Association between Corrected QT Interval and C-Reactive Protein in Patients with Inflammatory Bowel Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Exclusion Criteria
- History of cardiovascular, metabolic, and endocrine diseases;
- Electrolyte disturbances;
- Assumption of drugs affecting QT interval (with the exception of those required to cure IBD).
2.3. Control Group
2.4. ECG Analysis
2.5. Statistical Analysis
2.6. Systematic Review
3. Results
3.1. Study Population and Baseline Assessment
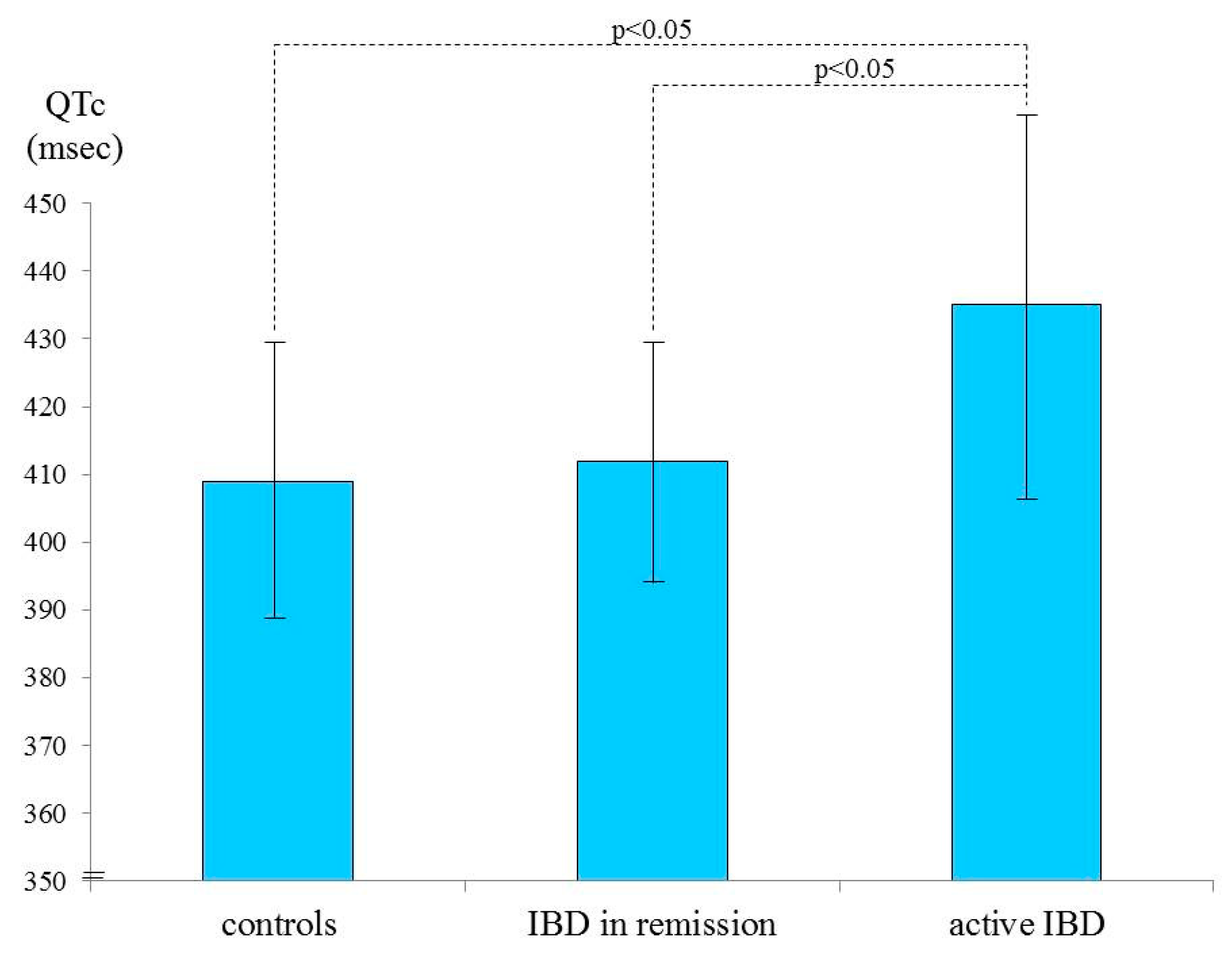
3.2. Case-Control QTc Comparison
3.3. QTc and Disease Activity
3.4. Systematic Review
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- de Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Harbord, M.; Annese, V.; Vavricka, S.R.; Allez, M.; Barreiro-de Acosta, M.; Boberg, K.M.; Burisch, J.; De Vos, M.; De Vries, A.M.; Dick, A.D.; et al. The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J. Crohns Colitis. 2016, 10, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Gautam, V.; Naseem, S. Acute-phase proteins: As diagnostic tool. J. Pharm. Bioallied Sci. 2011, 3, 118–127. [Google Scholar] [CrossRef]
- Vermeire, S.; Van Assche, G.; Rutgeerts, P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm. Bowel Dis. 2004, 10, 661–665. [Google Scholar] [CrossRef] [Green Version]
- Mosli, M.H.; Zou, G.; Garg, S.K.; Feagan, S.G.; MacDonald, J.K.; Chande, N.; Sandborn, W.J.; Feagan, B.G. C-Reactive Protein, Fecal Calprotectin, and Stool Lactoferrin for Detection of Endoscopic Activity in Symptomatic Inflammatory Bowel Disease Patients: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2015, 110, 802–820. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, N.; Chai, J.T.; Fisher, E.A.; Choudhury, R.P. Inflammatory processes in cardiovascular disease: A route to targeted therapies. Nat. Rev. Cardiol. 2017, 14, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Candales, A.; Hernández Burgos, P.M.; Hernandez-Suarez, D.F.; Harris, D. Linking Chronic Inflammation with Cardiovascular Disease: From Normal Aging to the Metabolic Syndrome. J. Nat. Sci. 2017, 3, e341. [Google Scholar] [PubMed]
- Mason, J.C.; Libby, P. Cardiovascular disease in patients with chronic inflammation: Mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur. Heart J. 2015, 36, 482–489. [Google Scholar] [CrossRef] [Green Version]
- Romano, S.; Salustri, E.; Ruscitti, P.; Carubbi, F.; Penco, M.; Giacomelli, R. Cardiovascular and Metabolic Comorbidities in Rheumatoid Arthritis. Curr. Rheumatol. Rep. 2018, 20, 81. [Google Scholar] [CrossRef] [PubMed]
- Fumery, M.; Xiaocang, C.; Dauchet, L.; Gower-Rousseau, C.; Peyrin-Biroulet, L.; Colombel, J.F. Thromboembolic events and cardiovascular mortality in inflammatory bowel diseases: A meta-analysis of observational studies. J. Crohns Colitis. 2014, 8, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Chen, G.; Cai, D.; Zhao, S.; Cheng, J.; Shen, H. Inflammatory Bowel Disease and Risk of Ischemic Heart Disease: An Updated Meta-Analysis of Cohort Studies. J. Am. Heart Assoc. 2017, 6, e005892. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kullo, I.J.; Pardi, D.S.; Loftus, E.V., Jr. Epidemiology, risk factors and management of cardiovascular diseases in IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Bunu, D.M.; Timofte, C.E.; Ciocoiu, M.; Floria, M.; Tarniceriu, C.C.; Barboi, O.B.; Tanase, D.M. Cardiovascular Manifestations of Inflammatory Bowel Disease: Pathogenesis, Diagnosis, and Preventive Strategies. Gastroenterol. Res. Pract. 2019, 2019, 3012509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristensen, S.L.; Ahlehoff, O.; Lindhardsen, J.; Erichsen, R.; Jensen, G.V.; Torp-Pedersen, C.; Nielsen, O.H.; Gislason, G.H.; Hansen, P.R. Disease activity in inflammatory bowel disease is associated with increased risk of myocardial infarction, stroke and cardiovascular death—a Danish nationwide cohort study. PLoS ONE 2013, 8, e56944. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Lindhardsen, J.; Ahlehoff, O.; Erichsen, R.; Lamberts, M.; Khalid, U.; Torp-Pedersen, C.; Nielsen, O.H.; Gislason, G.H.; Hansen, P.R. Increased risk of atrial fibrillation and stroke during active stages of inflammatory bowel disease: A nationwide study. Europace 2014, 16, 477–484. [Google Scholar] [CrossRef]
- Rungoe, C.; Basit, S.; Ranthe, M.F.; Wohlfahrt, J.; Langholz, E.; Jess, T. Risk of ischaemic heart disease in patients with inflammatory bowel disease: A nationwide Danish cohort study. Gut 2013, 62, 689–694. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Ahlehoff, O.; Lindhardsen, J.; Erichsen, R.; Lamberts, M.; Khalid, U.; Nielsen, O.H.; Torp-Pedersen, C.; Gislason, G.H.; Hansen, P.R. Prognosis after first-time myocardial infarction in patients with inflammatory bowel disease according to disease activity: Nationwide cohort study. Circ. Cardiovasc. Qual. Outcomes 2014, 7, 857–862. [Google Scholar] [CrossRef] [Green Version]
- Close, H.; Mason, J.M.; Wilson, D.W.; Hungin, A.P.; Jones, R.; Rubin, G. Risk of Ischaemic Heart Disease in Patients with Inflammatory Bowel Disease: Cohort Study Using the General Practice Research Database. PLoS ONE 2015, 10, e0139745. [Google Scholar] [CrossRef] [Green Version]
- Pattanshetty, D.J.; Anna, K.; Gajulapalli, R.D.; Sappati-Biyyani, R.R. Inflammatory bowel “Cardiac” disease: Point prevalence of atrial fibrillation in inflammatory bowel disease population. Saudi J. Gastroenterol. 2015, 21, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Kirchgesner, J.; Beaugerie, L.; Carrat, F.; Andersen, N.N.; Jess, T.; Schwarzinger, M. Increased risk of acute arterial events in young patients and severely active IBD: A nationwide French cohort study. Gut 2018, 67, 1261–1268. [Google Scholar] [CrossRef]
- Le Gall, G.; Kirchgesner, J.; Bejaoui, M.; Landman, C.; Nion-Larmurier, I.; Bourrier, A.; Sokol, H.; Seksik, P.; Beaugerie, L. Clinical activity is an independent risk factor of ischemic heart and cerebrovascular arterial disease in patients with inflammatory bowel disease. PLoS ONE 2018, 13, e0201991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aniwan, S.; Pardi, D.S.; Tremaine, W.J.; Loftus, E.V., Jr. Increased Risk of Acute Myocardial Infarction and Heart Failure in Patients With Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2018, 16, 1607–1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panhwar, M.S.; Mansoor, E.; Al-Kindi, S.G.; Sinh, P.; Katz, J.; Oliveira, G.H.; Cooper, G.S.; Ginwalla, M. Risk of Myocardial Infarction in Inflammatory Bowel Disease: A Population-based National Study. Inflamm. Bowel Dis. 2019, 25, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, K.H.; Christodoulou, D.K.; Pappas, K.; Pappas, C.; Tsianos, E.V. Electrocardiograph abnormalities in patients with active inflammatory bowel disease. Ann. Gastroenterol. 2007, 20, 275–281. [Google Scholar]
- Curione, M.; Aratari, A.; Amato, S.; Colotto, M.; Barbato, M.; Leone, S.; Tego, A.; Panetti, D.; Parlapiano, C. A study on QT interval in patients affected with inflammatory bowel disease without cardiac involvement. Intern. Emerg. Med. 2010, 5, 307–310. [Google Scholar] [CrossRef]
- Dogan, Y.; Soylu, A.; Eren, G.A.; Poturoglu, S.; Dolapcioglu, C.; Sonmez, K.; Duman, H.; Sevindir, I. Evaluation of QT and P wave dispersion and mean platelet volume among inflammatory bowel disease patients. Int. J. Med. Sci. 2011, 8, 540–546. [Google Scholar] [CrossRef] [Green Version]
- Yorulmaz, E.; Sezgin, A.; Yorulmaz, H.; Adali, G.; Ciftci, H. Prolonged QT dispersion in inflammatory bowel disease. World J. Gastroenterol. 2013, 19, 65–71. [Google Scholar] [CrossRef]
- Pattanshetty, D.J.; Gajulapalli, R.D.; Anna, K.; Sappati Biyyani, R.S. Prevalence of QT interval prolongation in inflammatory bowel disease. Turk. J. Gastroenterol. 2016, 27, 136–142. [Google Scholar] [CrossRef]
- Bornaun, H.A.; Yılmaz, N.; Kutluk, G.; Dedeoğlu, R.; Öztarhan, K.; Keskindemirci, G.; Tulunoğlu, A.; Şap, F. Prolonged P-Wave and QT Dispersion in Children with Inflammatory Bowel Disease in Remission. Biomed Res. Int. 2017, 2017, 6960810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acar, B.U.; Yayla, C.; Coskun, O.; Kaplan, M.; Ozin, Y.; Kayacetin, E. Assessment of ventricular repolarization alterations in patients with Inflammatory Bowel Disease. Kuwait Med. J. 2019, 51, 393–398. [Google Scholar]
- Erolu, E.; Polat, E. Cardiac Repolarization Properties in Children with Inflammatory Bowel Disease. Cyprus J. Med. Sci. 2020, 5, 126–130. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Capecchi, P.L.; Laghi-Pasini, F. Long QT Syndrome: An Emerging Role for Inflammation and Immunity. Front. Cardiovasc. Med. 2015, 2, 26. [Google Scholar] [CrossRef] [Green Version]
- Lazzerini, P.E.; Capecchi, P.L.; Laghi-Pasini, F. Systemic inflammation and arrhythmic risk: Lessons from rheumatoid arthritis. Eur. Heart J. 2017, 38, 1717–1727. [Google Scholar] [CrossRef]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019, 13, 144–164. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, K.W.; Tremaine, W.J.; Ilstrup, D.M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 1987, 317, 1625–1629. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Sandborn, W.J.; Feagan, B.G.; Reinisch, W.; Olson, A.; Johanns, J.; Travers, S.; Rachmilewitz, D.; Hanauer, S.B.; Lichtenstein, G.R.; et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005, 353, 2462–2476. [Google Scholar] [CrossRef] [Green Version]
- Harvey, R.F.; Bradshaw, J.M. A simple index of Crohn’s-disease activity. Lancet 1980, 1, 514. [Google Scholar] [CrossRef]
- Bazett, H.C. An analysis of the time relations of electrocardiogram. Heart 1920, 7, 53–70. [Google Scholar] [CrossRef]
- Postema, P.G.; Wilde, A.A. The measurement of the QT interval. Curr. Cardiol. Rev. 2014, 10, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Rautaharju, P.M.; Surawicz, B.; Gettes, L.S. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part IV: The ST segment, T and U waves, and the QT interval: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J. Am. Coll. Cardiol. 2009, 53, 982–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabhu, S.D. Cytokine-induced modulation of cardiac function. Circ. Res. 2004, 95, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Bartekova, M.; Radosinska, J.; Jelemensky, M.; Dhalla, N.S. Role of cytokines and inflammation in heart function during health and disease. Heart Fail. Rev. 2018, 23, 733–758. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Capecchi, P.L.; Laghi-Pasini, F.; Boutjdir, M. Autoimmune channelopathies as a novel mechanism in cardiac arrhythmias. Nat. Rev. Cardiol. 2017, 14, 521–535. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Capecchi, P.L.; El-Sherif, N.; Laghi-Pasini, F.; Boutjdir, M. Emerging Arrhythmic Risk of Autoimmune and Inflammatory Cardiac Channelopathies. J. Am. Heart Assoc. 2018, 7, e010595. [Google Scholar] [CrossRef] [Green Version]
- Lazzerini, P.E.; Laghi-Pasini, F.; Boutjdir, M.; Capecchi, P.L. Cardioimmunology of arrhythmias: The role of autoimmune and inflammatory cardiac channelopathies. Nat. Rev. Immunol. 2019, 19, 63–64. [Google Scholar] [CrossRef]
- Capecchi, P.L.; Laghi-Pasini, F.; El-Sherif, N.; Qu, Y.; Boutjdir, M.; Lazzerini, P.E. Autoimmune and inflammatory K+ channelopathies in cardiac arrhythmias: Clinical evidence and molecular mechanisms. Heart Rhythm. 2019, 16, 1273–1280. [Google Scholar] [CrossRef]
- London, B.; Baker, L.C.; Lee, J.S.; Shusterman, V.; Choi, B.R.; Kubota, T.; McTiernan, C.F.; Feldman, A.M.; Salama, G. Calcium-dependent arrhythmias in transgenic mice with heart failure. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H431–H441. [Google Scholar] [CrossRef]
- Petkova-Kirova, P.S.; Gursoy, E.; Mehdi, H.; McTiernan, C.F.; London, B.; Salama, G. Electrical remodeling of cardiac myocytes from mice with heart failure due to the overexpression of tumor necrosis factor-alpha. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2098–H2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawada, H.; Niwano, S.; Niwano, H.; Yumoto, Y.; Wakisaka, Y.; Yuge, M.; Kawahara, K.; Izumi, T. Tumor necrosis factor-alpha downregulates the voltage gated outward K+ current in cultured neonatal rat cardiomyocytes: A possible cause of electrical remodeling in diseased hearts. Circ. J. 2006, 70, 605–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Velasco, M.; Ruiz-Hurtado, G.; Hurtado, O.; Moro, M.A.; Delgado, C. TNF-alpha downregulates transient outward potassium current in rat ventricular myocytes through iNOS overexpression and oxidant species generation. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H238–H245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wang, H.; Zhang, Y.; Gao, H.; Nattel, S.; Wang, Z. Impairment of HERG K(+) channel function by tumor necrosis factor-alpha: Role of reactive oxygen species as a mediator. J. Biol. Chem. 2004, 279, 13289–13292. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.H.; Rozanski, G.J. Effects of human recombinant interleukin-1 on electrical properties of guinea pig ventricular cells. Cardiovasc. Res. 1993, 27, 525–530. [Google Scholar] [CrossRef]
- Hagiwara, Y.; Miyoshi, S.; Fukuda, K.; Nishiyama, N.; Ikegami, Y.; Tanimoto, K.; Murata, M.; Takahashi, E.; Shimoda, K.; Hirano, T.; et al. SHP2-mediated signaling cascade through gp130 is essential for LIF-dependent I CaL, [Ca2+]i transient, and APD increase in cardiomyocytes. J. Mol. Cell. Cardiol. 2007, 43, 710–716. [Google Scholar] [CrossRef]
- Monnerat, G.; Alarcón, M.L.; Vasconcellos, L.R.; Hochman-Mendez, C.; Brasil, G.; Bassani, R.A.; Casis, O.; Malan, D.; Travassos, L.H.; Sepúlveda, M.; et al. Macrophage-dependent IL-1β production induces cardiac arrhythmias in diabetic mice. Nat. Commun. 2016, 7, 13344. [Google Scholar] [CrossRef] [Green Version]
- Aromolaran, A.S.; Srivastava, U.; Alí, A.; Chahine, M.; Lazaro, D.; El-Sherif, N.; Capecchi, P.L.; Laghi-Pasini, F.; Lazzerini, P.E.; Boutjdir, M. Interleukin-6 inhibition of hERG underlies risk for acquired long QT in cardiac and systemic inflammation. PLoS ONE 2018, 13, e0208321. [Google Scholar] [CrossRef] [Green Version]
- Pisoni, C.N.; Reina, S.; Arakaki, D.; Eimon, A.; Carrizo, C.; Borda, E. Elevated IL-1β levels in anti-Ro/SSA connective tissue diseases patients with prolonged corrected QTc interval. Clin. Exp. Rheumatol. 2015, 33, 715–720. [Google Scholar]
- Adlan, A.M.; Panoulas, V.F.; Smith, J.P.; Fisher, J.P.; Kitas, G.D. Association between corrected QT interval and inflammatory cytokines in rheumatoid arthritis. J. Rheumatol. 2015, 42, 421–428. [Google Scholar] [CrossRef]
- Lazzerini, P.E.; Laghi-Pasini, F.; Bertolozzi, I.; Morozzi, G.; Lorenzini, S.; Simpatico, A.; Selvi, E.; Bacarelli, M.R.; Finizola, F.; Vanni, F.; et al. Systemic inflammation as a novel QT-prolonging risk factor in patients with torsades de pointes. Heart 2017, 103, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, P.E.; Acampa, M.; Capecchi, P.L.; Fineschi, I.; Selvi, E.; Moscadelli, V.; Zimbone, S.; Gentile, D.; Galeazzi, M.; Laghi-Pasini, F. Antiarrhythmic potential of anticytokine therapy in rheumatoid arthritis: Tocilizumab reduces corrected QT interval by controlling systemic inflammation. Arthritis Care Res. (Hoboken) 2015, 67, 332–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, H.; Kobayashi, Y.; Yokoe, I.; Kitamura, N.; Nishiwaki, A.; Takei, M.; Giles, J.T. Heart Rate-corrected QT Interval Duration in Rheumatoid Arthritis and Its Reduction with Treatment with the Interleukin 6 Inhibitor Tocilizumab. J. Rheumatol. 2018, 45, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Engel, T.; Ben-Horin, S.; Beer-Gabel, M. Autonomic Dysfunction Correlates with Clinical and Inflammatory Activity in Patients with Crohn’s Disease. Inflamm. Bowel Dis. 2015, 21, 2320–2326. [Google Scholar] [CrossRef] [PubMed]
- Aarestrup, J.; Jess, T.; Kobylecki, C.J.; Nordestgaard, B.G.; Allin, K.H. Cardiovascular Risk Profile Among Patients With Inflammatory Bowel Disease: A Population-based Study of More Than 100 000 Individuals. J. Crohns Colitis. 2019, 13, 319–323. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, N.M.; Wang, L.; Herren, A.W.; Wang, J.; Shenasa, F.; Bers, D.M.; Lindsey, M.L.; Ripplinger, C.M. Atherosclerosis exacerbates arrhythmia following myocardial infarction: Role of myocardial inflammation. Heart Rhythm. 2015, 12, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Patterson, E.; Huang, S.; Garrett, M.W.; Kem, D.C. Tumor necrosis factor alpha, rapid ventricular tachyarrhythmias, and infarct size in canine models of myocardial infarction. J. Cardiovasc. Pharmacol. 2005, 45, 153–159. [Google Scholar] [CrossRef]
- De Jesus, N.M.; Wang, L.; Lai, J.; Rigor, R.R.; Stuart, S.D.; Bers, D.M.; Lindsey, M.L.; Ripplinger, C.M. Antiarrhythmic effects of interleukin 1 inhibition after myocardial infarction. Heart Rhythm. 2017, 14, 727–736. [Google Scholar] [CrossRef] [Green Version]
- Roubille, C.; Richer, V.; Starnino, T.; McCourt, C.; McFarlane, A.; Fleming, P.; Siu, S.; Kraft, J.; Lynde, C.; Pope, J.; et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 480–489. [Google Scholar] [CrossRef]
- Meek, I.L.; Vonkeman, H.E.; van de Laar, M.A. Cardiovascular case fatality in rheumatoid arthritis is decreasing; first prospective analysis of a current low disease activity rheumatoid arthritis cohort and review of the literature. BMC Musculoskelet. Disord. 2014, 15, 142. [Google Scholar] [CrossRef] [Green Version]
- Daperno, M.; Armuzzi, A.; Danese, S.; Fries, W.; Liguori, G.; Orlando, A.; Papi, C.; Principi, M.; Rizzello, F.; Viscido, A.; et al. Unmet Medical Needs in the Management of Ulcerative Colitis: Results of an Italian Delphi Consensus. Gastroenterol. Res. Pract. 2019, 2019, 3108025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frieri, G.; Galletti, B.; Di Ruscio, M.; Tittoni, R.; Capannolo, A.; Serva, D.; Latella, G.; Sollima, L.; Leocata, P.; Necozione, S.; et al. The prognostic value of histology in ulcerative colitis in clinical remission with mesalazine. Therap. Adv. Gastroenterol. 2017, 10, 749–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viscido, A.; Papi, C.; Latella, G.; Frieri, G. Has infliximab influenced the course and prognosis of acute severe ulcerative colitis? Biologics 2019, 13, 23–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorino, G.; Caprioli, F.; Daperno, M.; Mocciaro, F.; Principi, M.; Viscido, A.; Fantini, M.C.; Orlando, A.; Papi, C.; Annese, V.; et al. Use of biosimilars in inflammatory bowel disease: A position update of the Italian Group for the Study of Inflammatory Bowel Disease (IG-IBD). Dig. Liver Dis. 2019, 51, 632–639. [Google Scholar] [CrossRef]
- Michalak, A.; Mosińska, P.; Fichna, J. Common links between metabolic syndrome and inflammatory bowel disease: Current overview and future perspectives. Pharmacol. Rep. 2016, 68, 837–846. [Google Scholar] [CrossRef]
- Ciccone, A.; Gabrieli, D.; Cardinale, R.; Di Ruscio, M.; Vernia, F.; Stefanelli, G.; Necozione, S.; Melideo, D.; Viscido, A.; Frieri, G.; et al. Metabolic Alterations in Celiac Disease Occurring after Following a Gluten-Free Diet. Digestion 2019, 100, 262–268. [Google Scholar] [CrossRef]
- Verdugo-Meza, A.; Ye, J.; Dadlani, H.; Ghosh, S.; Gibson, D.L. Connecting the Dots Between Inflammatory Bowel Disease and Metabolic Syndrome: A Focus on Gut-Derived Metabolites. Nutrients 2020, 12, 1434. [Google Scholar] [CrossRef]



| Characteristic | N (%) |
|---|---|
| Gender (female/male) | 20/18 |
| Age (mean, years ± SD) | 46 ± 11 |
| Disease duration (mean, years ± SD) | 12 ± 8 |
| Ulcerative colitis | 26 (68.4) |
| Pancolitis | 10 (38.5) |
| Left-colitis | 13 (50.0) |
| Proctitis | 3 (11.5) |
| Crohn’s disease | 12 (31.6) |
| Ileo-colonic | 9 (75.0) |
| Ileal | 3 (25.0) |
| Active disease | 9 (23.7) |
| Remission | 29 (76.3) |
| Sustained | 19 (65.5) |
| Short-term | 10 (34.5) |
| Therapy ^ | |
| Mesalazine | 29 (76.3) |
| Steroids | 6 (15.8) |
| Thiopurines | 4 (10.5) |
| Biologics | 9 (23.7) * |
| CRP (mean, mg/dL ± SD) | 1.11 ± 1.41 |
| QTc (mean, ms ± SD) | 417.579 ± 22.055 |
| Active IBD (9 pts) | Remission (29 pts) | p | |
|---|---|---|---|
| CRP (mean, mg/dL ± SD) | 3.000 ± 1.888 | 0.524 ± 1.887 | 0.0045 |
| QTc (mean, ms ± SD) | 435.111 ± 27.306 | 412.138 ± 17.328 | 0.0047 |
| Study | IBD Pts/Controls N° | QTc Findings | Clinical Activity |
|---|---|---|---|
| Curione, 2010 [27] | 20/18 (no comorbidities) | Mean QTc significantly higher in IBD vs. controls | Not evaluated |
| Dogan, 2011 [28] | 69/38 (no comorbidities) | Mean QTc similar between IBD and controls | Active disease in all patients |
| Yorulmaz, 2013 [29] | 104/47 (no comorbidities) | QTc dispersion significantly higher in IBD vs. controls | Not specified |
| Pattanshetty, 2016 [30] | 142/- (>50% with comorbidities) | Prolonged QTc interval in 46.5% of IBD patients | Not evaluated |
| Bornaun, 2017 [31] | 36/36 (pediatric) | Mean QTc min significantly different between IBD and controls | Clinical remission in all patients |
| Acar, 2019 [32] | 100/100 (no comorbidities) | Mean QTc similar between IBD and controls | Clinical remission in all patients |
| Erolu, 2020 [33] | 25/20 (pediatric) | QTc dispersion higher in IBD vs. controls (especially in UC) | Clinical remission in all patients |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viscido, A.; Capannolo, A.; Petroni, R.; Stefanelli, G.; Zerboni, G.; De Martinis, M.; Necozione, S.; Penco, M.; Frieri, G.; Latella, G.; et al. Association between Corrected QT Interval and C-Reactive Protein in Patients with Inflammatory Bowel Diseases. Medicina 2020, 56, 382. https://doi.org/10.3390/medicina56080382
Viscido A, Capannolo A, Petroni R, Stefanelli G, Zerboni G, De Martinis M, Necozione S, Penco M, Frieri G, Latella G, et al. Association between Corrected QT Interval and C-Reactive Protein in Patients with Inflammatory Bowel Diseases. Medicina. 2020; 56(8):382. https://doi.org/10.3390/medicina56080382
Chicago/Turabian StyleViscido, Angelo, Annalisa Capannolo, Renata Petroni, Gianpiero Stefanelli, Giulia Zerboni, Massimo De Martinis, Stefano Necozione, Maria Penco, Giuseppe Frieri, Giovanni Latella, and et al. 2020. "Association between Corrected QT Interval and C-Reactive Protein in Patients with Inflammatory Bowel Diseases" Medicina 56, no. 8: 382. https://doi.org/10.3390/medicina56080382





