Clinical Outcomes of Complications Following Self-Expandable Metallic Stent Insertion for Benign Tracheobronchial Stenosis
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Airway Intervention Techniques
2.3. Airway Stents
2.4. Data Collection
2.5. Statistical Analysis
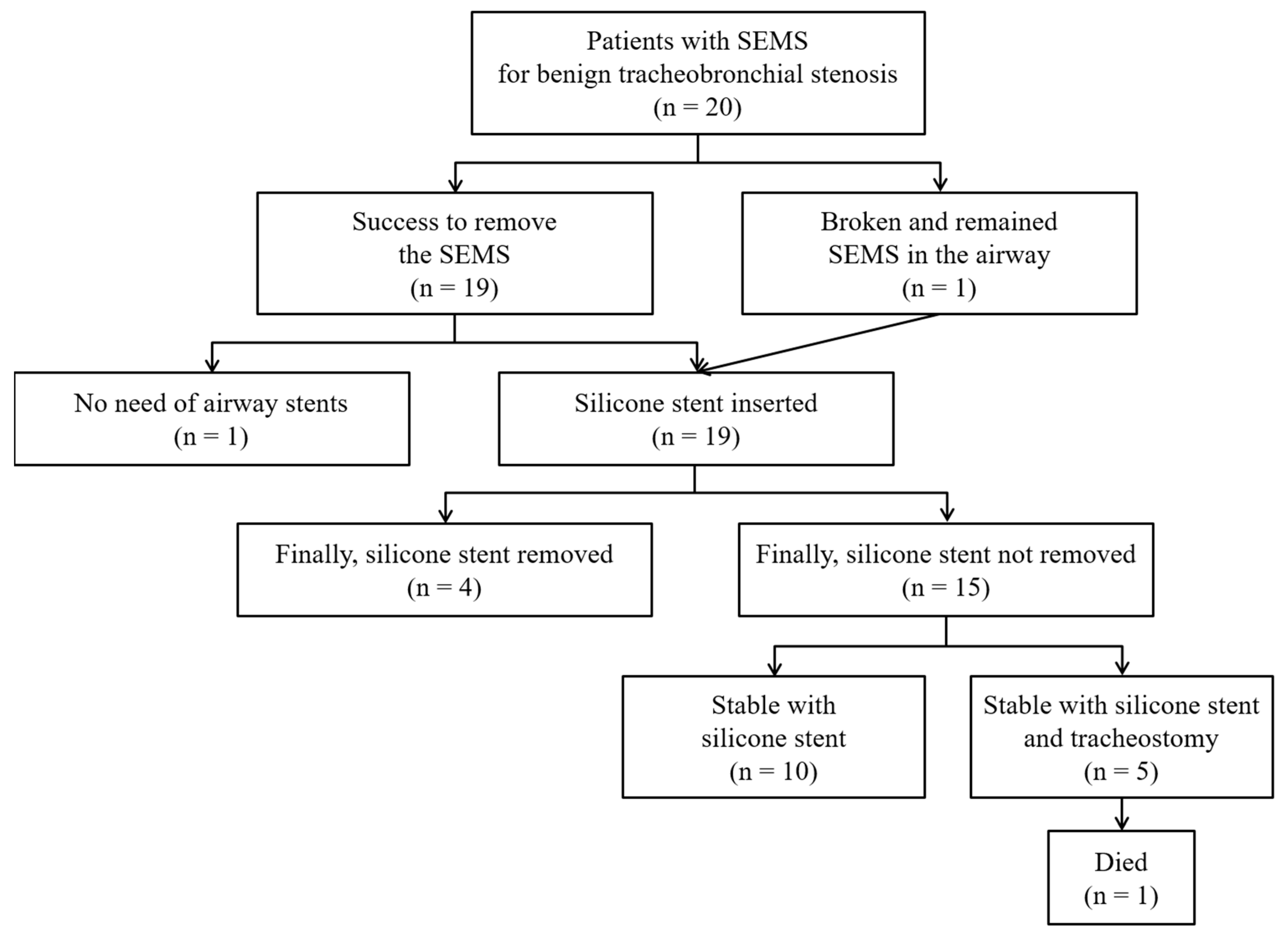
3. Results
3.1. Baseline Characteristics
3.2. Treatment Modalities
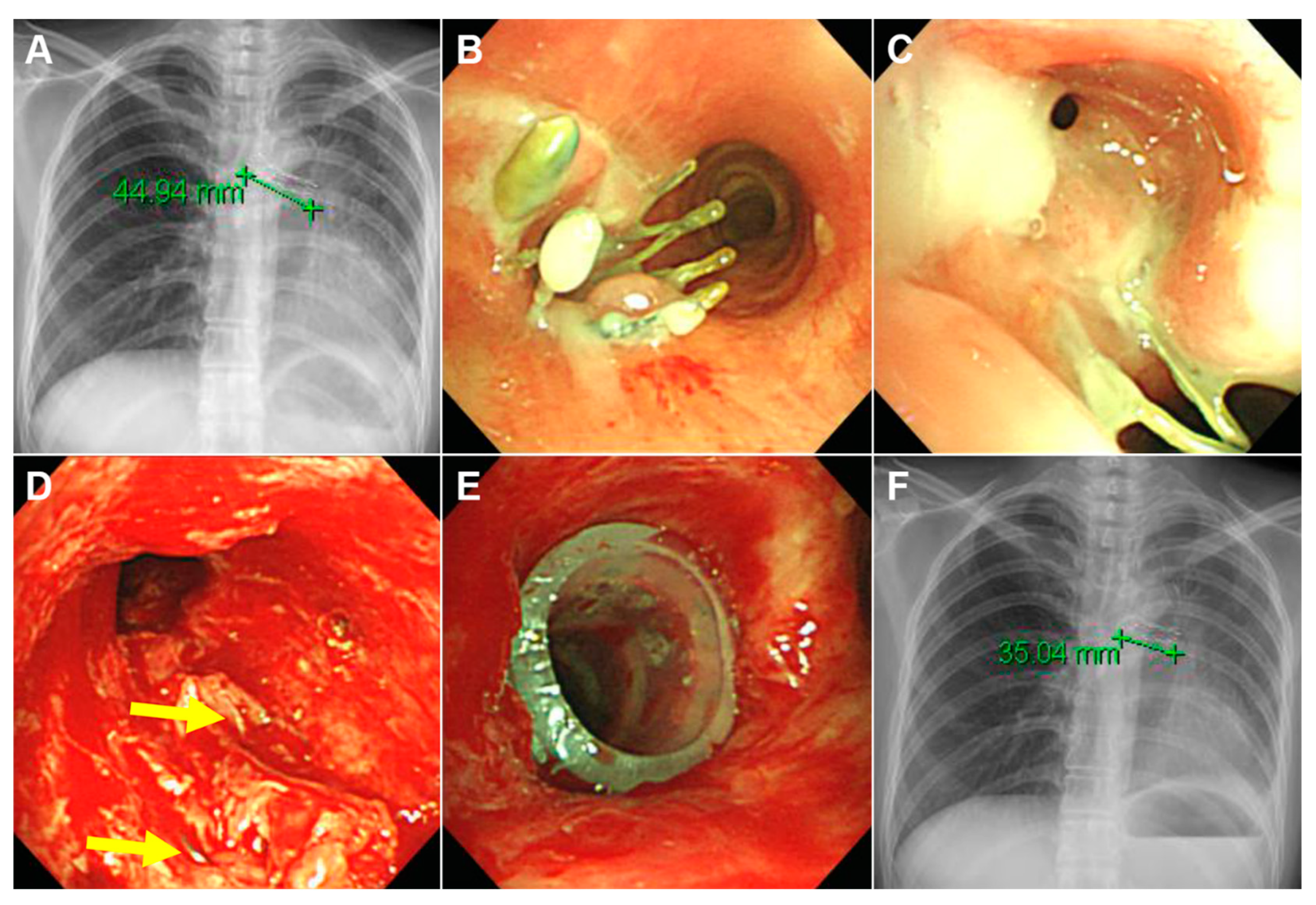
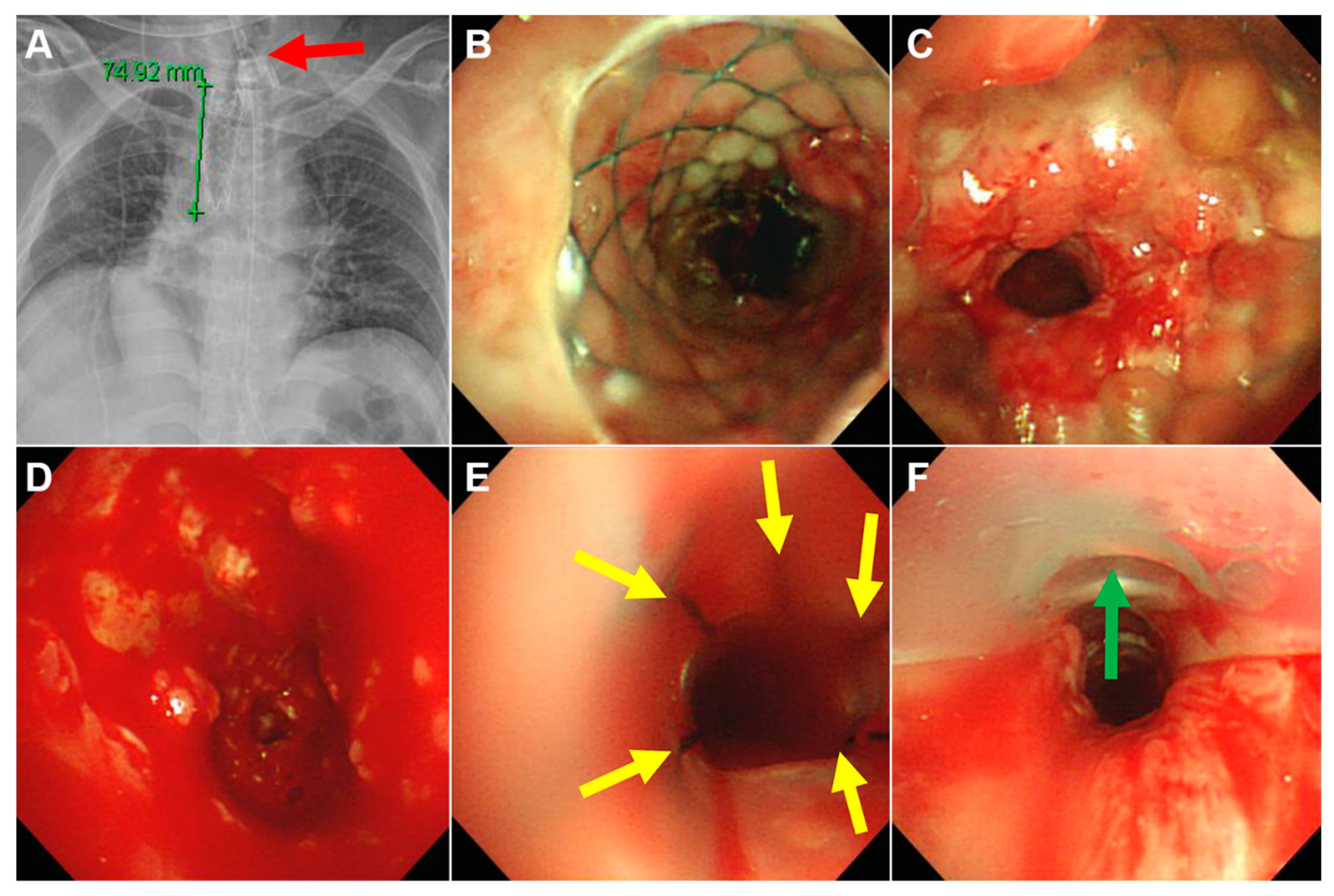
3.3. Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ASA | American Society of Anesthesiologists |
| CT | computed tomography |
| FDA | Food and Drug Administration |
| FEV1 | forced expiratory volume in one second |
| FVC | forced vital capacity |
| IQR | interquartile range |
| PITS | post-intubation tracheal stenosis |
| POTS | post-operative tracheal stenosis |
| PTBS | post-tuberculous tracheobronchial stenosis |
| PTTS | post-tracheostomy tracheal stenosis |
| SEMS | self-expandable metallic stents |
| TBS | tracheobronchial stenosis |
References
- Galluccio, G.; Lucantoni, G.; Battistoni, P.; Paone, G.; Batzella, S.; Lucifora, V.; Dello Iacono, R. Interventional endoscopy in the management of benign tracheal stenoses: Definitive treatment at long-term follow-up. Eur. J. Cardiothorac. Surg. 2009, 35, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.S.; Litle, V.; Yun, J.; Weiser, T.; Swanson, S.J. Airway stents. Ann. Thorac. Surg. 2008, 85, S792–S796. [Google Scholar] [CrossRef] [PubMed]
- Dumon, J.F. A dedicated tracheobronchial stent. Chest 1990, 97, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Breen, D.P.; Dutau, H. On-site customization of silicone stents: Towards optimal palliation of complex airway conditions. Respiration 2009, 77, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Tay, C.K.; Jeong, B.H.; Kim, H. Angulated Stents-A Novel Stent Improvisation to Manage Difficult Post-tuberculosis Bronchial Stenosis. ASAIO J. 2018, 64, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ballarin, J.I.; Diaz-Jimenez, J.P.; Castro, M.J.; Moya, J.A. Silicone stents in the management of benign tracheobronchial stenoses. Tolerance and early results in 63 patients. Chest 1996, 109, 626–629. [Google Scholar] [CrossRef]
- Ryu, Y.J.; Kim, H.; Yu, C.M.; Choi, J.C.; Kwon, Y.S.; Kwon, O.J. Use of silicone stents for the management of post-tuberculosis tracheobronchial stenosis. Eur. Respir. J. 2006, 28, 1029–1035. [Google Scholar] [CrossRef]
- Shin, B.; Kim, K.; Jeong, B.H.; Eom, J.S.; Song, W.J.; Kang, H.K.; Kim, H. Clinical significance of differentiating post-intubation and post-tracheostomy tracheal stenosis. Respirology 2017, 22, 513–520. [Google Scholar] [CrossRef]
- Dutau, H.; Musani, A.I.; Plojoux, J.; Laroumagne, S.; Astoul, P. The use of self-expandable metallic stents in the airways in the adult population. Expert Rev. Respir. Med. 2014, 8, 179–190. [Google Scholar] [CrossRef]
- Zias, N.; Chroneou, A.; Gonzalez, A.V.; Gray, A.W.; Lamb, C.R.; Riker, D.R.; Beamis, J.F., Jr. Changing patterns in interventional bronchoscopy. Respirology 2009, 14, 595–600. [Google Scholar] [CrossRef]
- Vinograd, I.; Klin, B.; Brosh, T.; Weinberg, M.; Flomenblit, Y.; Nevo, Z. A New Intratracheal Stent Made from Nitinol, an Alloy with Shape-Memory Effect. J. Thorac. Cardiovasc. Surg. 1994, 107, 1255–1261. [Google Scholar] [CrossRef]
- Dasgupta, A.; Dolmatch, B.L.; Abi-Saleh, W.J.; Mathur, P.N.; Mehta, A.C. Self-expandable metallic airway stent insertion employing flexible bronchoscopy: Preliminary results. Chest 1998, 114, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Alazemi, S.; Lunn, W.; Majid, A.; Berkowitz, D.; Michaud, G.; Feller-Kopman, D.; Herth, F.; Ernst, A. Outcomes, health-care resources use, and costs of endoscopic removal of metallic airway stents. Chest 2010, 138, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Colt, H.G.; Dumon, J.F. Airway stents. Present and future. Clin. Chest Med. 1995, 16, 465–478. [Google Scholar] [PubMed]
- Kim, H. Stenting therapy for stenosing airway disease. Respirology 1998, 3, 221–228. [Google Scholar] [CrossRef]
- Ryu, Y.J.; Kim, H.; Yu, C.M.; Choi, J.C.; Kwon, Y.S.; Kim, J.; Suh, S.W. Comparison of natural and Dumon airway stents for the management of benign tracheobronchial stenoses. Respirology 2006, 11, 748–754. [Google Scholar] [CrossRef]
- Kim, H.J.; Koh, W.J.; Suh, G.Y.; Chung, M.P.; Kim, J.G.; Suh, S.W.; Kwon, O.J. The Usefulness and Safety of Natural Stent in a Canine Model of Tracheal Stenosis. Tuberc. Respir. Dis. 2002, 53, 431–438. [Google Scholar] [CrossRef]
- Owens, W.D.; Felts, J.A.; Spitznagel, E.L., Jr. ASA physical status classifications: A study of consistency of ratings. Anesthesiology 1978, 49, 239–243. [Google Scholar] [CrossRef]
- Myer, C.M., 3rd; O’Connor, D.M.; Cotton, R.T. Proposed grading system for subglottic stenosis based on endotracheal tube sizes. Ann. Otol. Rhinol. Laryngol. 1994, 103, 319–323. [Google Scholar] [CrossRef]
- Kim, J.; Shin, J.H.; Kim, J.H.; Song, H.Y.; Song, S.Y.; Park, C.K. Metallic stent placement for the management of tracheal carina strictures and fistulas: Technical and clinical outcomes. Am. J. Roentgenol. 2014, 202, 880–885. [Google Scholar] [CrossRef]
- Cho, S.B.; Cha, S.A.; Choi, J.Y.; Lee, J.M.; Kang, H.H.; Moon, H.S.; Kim, S.W.; Yeo, C.D.; Lee, S.H. Serious Complications after Self-expandable Metallic Stent Insertion in a Patient with Malignant Lymphoma. Tuberc. Respir. Dis. 2015, 78, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Shin, J.H.; Kim, K.Y.; Lim, J.Y.; Kim, P.H.; Tsauo, J.; Kim, M.T.; Song, H.Y. Respiratory support with venovenous extracorporeal membrane oxygenation during stent placement for the palliation of critical airway obstruction: Case series analysis. J. Thorac. Dis. 2017, 9, 2599–2607. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gaissert, H.A.; Grillo, H.C.; Wright, C.D.; Donahue, D.M.; Wain, J.C.; Mathisen, D.J. Complication of benign tracheobronchial strictures by self-expanding metal stents. J. Thorac. Cardiovasc. Surg. 2003, 126, 744–747. [Google Scholar] [CrossRef]
- Lunn, W.; Feller-Kopman, D.; Wahidi, M.; Ashiku, S.; Thurer, R.; Ernst, A. Endoscopic removal of metallic airway stents. Chest 2005, 127, 2106–2112. [Google Scholar] [CrossRef]
- Swanson, K.L.; Edell, E.S.; Prakash, U.B.S.; Brutinel, W.M.; Midthun, D.E.; Utz, J.P. Complications of Metal Stent Therapy in Benign Airway Obstruction. J. Bronchol. 2007, 14, 90–94. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Yamaguchi, E.; Zhou, Y.; Li, D.; Zou, H.; Luo, L.; Ma, H. Endoscopic removal of metallic airway stents. J. Bronchol. Interv. Pulmonol. 2011, 18, 31–37. [Google Scholar] [CrossRef]
- Lim, S.Y.; Park, H.K.; Jeon, K.; Um, S.W.; Koh, W.J.; Suh, G.Y.; Chung, M.P.; Kwon, O.J.; Kim, H. Factors predicting outcome following airway stenting for post-tuberculosis tracheobronchial stenosis. Respirology 2011, 16, 959–964. [Google Scholar] [CrossRef]
- Chung, F.T.; Chen, H.C.; Chou, C.L.; Yu, C.T.; Kuo, C.H.; Kuo, H.P.; Lin, S.M. An outcome analysis of self-expandable metallic stents in central airway obstruction: A cohort study. J. Cardiothorac. Surg. 2011, 6, 46. [Google Scholar] [CrossRef]
- Serrano, C.; Laborda, A.; Lozano, J.M.; Caballero, H.; Sebastian, A.; Lopera, J.; de Gregorio, M.A. Metallic stents for tracheobronchial pathology treatment. Cardiovasc. Intervent. Radiol. 2013, 36, 1614–1623. [Google Scholar] [CrossRef]
- Dooms, C.; De Keukeleire, T.; Janssens, A.; Carron, K. Performance of Fully Covered Self-Expanding Metallic Stents in Benign Airway Strictures. Respiration 2009, 77, 420–426. [Google Scholar] [CrossRef]
- Fortin, M.; Lacasse, Y.; Elharrar, X.; Tazi-Mezalek, R.; Laroumagne, S.; Guinde, J.; Astoul, P.; Dutau, H. Safety and Efficacy of a Fully Covered Self-Expandable Metallic Stent in Benign Airway Stenosis. Respiration 2017, 93, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.F.; Xu, L.; Fan, L.L.; Cheng, D.Y.; Zheng, B.X. Long-term follow-up of self-expandable metallic stents in benign tracheobronchial stenosis: A retrospective study. BMC Pulm. Med. 2019, 19, 33. [Google Scholar] [CrossRef] [PubMed]
| Variables | n = 20 |
|---|---|
| Age, year | 40 (26–62) |
| Sex, female | 12 (60) |
| Comorbidities | |
| No | 6 (30) |
| Old tuberculosis | 7 (35) |
| Cerebrovascular disease | 4 (20) |
| Diabetes | 3 (15) |
| Lobectomy status * | 2 (10) |
| Leukemia | 1 (5) |
| Etiology of benign tracheobronchial stenosis | |
| Post-tuberculous tracheobronchial stenosis | 7 (35) |
| Post-intubation tracheal stenosis | 5 (25) |
| Post-tracheostomy tracheal stenosis | 5 (25) |
| Others † | 3 (15) |
| ASA physical status ‡ | |
| Class 1 | 2 (10) |
| Class 2 | 12 (60) |
| Class 3 | 4 (20) |
| Class 4 | 2 (10) |
| Intubation or tracheostomy due to respiratory failure before intervention | 4 (20) |
| Stenosis site | |
| Trachea | 14 (70) |
| Left main bronchus | 5 (25) |
| Bronchus intermedius | 1 (5) |
| Severity of stenosis (Myer and Cotton Grade) § | |
| I | 1 (5) |
| II | 2 (10) |
| III | 14 (70) |
| IV | 3 (15) |
| Spirometry (n = 8) | |
| FEV1/FVC | 69 (58–75) |
| FEV1, L | 1.95 (1.68–2.10) |
| FEV1, % predicted | 78 (67–88) |
| FVC, L | 2.79 (2.49–3.00) |
| FVC, % predicted | 86 (72–103) |
| Type of SEMS | |
| Covered SEMS | 19 (95) |
| Uncovered SEMS | 1 (5) |
| Length of SEMS, mm | 55 (45–78) |
| Duration of SEMS in place, months | 3 (2–8) |
| Reason for removal of SEMS | |
| Granulation tissue overgrowth | 20 (100) |
| Stent migration | 4 (20) |
| Stent fracture | 1 (5) |
| Variables | n = 20 |
|---|---|
| Duration of follow-up after the first intervention at our hospital, months | 40 (19–88) |
| Number of interventional bronchoscopies | 7 (2–10) |
| Treatment modalities * | |
| Successful removal of SEMS | 19 (95) |
| Silicone stent insertion | 19 (95) |
| Dumon or Natural stent | 17/19 (89) |
| Y-stent | 6/19 (32) |
| Montgomery T-tube | 2/19 (11) |
| Ballooning | 9 (45) |
| Laser therapy | 5 (25) |
| Tracheostomy tube insertion | 5 (25) |
| Variables | n = 20 |
|---|---|
| Immediate symptom relief | 20 (100) |
| Acute complications during the SEMS removal | 3 (15) |
| Mucosal tear and bleeding | 2 (10) * |
| Tracheo-esophageal fistula | 1 (5) † |
| Chronic complications during the follow-up | 20 (100) |
| Granulation tissue overgrowth | 16 (80) |
| Stent migration | 11/19 (58) ‡ |
| Mucostasis | 11 (55) |
| Malacia | 7 (35) |
| Tracheoesophageal fistula | 3 (15) |
| Restenosis after stent removal | 3/7 (43) § |
| Spirometry in the last state (n = 15) | |
| FEV1/FVC | 71 (65–84) |
| FEV1, L | 2.50 (1.89–2.86) |
| FEV1, % predicted | 88 (79–108) |
| FVC, L | 3.26 (2.61–3.85) |
| FVC, % predicted | 92 (77–104) |
| Final outcomes | |
| Persistent silicone stent placement | 15 (75) |
| Duration of stent placement, months | 65 (35–106) |
| Successful silicone stent removal | 4 (20) |
| Duration of stent placement, months | 12 (7–22) |
| Duration of follow-up after stent removal, months | 6 (4–26) |
| Successful removal of SEMS without additional stenting | 1 (5) ¶ |
| Permanent tracheostomy | 5 (25) |
| Surgical management | 0 (0) |
| Mortality | 1 (5) ǁ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, B.-H.; Ng, J.; Jeong, S.H.; Kim, H. Clinical Outcomes of Complications Following Self-Expandable Metallic Stent Insertion for Benign Tracheobronchial Stenosis. Medicina 2020, 56, 367. https://doi.org/10.3390/medicina56080367
Jeong B-H, Ng J, Jeong SH, Kim H. Clinical Outcomes of Complications Following Self-Expandable Metallic Stent Insertion for Benign Tracheobronchial Stenosis. Medicina. 2020; 56(8):367. https://doi.org/10.3390/medicina56080367
Chicago/Turabian StyleJeong, Byeong-Ho, Jeffrey Ng, Suk Hyeon Jeong, and Hojoong Kim. 2020. "Clinical Outcomes of Complications Following Self-Expandable Metallic Stent Insertion for Benign Tracheobronchial Stenosis" Medicina 56, no. 8: 367. https://doi.org/10.3390/medicina56080367
APA StyleJeong, B.-H., Ng, J., Jeong, S. H., & Kim, H. (2020). Clinical Outcomes of Complications Following Self-Expandable Metallic Stent Insertion for Benign Tracheobronchial Stenosis. Medicina, 56(8), 367. https://doi.org/10.3390/medicina56080367






