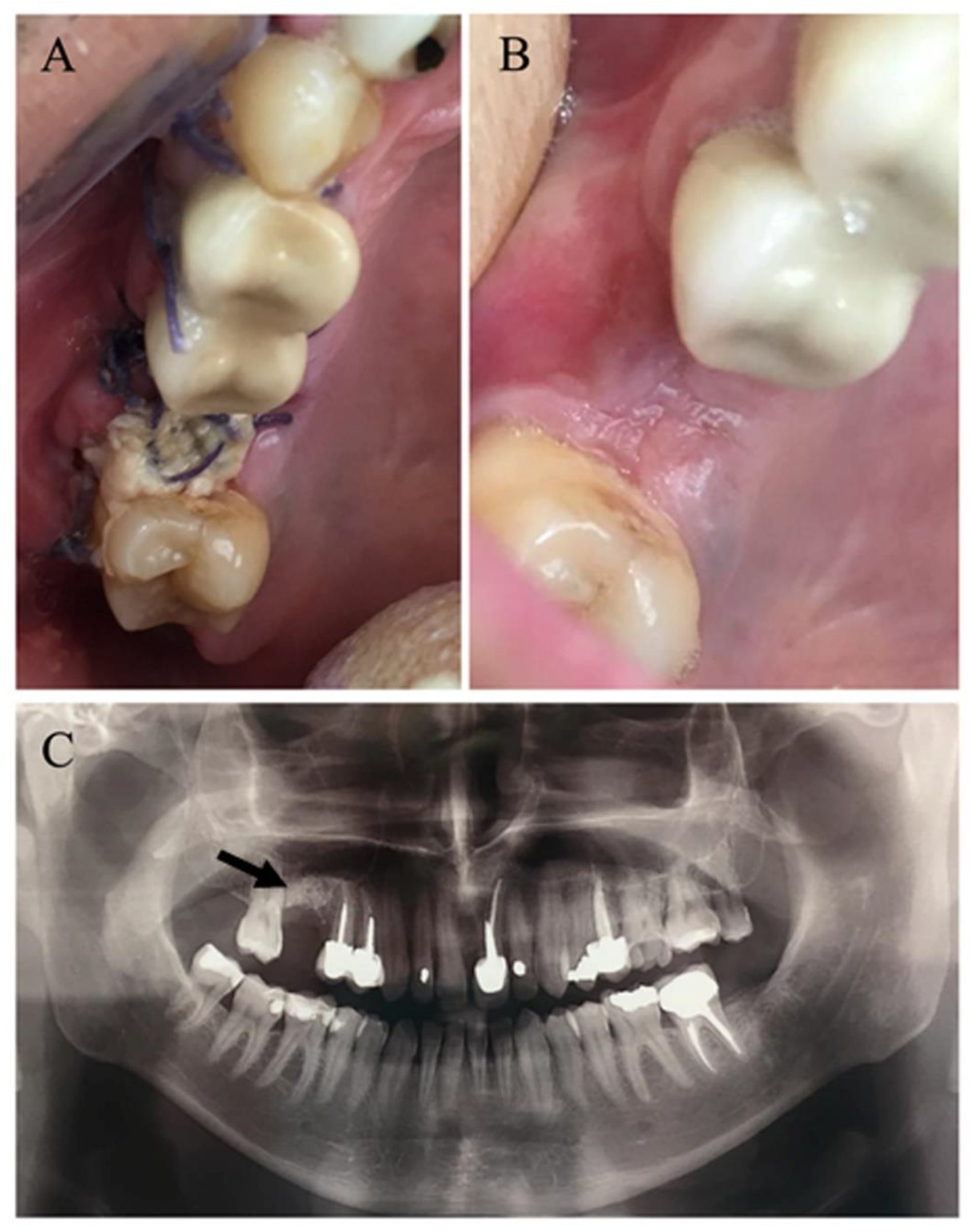
2.1. Surgical Procedure
2.2. Postoperative Care
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dym, H.; Wolf, J.C. Oroantral communication. Oral. Maxillofac. Surg. Clin. N. Am. 2012, 24, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Guven, O. A clinical study on oroantral fistulae. J. Craniomaxillofac. Surg. 1998, 26, 267–271. [Google Scholar] [CrossRef]
- Anavi, Y.; Gal, G.; Silfen, R.; Calderon, S. Palatal rotation-advancement flap for delayed repair of oroantral fistula: A retrospective evaluation of 63 cases. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2003, 96, 527–534. [Google Scholar] [CrossRef]
- Fonseca, R.J.; Barber, J.D.; Matheson, J.D. Oral and Maxillofacial Surgery, 2nd ed.; Saunders Elsevier: Philadelphia, PA, USA, 2009; pp. 214–215. [Google Scholar]
- Park, J.Y.; Chun, B.D.; Hwang, D.S. Versatility of the pedicled buccal fat pad flap of the management of oroantral fistula: A retrospective study of 25 cases. Maxillofac. Plast. Reconst. Surg. 2019, 41, 1–6. [Google Scholar] [CrossRef]
- Visscher, S.H.; Van Minnen, B.; Bos, R.R. Closure of oroantral communications: A review of the literature. J. Oral. Maxillofac. Surg. 2010, 68, 1384–1391. [Google Scholar] [CrossRef]
- Von Wowern, N. Correlation between the development of an oroantral fistula and the size of the corresponding bony defect. J. Oral. Surg. 1973, 31, 98–102. [Google Scholar]
- Von Wowern, N. Frequency of oroantral fistulae after perforation of the maxillary sinus. Scand. J. Dent. Res. 1970, 78, 394–396. [Google Scholar]
- Bravo, C.G.; Minzer, F.S.; Fernández, L. Odontogenic sinusitis, oro-antral fistula and surgical repair by Bichat’s fat pad: Literature review. Acta Otorrinolaringol. Esp. 2016, 67, 107–113. [Google Scholar] [CrossRef]
- Yalcin, S.; Oncu, B.; Emes, Y.; Atalay, B.; Aktas, I. Surgical treatment of oroantral fistulas: A clinical study of 23 cases. J. Oral. Maxillofac. Surg. 2011, 69, 333–339. [Google Scholar] [CrossRef]
- Procacci, P.; VAlfonsi, F.; Tonelli, P. Surgical treatment of oroantral communications. J. Craniofac. Surg. 2016, 27, 1190–1196. [Google Scholar] [CrossRef]
- Puglisi, S.; Privitera, S.; Maiolino, L.; Serra, A.; Garotta, M.; Blandino, G.; Speciale, A. Bacteriological findings and antimicrobial resistance in odontogenic and non-odontogenic chronic maxillary sinusitis. J. Med. Microbiol. 2011, 60, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Borogonovo, A.E.; Berardinelli, V.F.; Favale, M.; Maiorana, C. Surgical options in oroantral fistula treatment. Open. Dent. J. 2012, 6, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Abuabara, A.; Cortez, L.V.; Passeri, L.A.; De Moraes, M.; Moreira, R.W.F. Evaluation of different treatments of oroantralqoronasal communications: Experience of 112 cases. Int. J. Oral. Maxillofac. Surg. 2006, 35, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Al-Belasy, F.A. The relationship of “shisha” (water pipe) smoking to postextraction dry socket. J. Oral. Maxillofac. Surg. 2004, 62, 10–14. [Google Scholar] [CrossRef]
- Balaji, S.M. Tobacco smoking and surgical healing of oral tissues: A review. Indian J. Dent. Res. 2008, 19, 344–348. [Google Scholar] [CrossRef]
- Mayfield, L.; Soderholm, G.; Hallstrom, H.; Kullendorff, B.; Edwardsson, S.; Bratthall, G.; Brägger, U.; Attström, R. Guided tissue regeneration for the treatment of intraosseous defects using a bioabsorbable membrane: A controlled clinical study. J. Clin. Periodontol. 1998, 25, 585–595. [Google Scholar] [CrossRef]
- Sørensen, L.T. Wound healing and infection in surgery. The clinical impact of smoking and smoking cessation: A systematic review and meta-analysis. Arch. Surg. 2012, 147, 373–383. [Google Scholar] [CrossRef]
- Sørensen, L.T.; Karlsmark, T.; Gottrup, F. Abstinence from smoking reduces incisional wound infection: A randomized controlled trail. Ann. Surg. 2003, 238, 1–5. [Google Scholar] [CrossRef]
- Krueger, K.J.; Rohrich, R.J. Clearing the smoke: The scientific rationale for tobacco abstention with plastic surgery. Plast. Reconstr. Surg. 2001, 108, 1063–1073. [Google Scholar] [CrossRef]
- Rudmik, L.; Mace, J.C.; Smith, M.D. Smoking and endoscopic sinus surgery: Does smoking volume contribute to clinical outcome? Int. Forum. Allergy. Rhinol. 2011, 1, 145–152. [Google Scholar] [CrossRef]
- Briggs, R.D.; Wright, S.T.; Cordes, S.; Calhoun, K.H. Smoking in chronic rhinosinusitis: A predictor of poor long-term outcome after endoscopic sinus surgery. Laryngoscope 2004, 114, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Twito, D.; Sade, P. The effect of cigarette smoking habits on the outcome of dental implant treatment. Peerj 2014, 2, e546. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hurwitz, E.E.; Simon, M.; Vinta, S.R.; Abouleish, A.E.; Zehm, C.F.; Shabot, S.M.; Minhajuddin, A. Adding examples to the ASA-physical status classification improves correct assignment to patients. Anesthesiology 2017, 126, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Mehra, P.; Jeong, D. Maxillary sinusitis of odontogenic origin. Curr. Allergy Asthma Rep. 2009, 9, 238–243. [Google Scholar] [CrossRef]
- Rosenfeld, R.M.; Piccirillo, J.F.; Chandrasekhar, S.S.; Brook, I.; Kumar, K.A.; Kramper, M.; Orlandi, R.R.; Palmer, J.N.; Patel, Z.M.; Peters, A.; et al. Clinical practice guideline (update): Adult sinusitis. Otolaryngol. Head Neck Surg. 2015, 152, 1–39. [Google Scholar] [CrossRef]
- Vargas-Schaffer, G. Is the WHO analgesic ladder still valid? Twenty-four years of experience. Can. Fam. Physician. 2010, 56, 514–517. [Google Scholar]
- Miloro, M.; Ghali, G.E.; Larsen, P.E.; Waite, P.D. Peterson’s Principles of Oral and Maxillofacial Surgery, 3rd ed.; PMPH-USA: Raleigh, NC, USA, 2011; Volume 2, pp. 1437–1455. [Google Scholar]
- Visscher, S.H.; Room, M.R.F.; Sluiter, W.J.; Von Minnen, B.; Bos, R.R. Retrospective study on the treatment outcome of surgical closure of oroantral communications. J. Oral. Maxillofac. Surg. 2011, 69, 2956–2961. [Google Scholar] [CrossRef]
- Johnson, G.K.; Hill, M. Cigarette smoking and the periodontal patient. J. Periodontol. 2004, 75, 196–209. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Smoking and dental implants: A systematic review and meta-analysis. J. Dent. 2015, 43, 487–498. [Google Scholar] [CrossRef]
- Keenan, J.R.; Veitz-Keenan, A. The impact of smoking on failure rates, postoperative infection and marginal bone loss of dental implants. J. Evid. Based. Dent. 2016, 17, 4–5. [Google Scholar] [CrossRef]
- Moraschini, V.; Barboza, E.D. Success of dental implants in smokers and nonsmokers: A systematic review and meta-analysis. Int. J. Oral. Maxillofac. Surg. 2016, 45, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Alibrandi, A.; Rapisarda, E.; Matarese, G.; Williams, R.C.; Leonardi, R. Association of Vitamin d in patients with periodontitis: A cross-sectional study. J. Periodontal Res. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Matarese, G.; Ramaglia, L.; Pedullà, E.; Rapisarda, E.; Iorio-Siciliano, V. Association between periodontitis and glycosylated haemoglobin before diabetes onset: A cross-sectional study. Clin. Oral. Investig. 2019. [Google Scholar] [CrossRef]
- Jain, M.K.; Ramesh, C.; Sanker, K.; Lokesh, B.K.T. Pedicled buccal fat pad in the management of oroantral fistula: A clinical study of 15 cases. Int. J. Oral. Maxillofac. Surg. 2012, 41, 1025–1029. [Google Scholar] [CrossRef]
- Horowitz, G.; Koren, I.; Carmel, N.N.; Balaban, S.; Abu-Ghanem, S.; Fliss, D.M.; Reiser, V. One stage combined endoscopic and per-oral buccal fat pad approach for large oroantral fistula closure with secondary chronic maxillary sinusitis. Eur. Arch. Otorhinolaryngol. 2016, 273, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Gazal, G. Management of an emergency tooth extraction in diabetic patients on the dental chair. Saudi. Dent. J. 2020, 32, 1–6. [Google Scholar] [CrossRef] [PubMed]

| Variables | Non-Smokers | Smokers | p-Value |
|---|---|---|---|
| No. of patients | 59 | 38 | |
| Gender | |||
| Male | 32 (54.2%) | 26 (68.4%) | 0.2 |
| Female | 27 (45.8%) | 12 (31.6%) | |
| Age (years, mean ± SD) | 50.4 ± 16.0 | 51.3 ± 12.0 | 0.75 |
| ASA | |||
| I | 20 (33.9%) | 0 | |
| II | 35 (59.3%) | 34 (89.5%) | 0.001 |
| III | 4 (6.8%) | 4 (10.5%) |
| Etiology | Non-Smokers | Smokers |
|---|---|---|
| Non-elective surgery | ||
| Tooth extraction | 36 (61.0%) | 18 (47.4%) |
| Odontogenic infection | 14 (23.7%) | 6 (15.8%) |
| Pathology | 2 (3.4%) | 0 |
| Total | 52 (88.1%) | 24 (62.5%) |
| Elective Surgery | ||
| Preprosthetic surgery ** | 7 (11.9%) | 14 (36.8%) |
| Preoperative Signs and Symptoms | Non-Smokers | Smokers | P Value |
|---|---|---|---|
| OAF size | |||
| Soft tissue fistula diameter (Mean ± SD, mm) | 4.3 ± 3.2 | 5.7 ± 4.2 | 0.13 |
| Bone defect diameter (Mean ± SD, mm) | 13.9 ± 9.7 | 14.0 ± 9.9 | 0.97 |
| Soft tissue deficit/underlying bony defect (Mean ± SD) * | 0.4 ± 0.6 | 1.5 ± 5.3 | 0.35 |
| Maxillary sinusitis—clinical symptoms | 47 (79.7%) | 32 (84.2%) | 0.79 |
| S/P FESS | 4 (8.5%) | 5 (15.6%) | 0.3 |
| Radiographic sinus pathology ** | 49 (83.0%) | 35 (92.1%) | 0.35 |
| Foreign body inside the sinus | 7 (11.9%) | 8 (21.0%) | 0.13 |
| Implant | 2 (3.4%) | 1 (2.6%) | |
| Bone graft | 3 (5.1%) | 4 (10.5%) | |
| Tooth Root | 0 | 3 (7.9%) | |
| Other | 2 (3.4%) | 0 |
| Variable | Non-Smokers | Smokers | p Value |
|---|---|---|---|
| Operative time (Mean ± SD, Minutes) | 76.4 ± 25.9 | 74.6 ± 25.2 | 0.74 |
| Flap type | 0.71 | ||
| Palatal flap | 32 (54.2%) | 16 (42.1%) | |
| Buccal advancement flap | 7 (11.9%) | 6 (15.8%) | |
| Buccal fat pad + Buccal flap | 16 (27.1%) | 13 (34.2%) | |
| Buccal fat pad + Buccal flap + Palatal flap | 4 (6.8%) | 3 (7.9%) | |
| Caldwell-Luc operation | 48 (81.4%) | 23 (60.5%) | 0.03 |
| Variable | Non-Smokers | Smokers | p Value |
|---|---|---|---|
| Follow up (Mean ± SD, Months) | 7.3 ± 11.6 | 8.5 ± 12.8 | 0.65 |
| Hospitalization period (Mean ± SD, Days) | 4.0 ± 1.9 | 3.6 ± 1.7 | 0.34 |
| Pain level during hospital stay * | 0.05 | ||
| None | 17 (28.8%) | 16 (48.5%) | |
| Mild | 35 (72.9%) | 13 (34.2%) | |
| Moderate | 6 (10.2%) | 5 (13.2%) | |
| Severe | 1 (1.69%) | 4 (10.53%) | |
| Tramal (Mean ± SD, Dose) | 0.1 ± 0.36 | 0.6 ± 1.5 | 0.06 |
| Postoperative Complications | Non-Smokers | Smokers | p Value |
|---|---|---|---|
| Bleeding | 2 (3.4%) | 0 | 0.52 |
| Infection | 2 (3.4%) | 4 (10.5%) | 0.2 |
| Pain | 3 (5.1%) | 5 (13.2%) | 0.26 |
| Sensory disturbance | 0 | 3 (7.9%) | 0.06 |
| Epiphora | 1 (1.7%) | 0 | 1 |
| Delayed soft tissue healing | 0 | 2 (5.3%) | 0.15 |
| Sinonasal symptoms | 4 (6.8%) | 1 (2.6%) | 0.64 |
| Residual OAC | 4 (6.8%) | 6 (15.8%) | 0.18 |
| Spontaneous closure of residual OAF | 4 (100%) | 5 (83.3%) | |
| Failure | 0 | 1 (2.6%) | 0.39 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sella, A.; Ben-Zvi, Y.; Gillman, L.; Avishai, G.; Chaushu, G.; Rosenfeld, E. Evaluation of Surgical Treatment of Oroantral Fistulae in Smokers Versus Non-Smokers. Medicina 2020, 56, 310. https://doi.org/10.3390/medicina56060310
Sella A, Ben-Zvi Y, Gillman L, Avishai G, Chaushu G, Rosenfeld E. Evaluation of Surgical Treatment of Oroantral Fistulae in Smokers Versus Non-Smokers. Medicina. 2020; 56(6):310. https://doi.org/10.3390/medicina56060310
Chicago/Turabian StyleSella, Adi, Yehonatan Ben-Zvi, Leon Gillman, Gal Avishai, Gavriel Chaushu, and Eli Rosenfeld. 2020. "Evaluation of Surgical Treatment of Oroantral Fistulae in Smokers Versus Non-Smokers" Medicina 56, no. 6: 310. https://doi.org/10.3390/medicina56060310
APA StyleSella, A., Ben-Zvi, Y., Gillman, L., Avishai, G., Chaushu, G., & Rosenfeld, E. (2020). Evaluation of Surgical Treatment of Oroantral Fistulae in Smokers Versus Non-Smokers. Medicina, 56(6), 310. https://doi.org/10.3390/medicina56060310







