Changes in Muscle Mass and Composition by Exercise and Hypoxia as Assessed by DEXA in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mouse Models of Muscle Mass Loss and Gain
2.2. DEXA Acquisition
2.3. DEXA Analysis and Quantification
2.4. Calculation of Changes in Tissue Fat and Fat-Free Mass
2.5. Regulators of Muscle Growth and Atrophy
2.6. Statistical Analysis
3. Results
3.1. Changes in Body Weight with Free-Wheel Running and Hypoxia
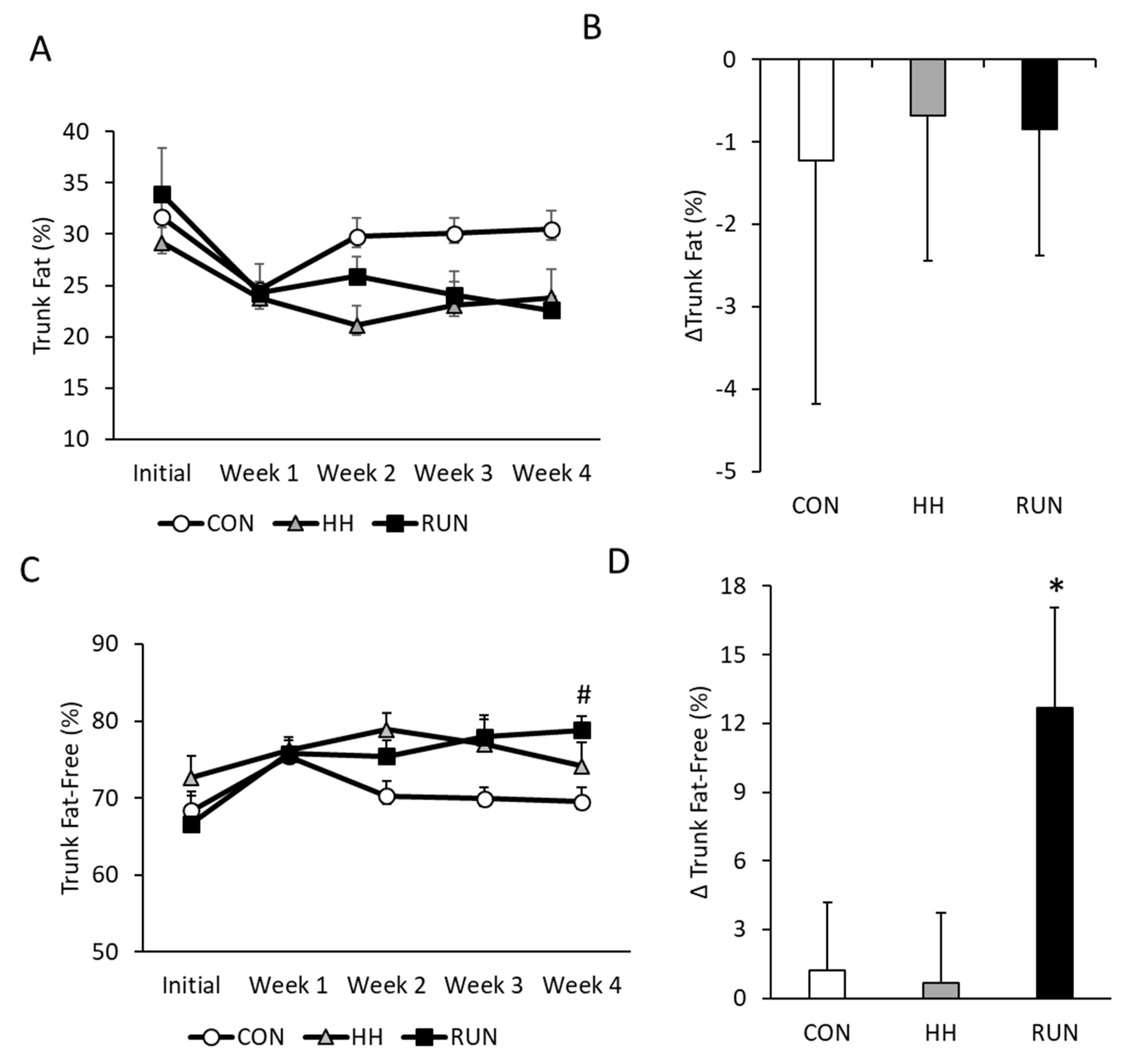
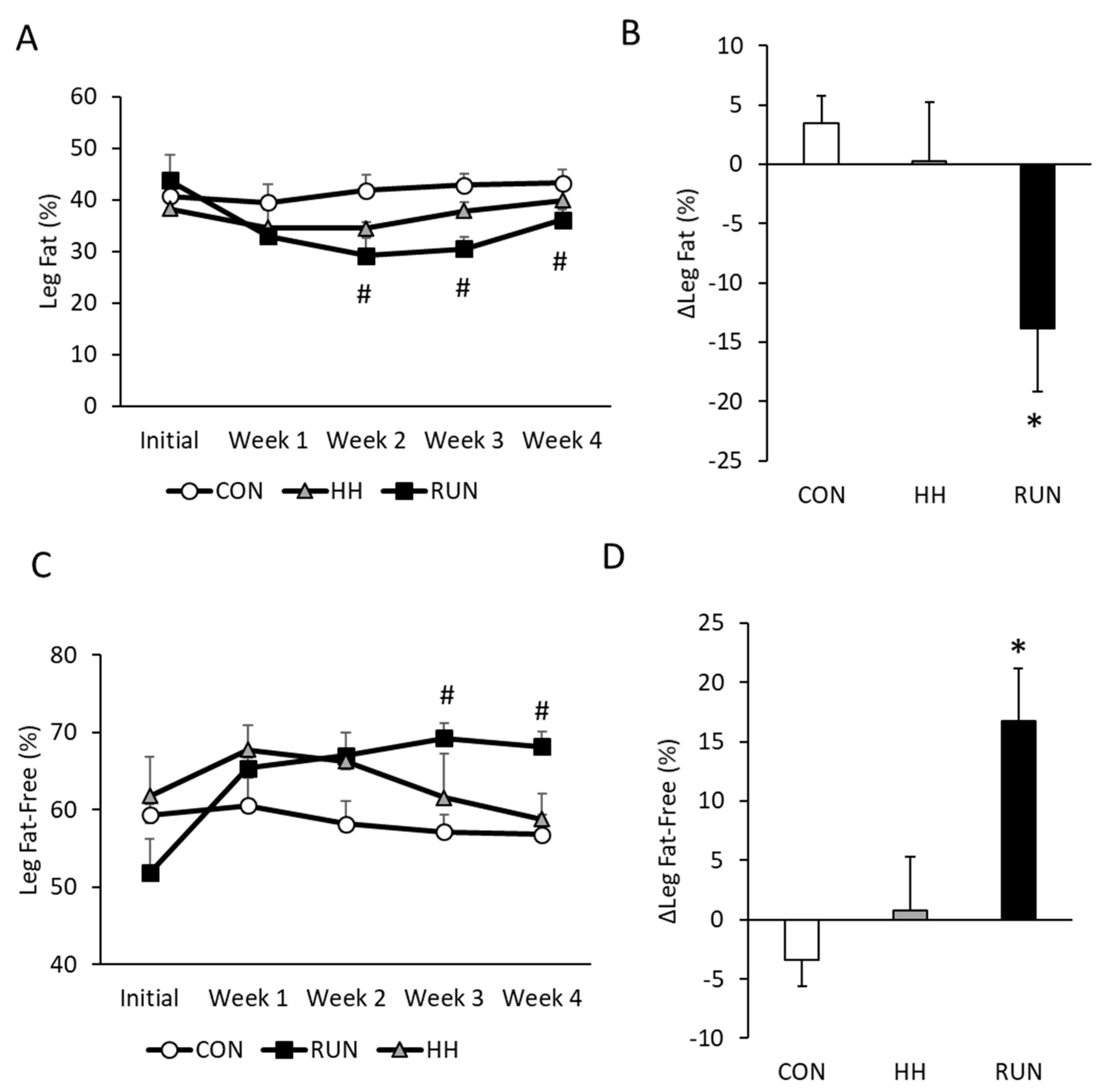
3.2. Temporal Changes in Trunk and Leg Fat by DEXA
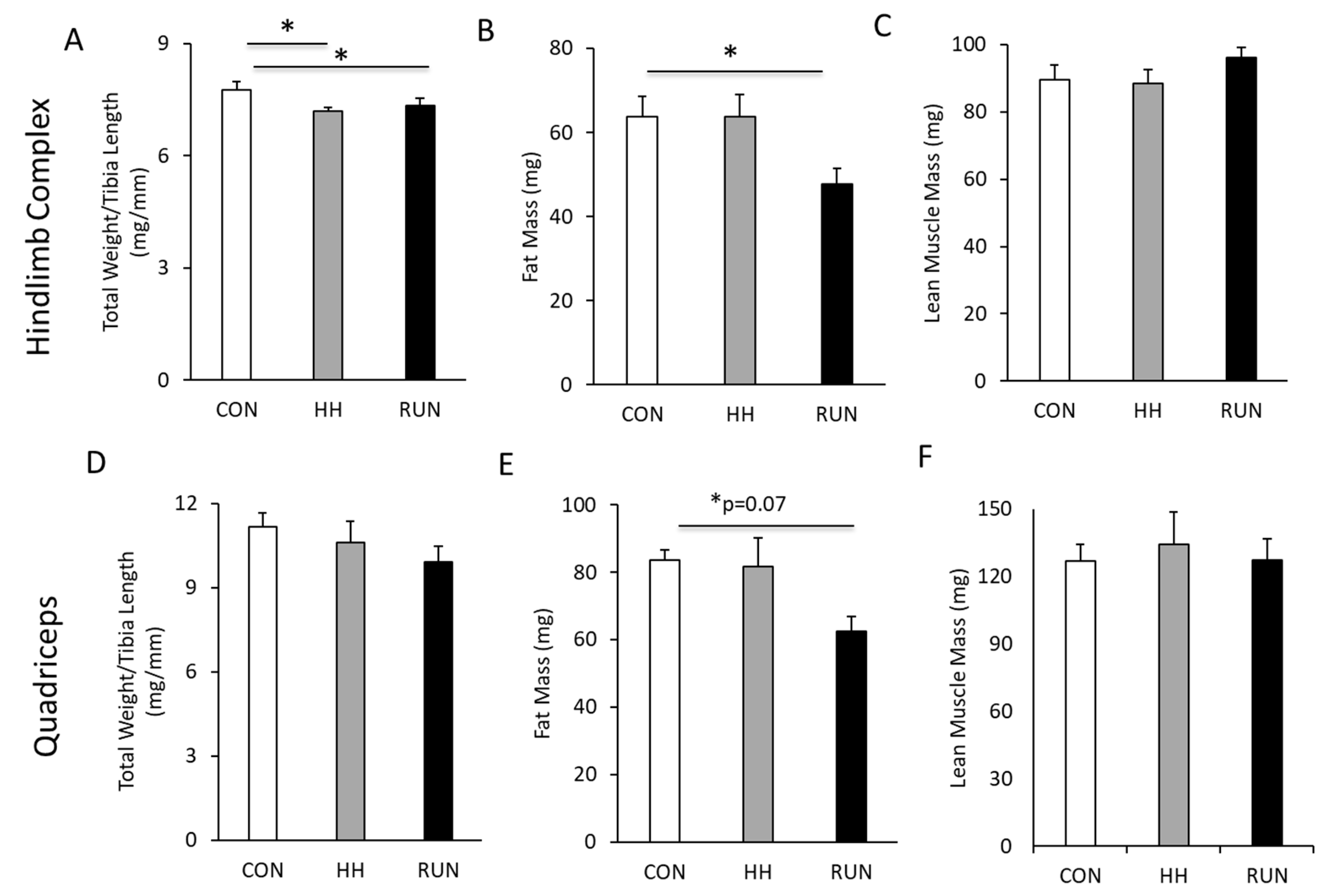
3.3. Hind Limb Complex and Quadriceps Mass, Fat-Free, and Fat-Mass
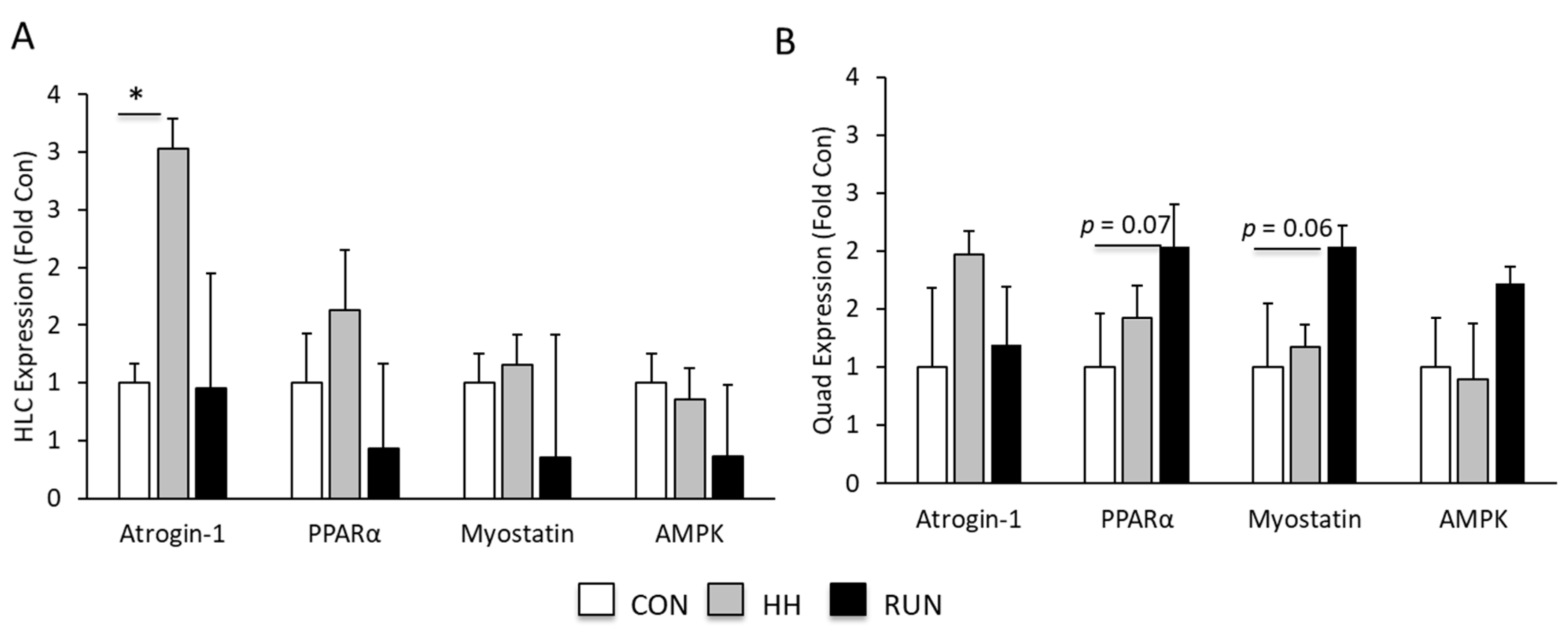
3.4. Molecular Mediators of Muscle Gains and Loss
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Garcia, J.M. Sarcopenia, cachexia and aging: Diagnosis, mechanisms and therapeutic options—A mini-review. Gerontology 2014, 60, 294–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anker, S.D.; Steinborn, W.; Strassburg, S. Cardiac cachexia. Ann. Med. 2004, 36, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.C.; Glass, D.J.; Guttridge, D.C. Cancer cachexia: Mediators, signaling, and metabolic pathways. Cell Metab. 2012, 16, 153–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dirks, M.L.; Wall, B.T.; van de Valk, B.; Holloway, T.M.; Holloway, G.P.; Chabowski, A.; Goossens, G.H.; van Loon, L.J. One Week of Bed Rest Leads to Substantial Muscle Atrophy and Induces Whole-Body Insulin Resistance in the Absence of Skeletal Muscle Lipid Accumulation. Diabetes 2016, 65, 2862–2875. [Google Scholar] [CrossRef] [Green Version]
- Fujita, Y.; Nakamura, Y.; Hiraoka, J.; Kobayashi, K.; Sakata, K.; Nagai, M.; Yanagawa, H. Physical-strength tests and mortality among visitors to health-promotion centers in Japan. J. Clin. Epidemiol. 1995, 48, 1349–1359. [Google Scholar] [CrossRef]
- Beaudart, C.; Zaaria, M.; Pasleau, F.; Reginster, J.Y.; Bruyere, O. Health Outcomes of Sarcopenia: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169548. [Google Scholar] [CrossRef] [Green Version]
- Laukkanen, P.; Heikkinen, E.; Kauppinen, M. Muscle strength and mobility as predictors of survival in 75-84-year-old people. Age Ageing 1995, 24, 468–473. [Google Scholar] [CrossRef]
- Borga, M.; West, J.; Bell, J.D.; Harvey, N.C.; Romu, T.; Heymsfield, S.B.; Dahlqvist Leinhard, O. Advanced body composition assessment: From body mass index to body composition profiling. J. Investig. Med. 2018, 66, 1–9. [Google Scholar] [CrossRef]
- Cartee, G.D.; Hepple, R.T.; Bamman, M.M.; Zierath, J.R. Exercise Promotes Healthy Aging of Skeletal Muscle. Cell Metab. 2016, 23, 1034–1047. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, M.; Hojo, Y.; Inoue, K.; Matsui, T.; Kawato, S.; McEwen, B.S.; Soya, H. Mild exercise increases dihydrotestosterone in hippocampus providing evidence for androgenic mediation of neurogenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 13100–13105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, R.L.; Jeon, J.Y.; Liu, F.F.; Maratos-Flier, E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E586–E594. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, S.B.; Phillips, S.M.; Atherton, P.J.; Patel, R.; Yarasheski, K.E.; Tarnopolsky, M.A.; Rennie, M.J. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J. Physiol. 2008, 586, 3701–3717. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.K.; Al-Khalili, L.; Guigas, B.; Leng, Y.; Garcia-Roves, P.M.; Krook, A. Role of AMP kinase and PPARdelta in the regulation of lipid and glucose metabolism in human skeletal muscle. J. Biol. Chem. 2007, 282, 19313–19320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petr, M.; Maciejewska-Skrendo, A.; Zajac, A.; Chycki, J.; Stastny, P. Association of Elite Sports Status with Gene Variants of Peroxisome Proliferator Activated Receptors and Their Transcriptional Coactivator. Int. J. Mol. Sci. 2019, 21, 162. [Google Scholar] [CrossRef] [Green Version]
- de Theije, C.; Costes, F.; Langen, R.C.; Pison, C.; Gosker, H.R. Hypoxia and muscle maintenance regulation: Implications for chronic respiratory disease. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 548–553. [Google Scholar] [CrossRef]
- Hoppeler, H.; Kleinert, E.; Schlegel, C.; Claassen, H.; Howald, H.; Kayar, S.R.; Cerretelli, P. Morphological adaptations of human skeletal muscle to chronic hypoxia. Int. J. Sports Med. 1990, 11 (Suppl. 1), S3–S9. [Google Scholar] [CrossRef]
- MacDougall, J.D.; Green, H.J.; Sutton, J.R.; Coates, G.; Cymerman, A.; Young, P.; Houston, C.S. Operation Everest II: Structural adaptations in skeletal muscle in response to extreme simulated altitude. Acta Physiol. Scand. 1991, 142, 421–427. [Google Scholar] [CrossRef]
- Sillau, A.H.; Banchero, N. Effects of hypoxia on capillary density and fiber composition in rat skeletal muscle. Pflug. Arch. 1977, 370, 227–232. [Google Scholar] [CrossRef]
- Hayot, M.; Rodriguez, J.; Vernus, B.; Carnac, G.; Jean, E.; Allen, D.; Goret, L.; Obert, P.; Candau, R.; Bonnieu, A. Myostatin up-regulation is associated with the skeletal muscle response to hypoxic stimuli. Mol. Cell Endocrinol. 2011, 332, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Torres, M.; Montserrat, J.M.; Pavia, J.; Dalmases, M.; Ros, D.; Fernandez, Y.; Barbe, F.; Navajas, D.; Farre, R. Chronic intermittent hypoxia preserves bone density in a mouse model of sleep apnea. Respir. Physiol. Neurobiol. 2013, 189, 646–648. [Google Scholar] [CrossRef]
- Friedman, M.A.; Szczepankiewicz, R.P.; Kohn, D.H. Combined mineral-supplemented diet and exercise increases bone mass and strength after eight weeks and maintains increases after eight weeks detraining in adult mice. PLoS ONE 2018, 13, e0204470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konopka, A.R.; Harber, M.P. Skeletal muscle hypertrophy after aerobic exercise training. Exerc. Sport Sci. Rev. 2014, 42, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.H.; Oliver, S.J.; Hillyer, K.; Sanders, S.; Smith, Z.; Williams, C.; Yates, D.; Ginnever, H.; Scanlon, E.; Roberts, E.; et al. Body composition at high altitude: A randomized placebo-controlled trial of dietary carbohydrate supplementation. Am. J. Clin. Nutr. 2009, 90, 1193–1202. [Google Scholar] [CrossRef] [Green Version]
- Bartling, B.; Al-Robaiy, S.; Lehnich, H.; Binder, L.; Hiebl, B.; Simm, A. Sex-related differences in the wheel-running activity of mice decline with increasing age. Exp. Gerontol. 2017, 87, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Bohuslavova, R.; Kolar, F.; Kuthanova, L.; Neckar, J.; Tichopad, A.; Pavlinkova, G. Gene expression profiling of sex differences in HIF1-dependent adaptive cardiac responses to chronic hypoxia. J. Appl. Physiol. 2010, 109, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.L.; Harrison, B.C.; Maass, A.; Bell, M.L.; Byrnes, W.C.; Leinwand, L.A. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J. Appl. Physiol. 2001, 90, 1900–1908. [Google Scholar] [CrossRef] [Green Version]

| BW | Trunk | Leg | HLC Mass | HLC FFM | HLC FM | HLC Gene Expression | Quad Mass | Quad FFM | Quad FM | Quad Gene Expression | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | ↔ | ↔ | ↔ |  |  |  |  |  |  |  |  |
| HH | ↓ ↓ | ↔ | ↔ | ↓ | ↔ | ↔ | ↑↑ atrophy | ↔ | ↔ | ↔ | ↔ |
| RUN | ↓ | ↑ fat-free | ↑ fat-free | ↓ | ↔ | ↓ | ↔ | ↔ | ↔ | ↓ | ↑ growth |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McNair, B.D.; Marcello, N.A.; Smith, D.T.; Schmitt, E.E.; Bruns, D.R. Changes in Muscle Mass and Composition by Exercise and Hypoxia as Assessed by DEXA in Mice. Medicina 2020, 56, 446. https://doi.org/10.3390/medicina56090446
McNair BD, Marcello NA, Smith DT, Schmitt EE, Bruns DR. Changes in Muscle Mass and Composition by Exercise and Hypoxia as Assessed by DEXA in Mice. Medicina. 2020; 56(9):446. https://doi.org/10.3390/medicina56090446
Chicago/Turabian StyleMcNair, Benjamin D., Nicholas A. Marcello, Derek T. Smith, Emily E. Schmitt, and Danielle R. Bruns. 2020. "Changes in Muscle Mass and Composition by Exercise and Hypoxia as Assessed by DEXA in Mice" Medicina 56, no. 9: 446. https://doi.org/10.3390/medicina56090446





