Abstract
Background and Objectives: An effective flushing technique is essential to reduce intravenous (IV)-related complications and improve patient care. New technology should contribute to such improvements, while reducing costs and increasing care efficiency. This study evaluated the efficacy, safety, and convenience of a new flushing technique using a Baro Flush™ controller. Materials and Methods: We evaluated the efficacy and safety of Baro Flush™ by measuring the infusion flushing volume and pressure in vitro. Afterwards, we prospectively enrolled 3000 patients with flushing and assigned 1500 patients with a new technique for flushing and 1500 with a conventional flushing method, which was performed by 48 registered nurses (RNs) at the Gil Medical Center in June 2018. The efficacy, safety, and convenience of the new flushing method were evaluated though a questionnaire survey. Results: The average flushing pressure was 12.5 ± 0.6 psi (86.18 ± 4.14 kPa) with 1.2 ± 0.2 mL per flush, as recommended by the Centers for Disease Control and Prevention based on 85 experiments. No IV-catheter-related complications were reported by the RNs during the study. More than 80% of the RNs reported that the new flushing method was easier to learn, improved care efficacy, and was more convenient than conventional flushing. Conclusions: The new flushing method using a Baro Flush™ controller showed improved efficacy, safety, and convenience compared with the conventional flushing method, and no IV-catheter-related complications occurred, including occlusion and inflammation. The new flushing method promises to reduce IV-catheter-related complications and shows improved efficacy, safety, and convenience.
1. Introduction
Venous cannulation via a central or peripheral intravenous (IV) catheter is one of the most frequently performed health care procedures, with up to 80% of all hospitalized patients requiring medication, nutrition, or transfusion through IV catheters [1,2,3]. Although IV catheter insertion, and maintenance are common procedures, studies have reported that IV-catheter-related complication rates range from 2% to 80%, where complications include phlebitis (inflammation of the vein wall), occlusions (blockage), infiltration/extravasation (fluid moving into the third compartment), and, rarely, infections [2,4,5,6].
According to the Centers for Disease Control and Prevention (CDC), it is important to flush the catheter under positive pressure to reduce IV-catheter-related complications, maintain patency, and prevent the mixing of incompatible medications [4,7,8,9]. Although there is no consensus on the optimal frequency of catheter flushing [7], the Royal College of Nursing recommends flushing more than two times a day [10] to prevent peripheral IV-catheter-related complications [4,9,11,12]. Because each flushing procedure has to be done using aseptic techniques, IV-catheter flushing is a burden on registered nurses (RNs) and is also time consuming and expensive [11,13,14].
Conventional flushing methods usually involve several steps: preparing the flushing fluid (sterile 0.9% sodium chloride) in a 10 mL syringe; cleaning the access port with a single-use 70% alcohol-impregnated swab or 2% alcohol chlorhexidine thoroughly for at least 15 s, and allowing it to dry before accessing the system [7,10,15]. Often, physicians and RNs find it hard to follow these recommendations, since IV-catheter flushing techniques should be done with aseptic and complex procedures [13]. According to a recent report that examined the real-world practice of IV-catheter insertion and maintenance, more than 15% of clinicians did not follow the recommendations [16,17,18]. Therefore, simple, convenient, infection-free techniques are needed.
Flushing with an aseptic non-touch technique using a prefilled syringe was introduced as an alternative to conventional methods; although it eliminated preparation of the flushing fluid, it still needed several complex steps [19]. Positive- or negative-pressure mechanical valves with needleless connectors were also introduced. However, owing to the increased rates of IV-catheter-related infection, the CDC recommended against physicians using these methods [20,21].
Therefore, we evaluated the efficacy, convenience, and safety of the new Baro Flush™ flushing method in a prospective study population.
2. Methods
2.1. Characteristics of the New Flushing Method Using the Baro Flush™ Controller
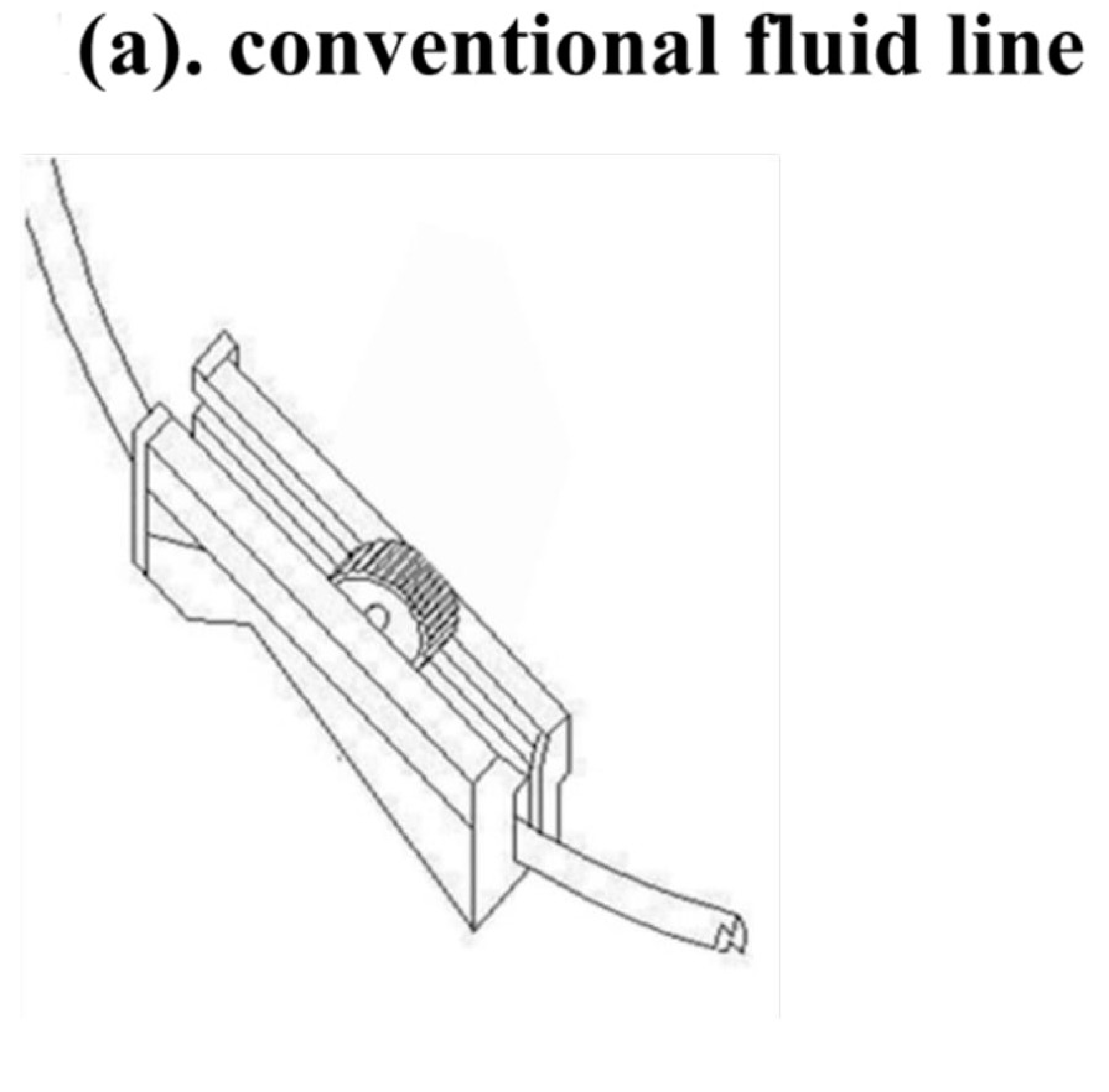
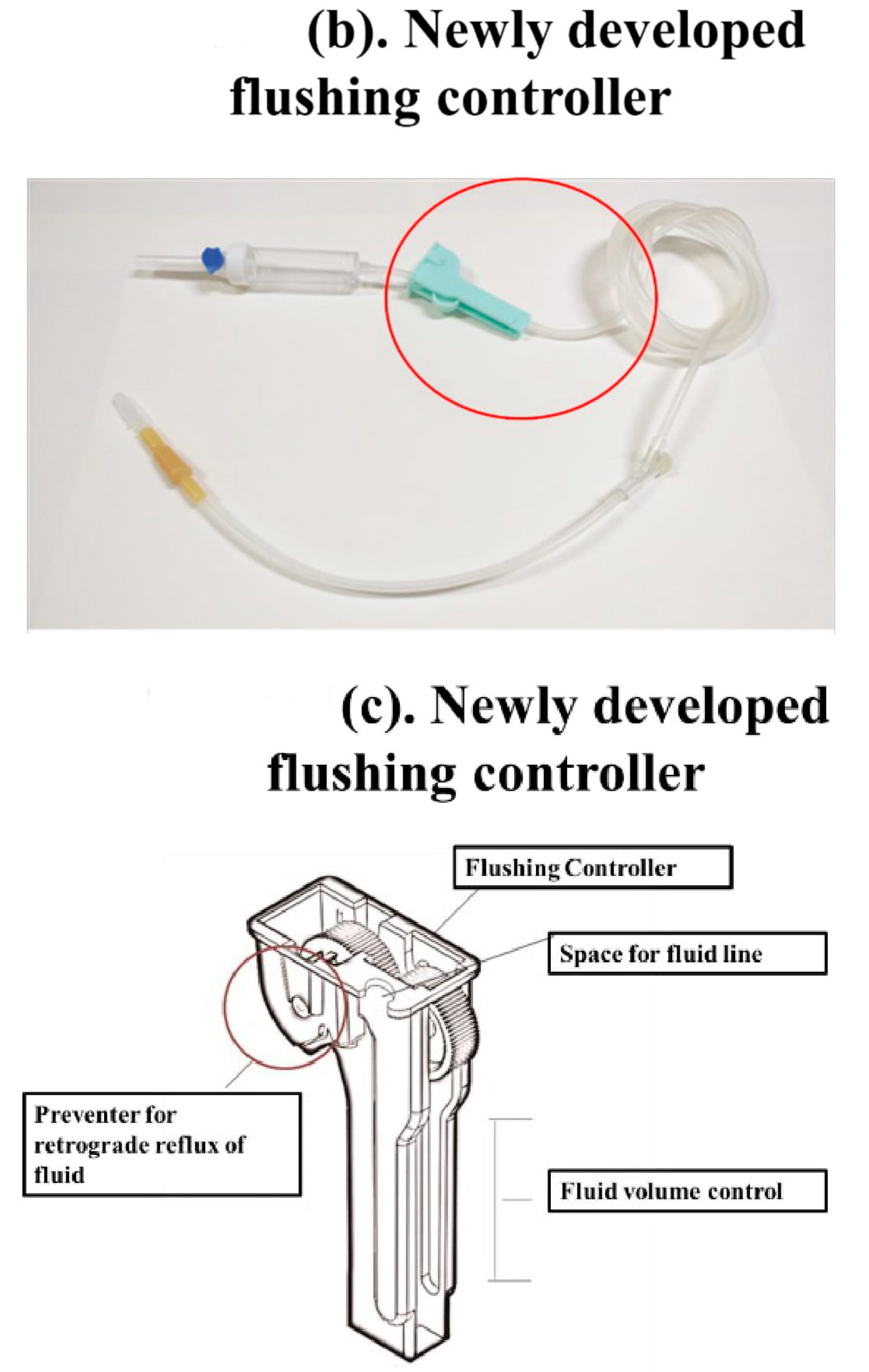
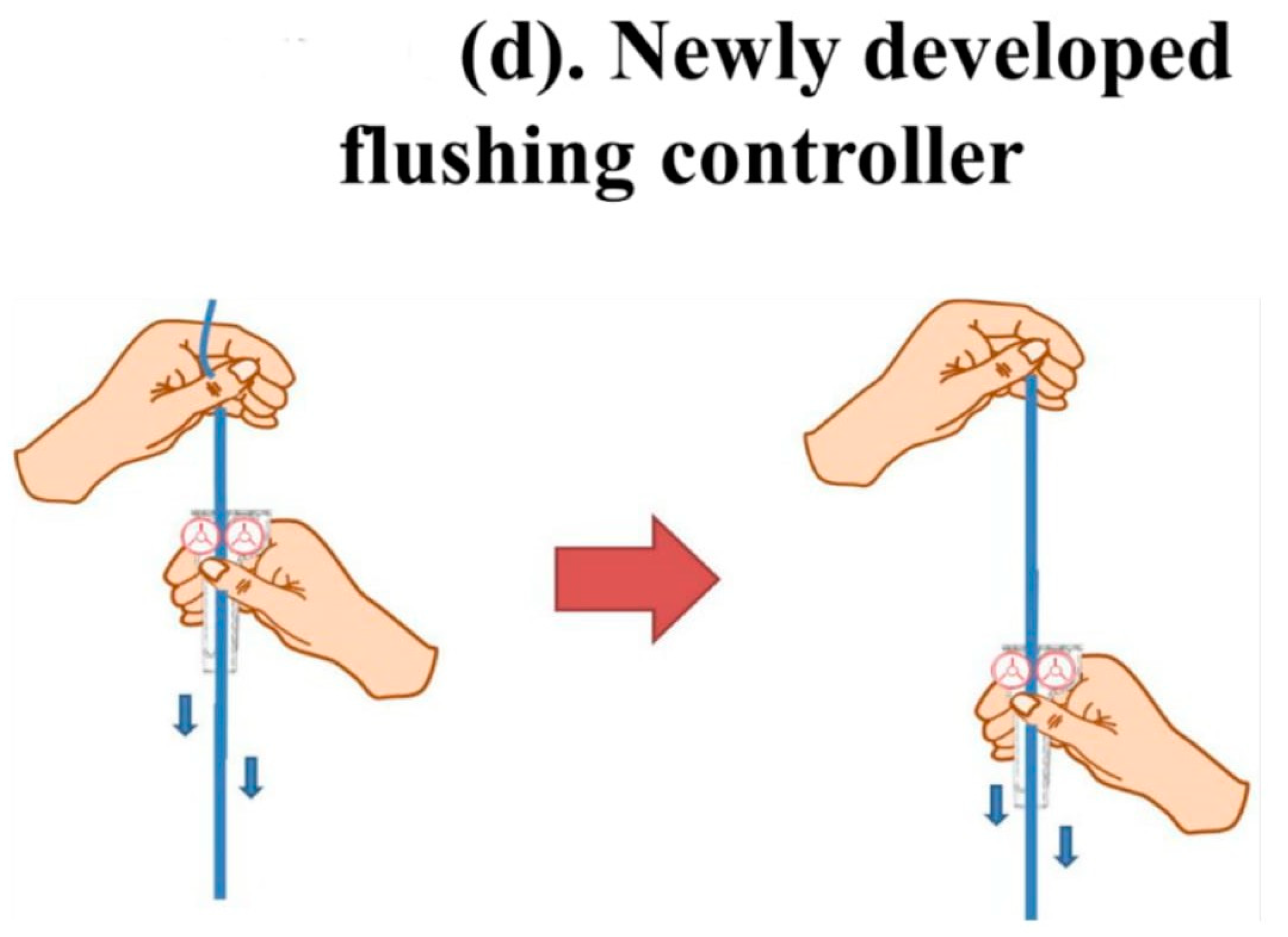
The new flushing set is a modification of a conventional IV set, especially the fluid volume controller. To manipulate the flushing controller, the flushing controller is pulled along a conventional fluid line and down and up twice to generate sufficient flushing volume and pressure (Figure 1).
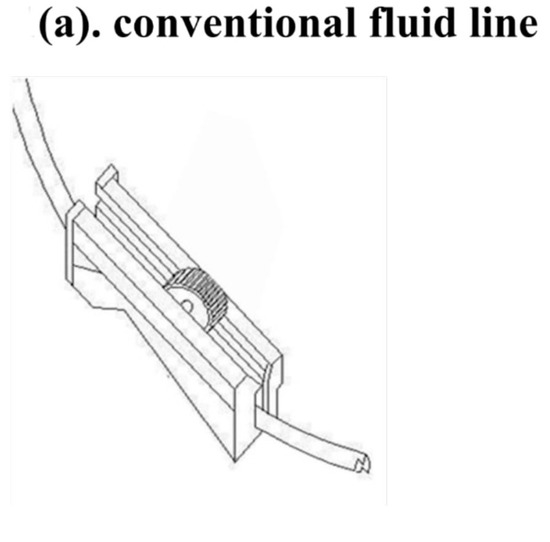
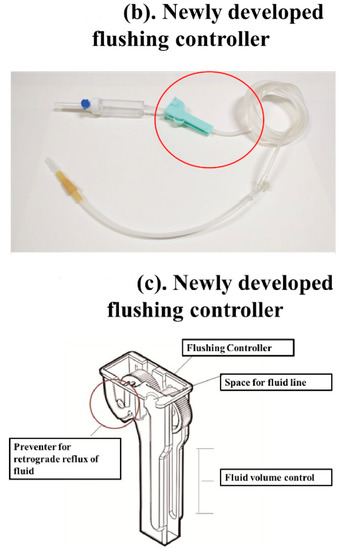
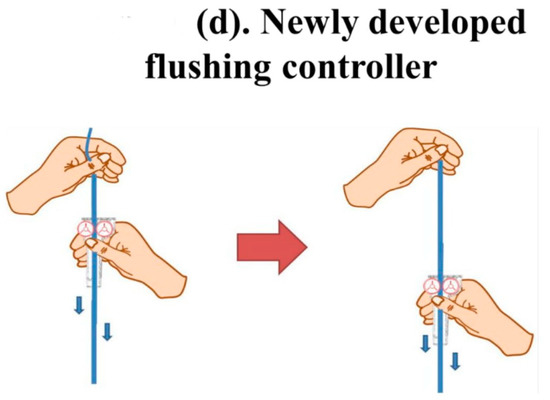
Figure 1.
The new Baro Flush™ flushing controller. (a) Conventional fluid line. (b) The Baro Flush™ controller. (c) Illustration of Baro Flush™, and (d) operation of Baro Flush™. The externally located Baro Flush™ flushing controller was modified from a conventional fluid volume controller and has the added function of flushing. To operate Baro Flush™, the controller is rolled down and up the intravenous (IV) line twice.
To evaluate efficacy, convenience, and safety of the new Baro Flush™, we conducted a study in two steps. In the first step, we conducted an in vitro study to find the proper flushing volume and pressure before applying it directly into patients. In the second step, we prospectively enrolled 3000 patients who were admitted into the Gil Medical Center (GMC) and assigned into 1500 patients with the conventional flushing method and 1500 patients with the new flushing technique.
2.2. Efficacy and Safety of the New Flushing Method Using the Controller
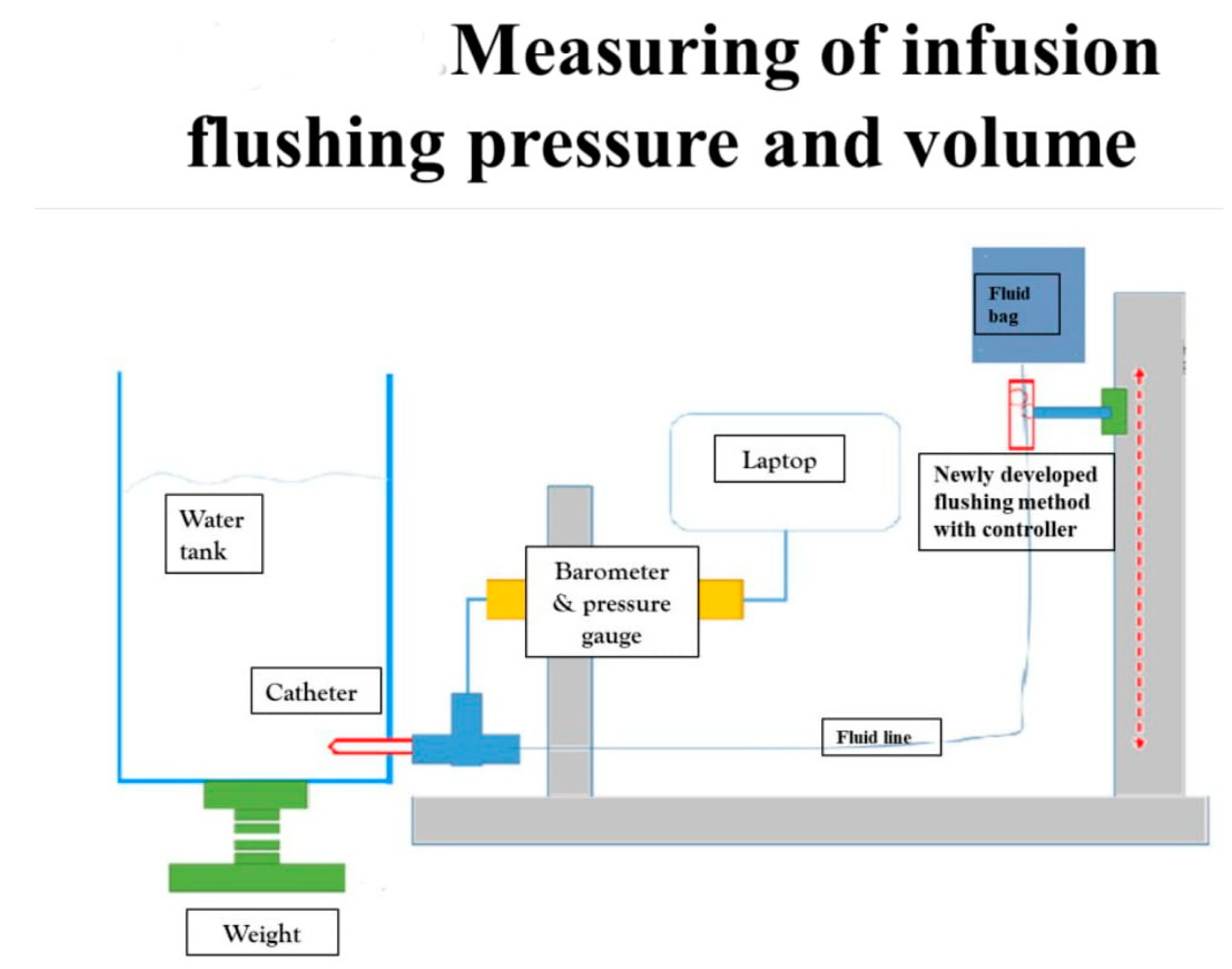
We evaluated the efficacy and safety of the new Baro Flush™ flushing method by measuring the infusion flushing pressure and volume in vitro. CDC guidelines recommend an infusion pressure of less than 25 psi and a minimum flushing volume of 2 mL for one flush [7,8,22]. To measure the flushing pressure using Baro Flush™, we connected a barometer directly to the system. To measure the flushing volume, we devised the experiment shown in Figure 2. The experiment involved (1) a water tank, (2) a fluid bag supplying water to the water tank, (3) a pressure gauge, and (4) connecting fluid lines. We weighed the water in the tank to calculate the flushing volume.
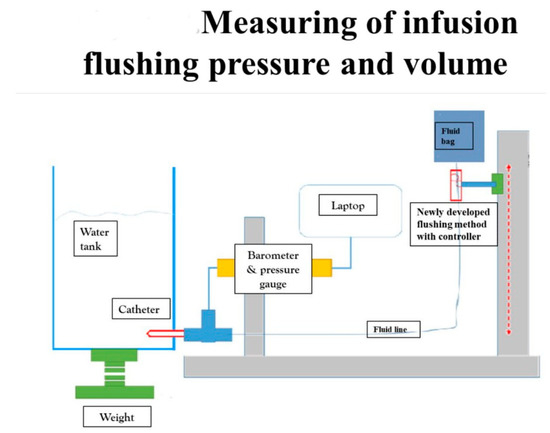
Figure 2.
Measuring the infusion flushing pressure and volume. To measure the flushing pressure with our new flushing method, we used (1) a water tank, (2) fluid bag, (3) barometer, and (4) connecting fluid lines. Using our new flushing controller, we measured the flushing volume and pressure on the water tank with the pressure gauge.
We repeated this experiment 85 times by having 13 RNs and four physicians perform the experiment five times each.
2.3. Assessing the Learnability, Effectiveness, and Convenience of the New Method
We prospectively enrolled 48 RNs at the GMC who performed 3000 cases of IV-catheter flushes in June 2018: 1500 cases (1500 patients) using the Baro Flush™ and 1500 cases (1500 patients) using the conventional method.
We prospectively enrolled 3000 consecutive patients who were admitted into the GMC during the study period, who agreed to be enrolled in this study, and who signed into written consent.
The inclusion criteria for registered nurses is as below: First, they had to agree to participate in this study and sign written informed consent. Second, they had to work as registered nurses in the ward in the GMC. Third, they had to have experience using flushing sets for patients. Fourth, they had to have experience flushing for patients. The inclusion criteria for enrolled patients was as follows: they were supposed to have an intravenous catheter more than 48 h after admission.
Exclusion criteria for enrolled patients and registered nurses were as follows: first, those who refused to participate in this study; second, those who were not supposed to have an intravenous catheter in less than 48 h. Since Baro FlushTM was made for any patient who uses an intravenous catheter, we did not set any other exclusion criteria for this study except for the aforementioned one to avoid allocation bias.
Using a questionnaire, the 48 RNs evaluated the efficacy, convenience, and safety of the new, and conventional flushing methods. The questionnaire included four items: (1) learnability, (2) efficacy, (3) convenience, and (4) safety. Complications of the new flushing technique using the controller were closely monitored by qualified RNs.
We monitored patients with flushing-related complications during the admission period and more than one week after discharge.
2.4. Statistical Analysis
The surveys consisted of close-ended multiple-choice questions and hybrid questions (i.e., rate the importance or provide numbers), which are summarized descriptively in proportion with their 95% confidence intervals. The nonparametric Mann–Whitney U test was used for the analysis. Categorical variables were reported as a percentage and analyzed using the chi-square or Fisher’s exact test. A p-value ≤ 0.05 was deemed to be significant. All statistical analysis was performed using SPSS software (ver. 20.0; SPSS Inc., Chicago, IL, USA).
2.5. Ethics
The study design, survey protocol, and related documentation were reviewed and approved by the GMC Research Ethics Board (GMC IRB number: GBIRB2017-124).
3. Result
3.1. Efficacy and Safety of the New Flushing Method Using the Baro Flush™ Controller
Using Baro Flush™, the average ± standard deviation of flushing infusion pressure was 12.5 ± 0.6 psi, (86.18 ± 4.14 kPa), and the mean flushing volume was 1.2 ± 0.2 mL per flush based on 85 experiments.
No case of occlusion or phlebitis was reported in either group during the study period.
3.2. The Learnability, Effectiveness, and Convenience of the New Method Assessed by RNs
Regarding the convenience of Baro Flush™, 70.0% (n = 33) of the participants indicated that the new method had good to excellent convenience compared with the conventional method (Figure S1a,b, Table 1 and Table 2). Regarding learnability, 100% (n = 48) of the participants indicated that the new method was easier to learn and use than the conventional flushing method (Figure S1a,b, Table 2). Moreover, more than 95% of the participants indicated that the new method improved the quality, safety, and speed of flushing.

Table 1.
Questionnaire survey used to assess the learnability, effectiveness, and convenience of the new Baro Flush™ method used by registered nurses (RNs).

Table 2.
Questionnaire comparing Baro Flush™ with the conventional flushing method used by RNs (n = 48).
4. Discussion
This study evaluated a new flushing method using the Baro Flush™ controller without the need for flushing syringes and evaluated its efficacy, convenience, and safety compared with the conventional flushing technique. The new flushing method reduced the risk of IV-catheter-related irritation and infection compared with the conventional method. Moreover, the learnability, efficacy, and convenience of the new method were higher than for the conventional method (p < 0.01). Therefore, this method is a promising alternative to the conventional flushing method.
There were several differences between our newly developed flushing device and the conventional IV infusion pump system. First, our newly developed flushing device is to be used for effective flushing not to be used for continuous drug infusion. While the conventional device, the IV infusion pump, is generally used for continuous drug infusion or transfusion, our newly developed device [23,24], the Baro Flush™, is to be used for effective flushing with proper pressure and volume. Baro Flush™ is made for effective and convenient flushing in a simple way, especially when patients have to keep their intravenous catheter for long-term infusion with proper scheduled flushing. Second, the conventional IV infusion pump is so expensive that it is impossible to apply an IV infusion pump to all patients. However, the Baro Flush™ is a simple but effective device. In this regard, our new device is somewhat different from automated IV infusion pumps.
There have been several attempts to overcome the pitfalls of conventional flushing methods. Flushing with prefilled syringes and positive- or negative-pressure mechanical valve needleless connectors has been introduced in clinical practice. When using prefilled syringes, RNs and clinicians do not need to prepare the flushing syringes [19]. Otherwise, there is no difference from the conventional method. Moreover, considering cost-effectiveness, flushing with a prefilled syringe is expensive [19]. A recent report on real-world IV-catheter management found that RNs and clinicians do not follow the recommendation “one needle, one syringe, and only one time” [16]. Moreover, a recent observational study found that only 25% of RNs performed handwashing or gloving before or after an injection [13]. Therefore, more convenient aseptic techniques for real-world situations are needed. Another alternative method, i.e., the use of positive- or negative-pressure mechanical valve needleless connectors, has been withdrawn due to an increased risk of bloodstream infections.
As an alternative, we evaluated an external flushing controller, the Baro Flush™, which is a modified version of the conventional fluid volume controller with the added function of flushing. The Baro Flush™ procedure consists of pulling the controller down and up along the IV line twice. Consequently, no aseptic procedures are needed for managing the controller, and no flushing syringes or accessing ports are required. This simplified technique is easy to learn and use and is effective. RNs reported that the new method was superior to the conventional method in terms of learnability, efficacy, convenience, and safety.
The average flushing pressure using Baro Flush™ in 85 experiments was 12.5 psi with 1.2 mL per flush. According to CDC guidelines [7], the infusion pressure should never exceed 25 psi to prevent damage to blood vessels, and a minimum flushing volume of 2 mL of flushing solution is considered to be sufficient. Therefore, the CDC guidelines recommend using a 10-mL syringe with 2 mL of flushing fluid for conventional flushing. Because the Baro Flush™ method provides the recommended flushing pressure and volume, it is safe for use. During our study, there were no complications, such as peripheral IV-catheter occlusion, inflammation, or thrombophlebitis using the new flushing method in 1500 cases. Our flushing method is simple and easy to learn compared with conventional flushing methods, which should reduce the error rate. In addition, our flushing method does not require the use of gloves, syringes, or other costly items.
Our study had several limitations. Although 3000 cases of flushing were examined, the study was conducted in a single center. Therefore, a larger study population in a multicenter randomized controlled trial is needed. Second, we monitored complications only for up to 7 days. However, IV-catheter-related complications usually occur within 7 days [7].
5. Conclusions
In conclusion, the new flushing method using a flushing controller is a promising alternative to the conventional flushing method in terms of efficacy, safety, and convenience of use to prevent IV-catheter-related complications.
Supplementary Materials
The following are available online at https://www.mdpi.com/1648-9144/56/8/393/s1, Figure S1: Questionnaire survey for assessing the learnability, effectiveness, and convenience of the new Baro Flush™ method by registered nurses (RNs).
Author Contributions
Y.I.C., D.K.P., and Y.J.K. contributed to study concept and design, acquisition analysis and interpretation of data, drafting of the manuscript, and obtaining funding. J.H.C., J.-W.C., and K.O.K. contributed to study analysis and interpretation of data and critically revised the manuscript. K.A.K. and H.Y.C. contributed to the study design and critically revised the manuscript. All authors read and approved the final manuscript. The authors do not declare any conflict of interest.
Funding
This work was supported by technology innovation program (10085750) funded by Ministry of Trade, Industry& Energy (MOTIE, Korea).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zingg, W.; Pittet, D. Peripheral venous catheters: An under-evaluated problem. Int. J. Antimicrob. Agents 2009, 34, S38–S42. [Google Scholar] [CrossRef]
- Devrim, I.; Oruc, Y.; Demirağ, B.; Kara, A.; Düzgöl, M.; Uslu, S.; Yasar, N.; Köker, S.A.; Töret, E.; Bayram, N.; et al. Central line bundle for prevention of central line–associated bloodstream infection for totally implantable venous access devices (ports) in pediatric cancer patients. J. Vasc. Access 2018, 19, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Moureau, N.L. Vessel Health and Preservation: The Right Approach for Vascular Access; Springer Nature: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Marsh, N.; Webster, J.; Mihala, G.; Rickard, C. Devices and dressings to secure peripheral venous catheters to prevent complications. Cochrane Database Syst. Rev. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Abolfotouh, M.; Salam, M.; Mustafa, A.B.; White, D.; Balkhy, H.H. Prospective study of incidence and predictors of peripheral intravenous catheter-induced complications. Ther. Clin. Risk Manag. 2014, 10, 993–1001. [Google Scholar] [CrossRef]
- Ruiz-Giardín, J.; Chamorro, I.O.; Ríos, L.V.; Aroca, J.J.; Arata, I.G.; López, J.V.S.; Santillán, M.G. Blood stream infections associated with central and peripheral venous catheters. BMC Infect. Dis. 2019, 19, 841. [Google Scholar] [CrossRef]
- Garrett, J.H., Jr. A Review of the CDC Recommendations for Prevention of HAIs in Outpatient Settings. AORN J. 2015, 101, 519–528. [Google Scholar] [CrossRef]
- Conway, M.A.; McCollom, C.; Bannon, C. Central Venous Catheter Flushing Recommendations. J. Pediatr. Oncol. Nurs. 2014, 31, 185–190. [Google Scholar] [CrossRef]
- Kleidon, T.; Keogh, S.; Flynn, J.; Schults, J.; Mihala, G.; Rickard, C.M. Flushing of peripheral intravenous catheters: A pilot, factorial, randomised controlled trial of high versus low frequency and volume in paediatrics. J. Paediatr. Child Heal. 2020, 56, 22–29. [Google Scholar] [CrossRef]
- Mihala, G.; Ray-Barruel, G.; Chopra, V.; Webster, J.; Wallis, M.; Marsh, N.; McGrail, M.R.; Rickard, C.M. Phlebitis Signs and Symptoms with Peripheral Intravenous Catheters. J. Infus. Nurs. 2018, 41, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Goossens, G. Flushing and Locking of Venous Catheters: Available Evidence and Evidence Deficit. Nurs. Res. Pr. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rickard, C.M.; Webster, J.; Wallis, M.; Marsh, N.; McGrail, M.R.; French, V.; Foster, L.; Gallagher, P.; Gowardman, J.R.; Zhang, L.; et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: A randomised controlled equivalence trial. Lancet 2012, 380, 1066–1074. [Google Scholar] [CrossRef]
- Al-Rawajfa, O.M.; Tubaishat, A. A concealed observational study of infection control and safe injection practices in Jordanian governmental hospitals. Am. J. Infect. Control. 2017, 45, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Carr, P.J.; Rippey, J.C.; Cooke, M.; Higgins, N.; Trevenen, M.; Foale, A.; Rickard, C.M. From insertion to removal: A multicenter survival analysis of an admitted cohort with peripheral intravenous catheters inserted in the emergency department. Infect. Control Hosp. Epidemiol. 2018, 39, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, V.D.; Maki, D.G.; Rodrigues, C.; Álvarez-Moreno, C.; Leblebicioglu, H.; Sobreyra-Oropeza, M.; Berba, R.; Madani, N.; Medeiros, E.A.; Cuéllar, L.E.; et al. Impact of International Nosocomial Infection Control Consortium (INICC) Strategy on Central Line–Associated Bloodstream Infection Rates in the Intensive Care Units of 15 Developing Countries. Infect. Control. Hosp. Epidemiol. 2010, 31, 1264–1272. [Google Scholar] [CrossRef]
- Kossover-Smith, R.A.; Coutts, K.; Hatfield, K.; Cochran, R.; Akselrod, H.; Schaefer, M.K.; Perz, J.F.; Bruss, K. One needle, one syringe, only one time? A survey of physician and nurse knowledge, attitudes, and practices around injection safety. Am. J. Infect. Control. 2017, 45, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Sacks, G.D.; Diggs, B.S.; Hadjizacharia, P.; Green, N.; Salim, A.; Malinoski, D.J. Reducing the rate of catheter-associated bloodstream infections in a surgical intensive care unit using the Institute for Healthcare Improvement central line bundle. Am. J. Surg. 2014, 207, 817–823. [Google Scholar] [CrossRef]
- Keogh, S.; Shelverton, C.; Flynn, J.; Davies, K.; Marsh, N.; Rickard, C. An observational study of nurses’ intravenous flush and medication practice in the clinical setting. Vasc. Access 2017, 3, 3–10. [Google Scholar]
- Gerçeker, G.Ö.; Sevgili, S.A.; Yardımcı, F. Impact of flushing with aseptic non-touch technique using pre-filled flush or manually prepared syringes on central venous catheter occlusion and bloodstream infections in pediatric hemato-oncology patients: A randomized controlled study. Eur. J. Oncol. Nurs. 2018, 33, 78–84. [Google Scholar]
- Sweet, M.A.; Cumpston, A.; Briggs, F.; Craig, M.; Hamadani, M. Impact of alcohol-impregnated port protectors and needleless neutral pressure connectors on central line–associated bloodstream infections and contamination of blood cultures in an inpatient oncology unit. Am. J. Infect. Control. 2012, 40, 931–934. [Google Scholar] [CrossRef]
- Btaiche, I.F.; Kovacevich, D.S.; Khalidi, N.; Papke, L.F. The effects of needleless connectors on catheter-related bloodstream infections. Am. J. Infect. Control. 2011, 39, 277–283. [Google Scholar] [CrossRef]
- Lee, O.K.E.; Johnston, L. A Systematic Review for Effective Management of Central Venous Catheters and Catheter Sites in Acute Care Paediatric Patients. Worldviews Evid.-Based Nurs. 2005, 2, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Dadpour, B.; Vahabzadeh, M.; Mostafazadeh, B. Comparison of the efficacy of an infusion pump or standard IV push injection to deliver naloxone in treatment of opioid toxicity. Acute Crit. Care 2020, 35, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chan, F.-Y.; Or, C.K.; Sun, D.T.-F.; Lai, W.-S.; So, H.-Y. Heuristic evaluation and simulated use testing of infusion pumps to inform pump selection. Int. J. Med Inform. 2019, 131, 103932. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).