In Vivo Biological Evaluation of Orthodontically Moved Incisors after Replantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Histomorphometric Analysis
- (a)
- Repair: small resorption areas repaired by cement neoformation;
- (b)
- Root resorption: inflammatory resorption areas with the presence of an inflammatory infiltrate in addition to the multinucleated cells; and
- (c)
- Ankylosis: deposition of bone tissue juxtaposed to the cementum layer.
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Adams, F.R. Traumatized and fractured young teeth. JADA 1944, 31, 41–248. [Google Scholar] [CrossRef]
- Matos, F.S.; Godolphim, F.J.; Correia, A.M.O.; Junior, R.L.C.A.; Paranhos, L.R.; Rode, S.M.; Ribeiro, M.A.G. Effect of laser photobiomodulation on the periodontal repair process of replanted teeth. Dent. Traumatol. 2016, 32, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, J.O. Challenges in clinical dental traumatology. Endod. Dent. Traumatol. 1985, 1, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, J.O.; Lovschall, H. Response of Oral Tissues to Trauma. In Textbook and Color Atlas of Traumatic Injuries to the Teeth, 4th ed.; Andreasen, J.O., Andreasen, F.M., Andersson, L., Eds.; Blackwell/Munksgaard: Oxford, UK, 2007; pp. 217–254. [Google Scholar]
- Hasanuddin, S.; Sharada Reddy, J. Sequelae of delayed replantation of maxillary permanent incisors after avulsion: A case series with 24-month follow-up and clinical review. J. Indian Soc. Pedod. Prev. Dent. 2018, 36, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Krug, R.; Kremeier, K.; Krastl, G. Long-term retention of avulsed maxillary permanent incisors replantes after prolonged non-physiological storage. Dent. Traumatol. 2019, 35, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Ruizhen, F.; Siyi, L.; Lei, G.; Li’an, W. Decoronation management of the replacement resorption after delayed replantation of avulsed teeth—Case report with 4-year follow-up. Hua Xi Kou Qiang Yi Xue Za Zhi 2017, 35, 665–669. [Google Scholar] [PubMed]
- Gonçalves, P.S.P.; Ionta, F.Q.; Rios, D.; Oliveira, D.S.B.; Couto-Filho, C.E.G.; Honório, H.M. Reimplantation of na avulsed mature permanente toorh after 6 days: A 1-year follow-up. Gen. Dent. 2018, 66, 71–75. [Google Scholar]
- Mackie, I.C. The avulsed tooth. Br. Dent. J. 1987, 163, 359. [Google Scholar] [CrossRef]
- Walia, T.; Chandwani, N. Long-term management of an ankylosed young permanent incisor replanted within 2 h of avulsion: A case with a 10-year follow-up. J. Indian Soc. Pedod. Prev. Dent. 2019, 37, 99–106. [Google Scholar] [CrossRef]
- Järvinen, S. Incisal overjet and traumatic injuries to upper permanent incisors. A retrospective study. Acta Odontol. Scand. 1978, 36, 359–362. [Google Scholar] [CrossRef]
- Brin, I.; Ben-Bassat, Y.; Heling, I.; Brezniak, N. Profile of an orthodontic patient at risk of dental trauma. Endod. Dent. Traumatol. 2000, 16, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Burden, D.J. An investigation of the association between overjet size, lip coverage and traumatic injury to maxillary incisors. Eur. J. Orthod. 1995, 17, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, C.M.; Tedestam, G. Etiological and predisposing factors related to traumatic injuries to permanent teeth. Swed. Dent. J. 1993, 17, 183–190. [Google Scholar] [PubMed]
- Petti, S.; Tarsitani, G. Traumatic injuries to anterior teeth in Italian schoolchildren: Prevalence and risk factors. Endod. Dent. Traumatol. 1996, 12, 294–297. [Google Scholar] [CrossRef]
- Antunes, L.A.; Gomes, I.F.; Almeida, H.M.; Silva, E.A.; Calasans-Maia, J.A.; Antunes, L.S. Increased overjet is a risk factor for dental trauma in preschool children. Indian J. Dent. Res. 2015, 26, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Arrai, G.P.; Rossi-Fedele, G.; Dogramaci, E.J. The association of overjet size and traumatic dental injuries—A systematic review and meta-analysis. Dent. Traumatol. 2019, 35, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Malmgren, O.; Goldson, L.; Hill, C.; Orwin, A.; Petrini, L.; Lundberg, M. Root resorption after orthodontic treatment of traumatized teeth. Am. J. Orthod. 1982, 82, 487–491. [Google Scholar] [CrossRef]
- Abrams, R.A. Replantation of an avulsed incisor: Case report. Quintessence Int. Dent. Dig. 1978, 9, 85–87. [Google Scholar]
- Abrams, R.A. Tooth replantation: 11-year follow up. Aust. Dent. J. 1987, 6, 427–429. [Google Scholar] [CrossRef]
- Myers, H.I.; Nassimben, L.; Alley, J.; Gehsig, J. Replantation of teeth in the hamster. Oral Surg. 1954, 7, 116–1129. [Google Scholar] [CrossRef]
- Skoglund, A. Pulpal changes in replanted and autotransplanted apicoectomized mature teeth of dogs. Int. J. Oral Surg. 1981, 10, 111–121. [Google Scholar] [CrossRef]
- Andreasen, J.O.; Kristerson, L. The effect of extra-alveolar root filling with calcium hydroxide on periodontal healing after replantation of permanent incisors in monkeys. J. Endod. 1981, 7, 349–353. [Google Scholar] [CrossRef]
- Blomlöf, L.; Lindskog, S.; Andersson, L.; Hedström, K.G.; Hammarström, L. Storage of experimentally avulsed teeth in milk prior to replantation. J. Dent. Res. 1983, 62, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Brown, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Vet. Clin. Pathol. 2012, 41, 24–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.J.; Clutton, R.E.; Lilley, E.; Hansen, K.E.A.; Brattelid, T. PREPARE: Guidelines for planning animal research and testing. Lab Anim. 2018, 52, 135–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 302–306. [Google Scholar] [CrossRef] [Green Version]
- Soma, L.R. Anesthetic and analgesic considerations in the experimental animal. In The Role of Animals in Biomedical Research; Annals of New York Academy Science: New York, NY, USA, 1983; Volume 406, pp. 32–47. [Google Scholar]
- Okamoto, T. Reimplante de dentes de crescimento contínuo após ressecção da papila dental e órgão do esmalte. Estudo Histológico. Rev. Ass. Paul. Cirurg. Dent. 1976, 30, 382–395. [Google Scholar]
- Okamoto, T.; Niccoli-Filho, W.; Sonode, C.K.; Martins, A.P.; Souza, R. Immediate replantation of maxillary incisors in rats: Effects of tooth immersion in sodium fluoride and subsequent removal of the periodontal ligament. Braz. Dent. J. 1999, 10, 73–79. [Google Scholar]
- Saito, C.T.M.H.; Guinelli, J.L.; Panzarini, S.R.; Garcia, V.G.; Okamoto, R.; Okamoto, T.; Sonoda, C.K.; Poi, W.R. Effect of low-level laser therapy on the healing process after tooth replantation: A histomorphometrical and immunohistochemical analysis. Dent. Traumatol. 2011, 27, 30–39. [Google Scholar] [CrossRef]
- Andreasen, J.O. Experimental dental traumatology: Development of a model for external root resorption. Endod. Dent. Traumatol. 1987, 3, 269–287. [Google Scholar] [CrossRef]
- Barbizam, J.V.B.; Massarwa, R.; Silva, L.A.B.; Silva, R.A.B.; Nelson-Filho, P.; Consolaro, A.; Cohenca, N. Histophatological evaluation of the effects of variable extraoral dry times and enamel matrix proteins (Enamel Matrix Derivatives) application on replanted dog’s teeth. Dent. Traumatol. 2015, 31, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, C.; Cohenca, N.; Romano, F.; Pucinelli, C.M.; Cohenca, N.; Romero, M.; Lucisano, M.P.; Silva, R.A.B.; Silva, L.A.B. The effect of immediate controlled force on periodontal healing of teeth replanted after short dry time in dogs. Dent. Traumatol. 2018, 34, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Brin, I.; Ben-Bassat, Y.; Heling, I.; Engelberg, A. The influence of orthodontics treatment on previously traumatized permanent incisors. Eur. J. Orthod. 1991, 13, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Malmgren, O.; Malmgren, B.; Goldson, L. Orthodontic management of the traumatized dentition. In Textbook and Color Atlas of Traumatic Injuries to the Teeth, 4th ed.; Andreasen, J.O., Andreasen, F.M., Andersson, L., Eds.; Blackwell Munksgaard: Oxford, UK, 2007; pp. 669–715. [Google Scholar]
- Bauss, O.; Freitag, S.; Röhling, J.; Rahman, A. Influence of orthodontic intrusion on pulpal vitality of previously traumatized maxillary permanent incisors. Am. J. Orthod. Dentofac. Orthop. 2008, 134, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Kindelan, S.A.; Day, P.F.; Kindelan, J.D.; Spencer, J.R.; Duggal, M.S. Dental trauma: An overview of its influence on the management of orthodontic treatment. Part 1. J. Orthod. 2008, 35, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Levander, E.; Malmgren, O. Evaluation of the risk of root resorption during orthodontic treatment: A study of upper incisors. Eur. J. Orthod. 1988, 10, 30–38. [Google Scholar] [CrossRef]
- Tondelli, P.M.; Mendonça, M.R.; Cuoghi, O.A.; Pereira, A.L.P.; Busato, M.C.A. Knowledge on dental trauma and orthodontic tooth movement held by a group of orthodontists. Braz. Oral Res. 2010, 24, 76–82. [Google Scholar] [CrossRef] [Green Version]
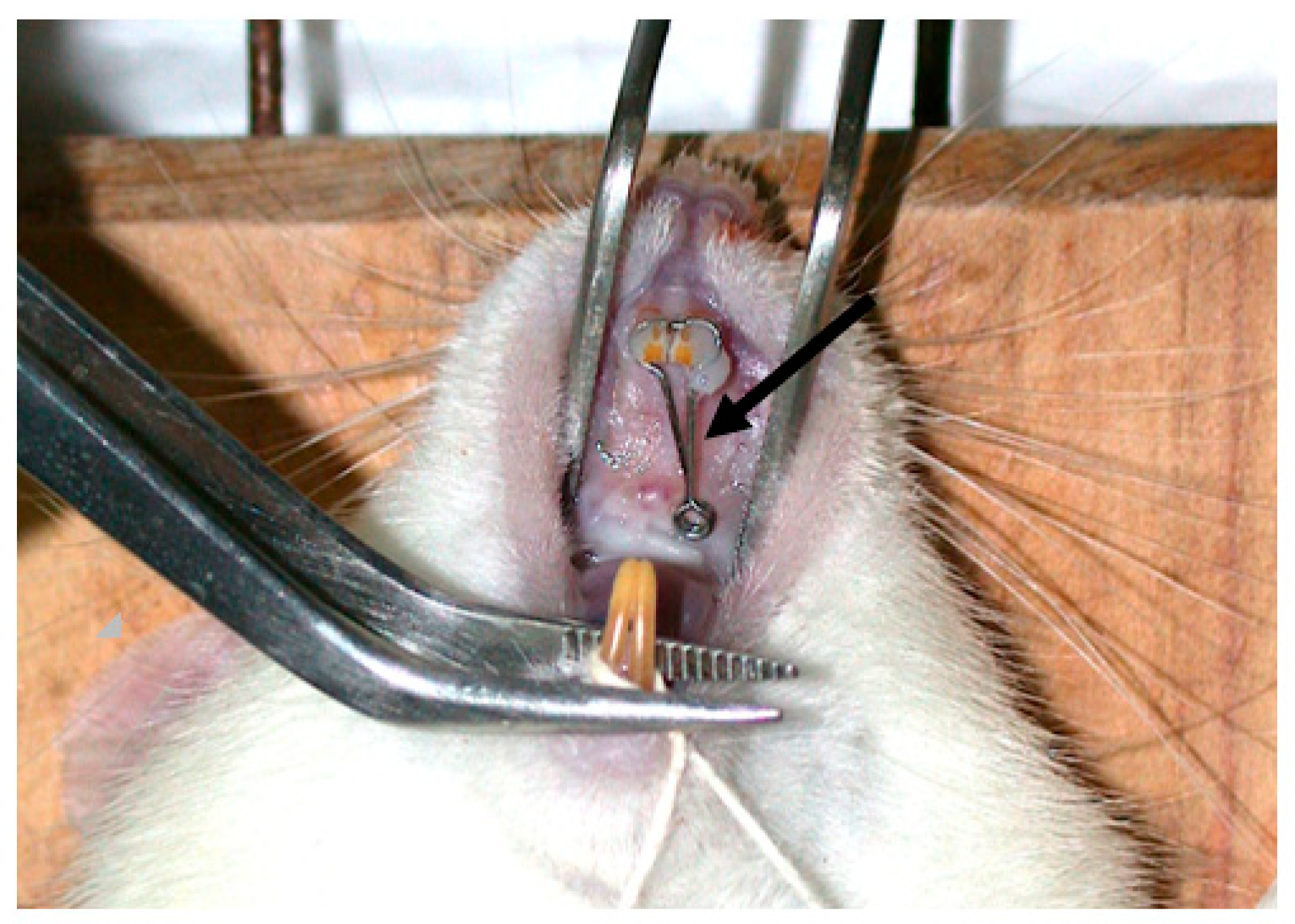

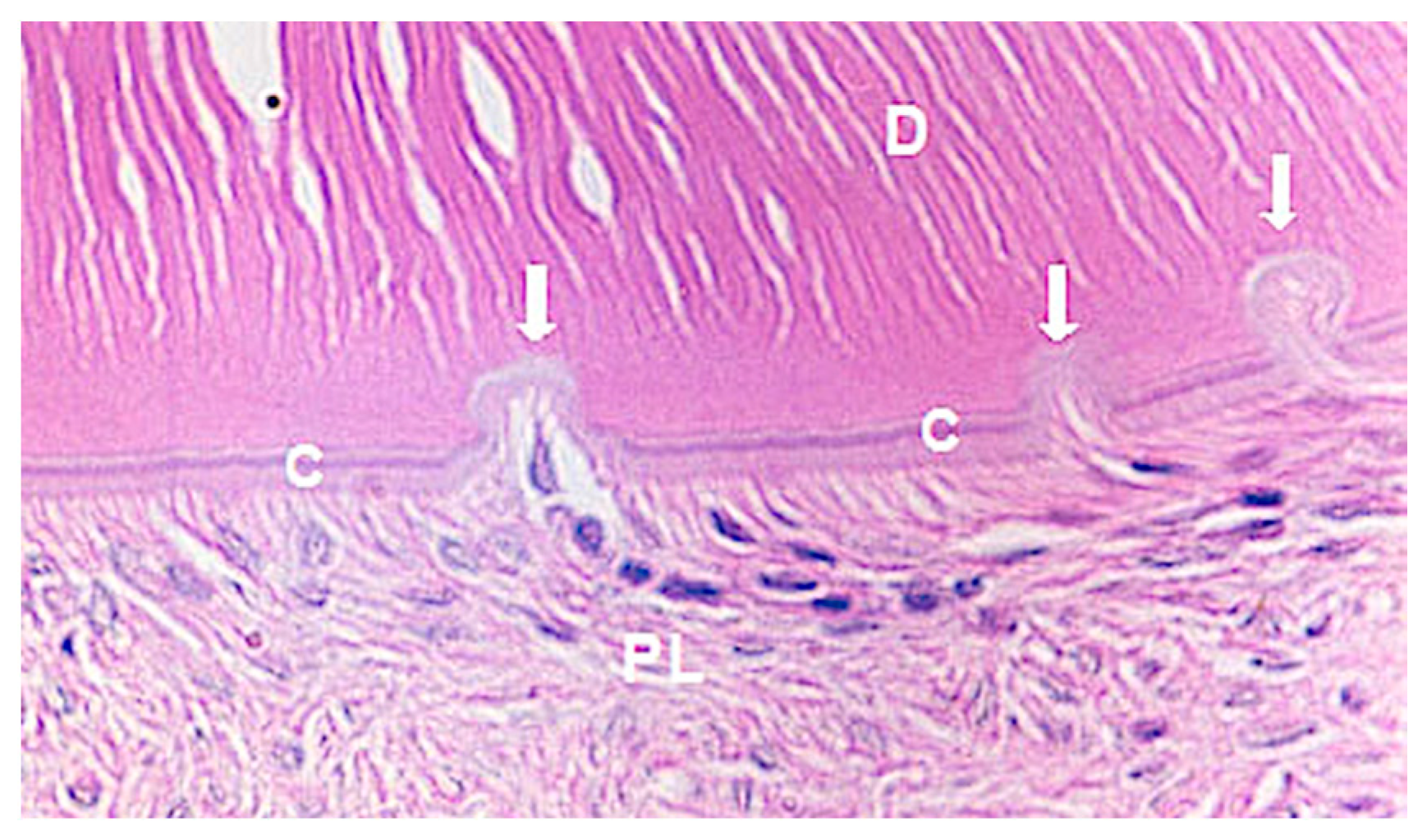

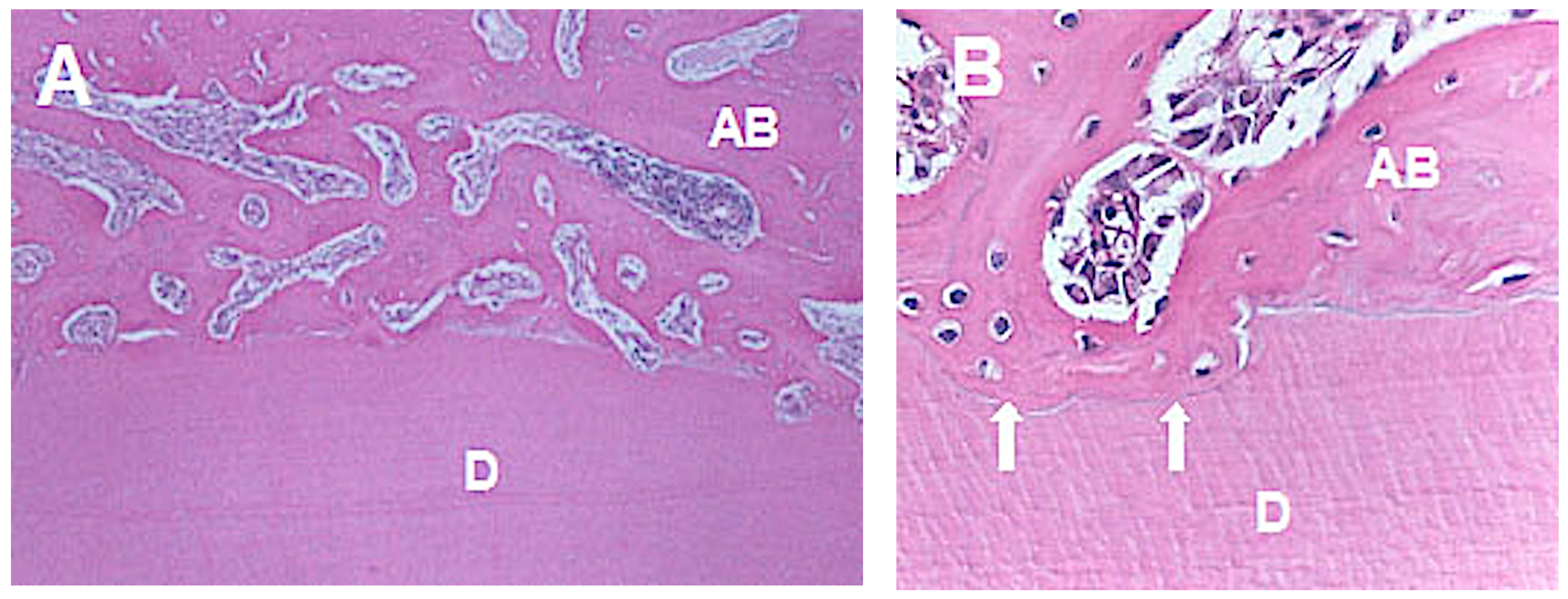
| Groups | n | Orthodontic Movement | Euthanasia |
|---|---|---|---|
| 1 | 15 | ---- | 30 days after onset of experiment |
| 2 | 15 | 30 days after reimplantation | 7 days after orthodontic movement |
| 3 | 15 | ---- | 60 days after onset of experiment |
| 4 | 15 | 60 days after reimplantation | 7 days after onset of orthodontic movement |
| Groups | Total | Histological Evaluation of the Pressure Side | ||||||
|---|---|---|---|---|---|---|---|---|
| Repair | Root Resorption | Ankylosis | ||||||
| n | % | n | % | n | % | n | % | |
| 1 (30 days-without movement) | 45 | 100 | 15 | 33.3 | 30 | 66.7 | 0 | 0.0 |
| 2 (30 days-with movement) | 45 | 100 | 0 | 0.0 | 36 | 80.0 | 9 | 20.0 |
| 3 (60 days-without movement) | 45 | 100 | 33 | 73.3 | 0 | 0.0 | 12 | 26.7 |
| 4 (60 days-with movement) | 45 | 100 | 0 | 0.0 | 28 | 62.2 | 17 | 37.8 |
| Total | 180 | 100 | 48 | 26.7 | 94 | 52.2 | 38 | 21.1 |
| Groups | Total | Histological Evaluation in the Tension Side | ||||||
|---|---|---|---|---|---|---|---|---|
| Repair | Root Resorption | Ankylosis | ||||||
| n | % | n | % | n | % | n | % | |
| 1 (30 days-without movement) | 45 | 100 | 14 | 31.1 | 31 | 68.9 | 0 | 0.0 |
| 2 (30 days-with movement) | 45 | 100 | 0 | 0.0 | 29 | 64.4 | 16 | 35.6 |
| 3 (60 days-without movement) | 45 | 100 | 31 | 68.9 | 0 | 0.0 | 14 | 31.1 |
| 4 (60 days-with movement) | 45 | 100 | 0 | 0.0 | 24 | 53.3 | 21 | 46.7 |
| Total | 180 | 100 | 45 | 25 | 84 | 46.7 | 51 | 28.3 |
| Post-Replantation Period | Histological Evaluation | Orthodontic Movement | Total | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | |||||||
| n | % | n | % | n | % | |||
| 30 days | Repair | 29 | 32.2 | 0 | 0.0 | 29 | 16.1 | <0.001 |
| No repair* | 61 | 67.8 | 90 | 100 | 151 | 83.9 | ||
| Total | 90 | 100 | 90 | 100 | 180 | 100 | ||
| 60 days | Repair | 64 | 71.1 | 0 | 0.0 | 64 | 35.6 | <0.001 |
| No repair* | 24 | 26.7 | 90 | 100 | 116 | 64.4 | ||
| Total | 90 | 100 | 90 | 100 | 180 | 100 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calasans-Maia, J.d.A.; Calasans-Maia, M.D.; Stuani, M.B.S.; Alves, A.T.N.N.; Montemezzi, P.; Mourão, C.F.d.A.B.; Cal-Neto, J.P.e.; Ruellas, A.C.d.O. In Vivo Biological Evaluation of Orthodontically Moved Incisors after Replantation. Medicina 2020, 56, 421. https://doi.org/10.3390/medicina56090421
Calasans-Maia JdA, Calasans-Maia MD, Stuani MBS, Alves ATNN, Montemezzi P, Mourão CFdAB, Cal-Neto JPe, Ruellas ACdO. In Vivo Biological Evaluation of Orthodontically Moved Incisors after Replantation. Medicina. 2020; 56(9):421. https://doi.org/10.3390/medicina56090421
Chicago/Turabian StyleCalasans-Maia, Jose de Albuquerque, Monica Diuana Calasans-Maia, Maria Bernadete Sasso Stuani, Adriana Terezinha Neves Novellino Alves, Pietro Montemezzi, Carlos Fernando de Almeida Barros Mourão, Julio Pedra e Cal-Neto, and Antonio Carlos de Oliviera Ruellas. 2020. "In Vivo Biological Evaluation of Orthodontically Moved Incisors after Replantation" Medicina 56, no. 9: 421. https://doi.org/10.3390/medicina56090421






