The Risk Prediction of Coronary Artery Lesions through the Novel Hematological Z-Values in 4 Chronological Age Subgroups of Kawasaki Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Diagnostic Criteria
2.3. Z-Value Calculation
2.4. Statistical Analysis
2.5. Ethics
2.6. Echocardiography
3. Results
3.1. Patient Characteristics
3.2. Binominal Logistic Regressions and Optimal Cutoff Values of the Risk Factors for CAL in KD in Each KD Phase and Age Subgroup
3.3. Comparisons of Hematological Z-Values between KD and FC Subgroups in the Acute Phase
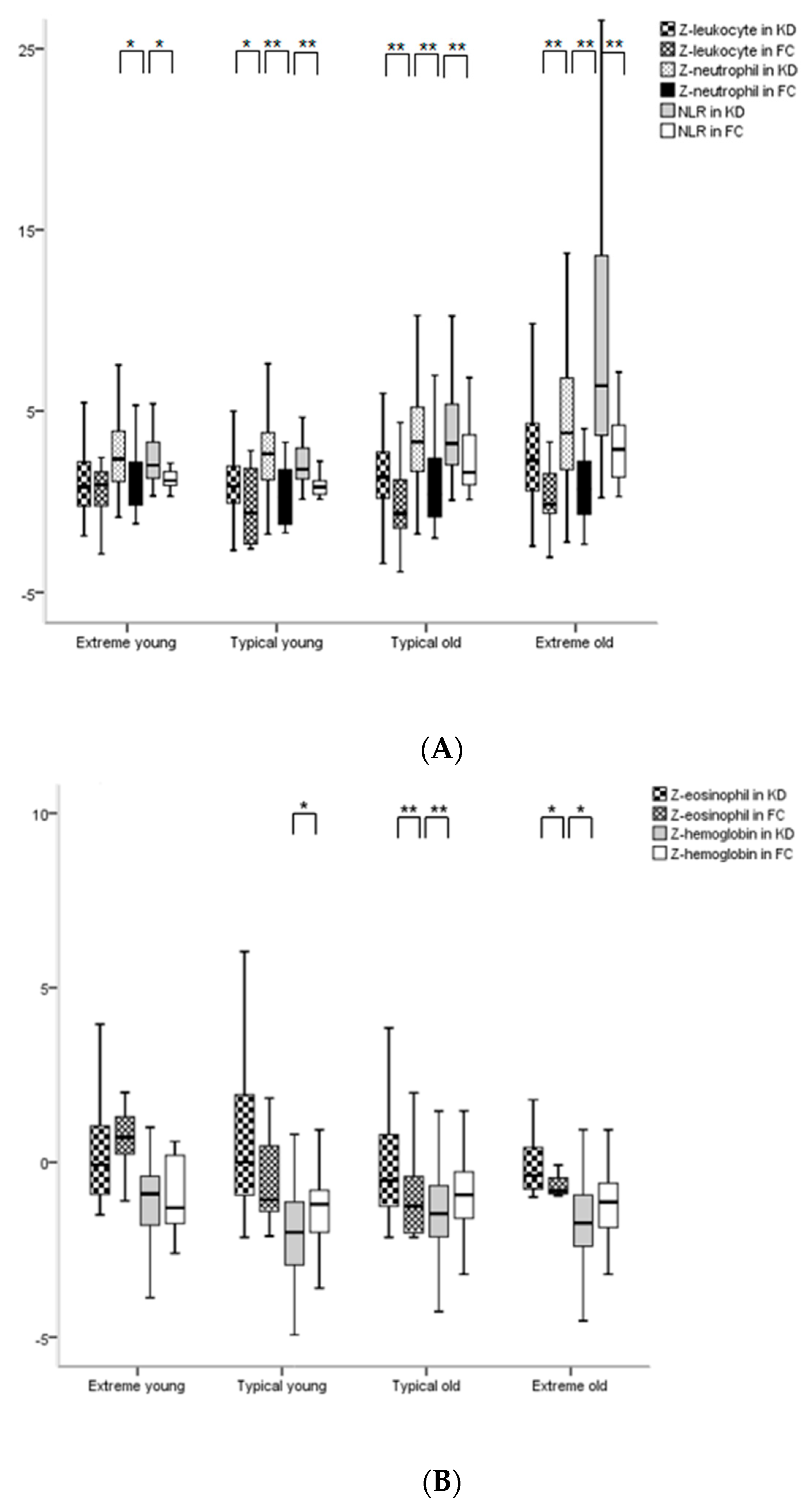
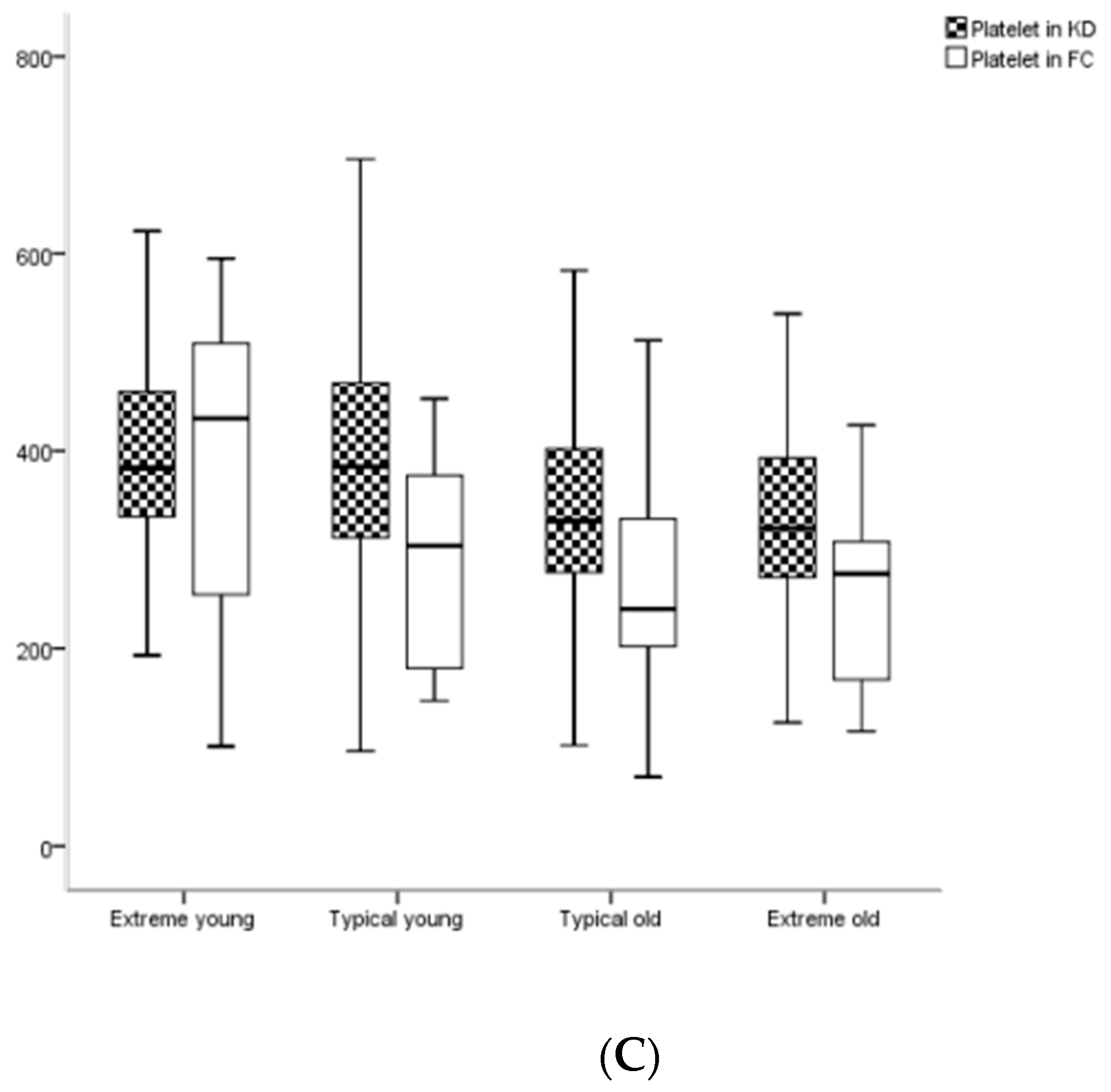
3.4. Comparisons of Hematological Z-Values among 4 Age Subgroups or among Phases in KD
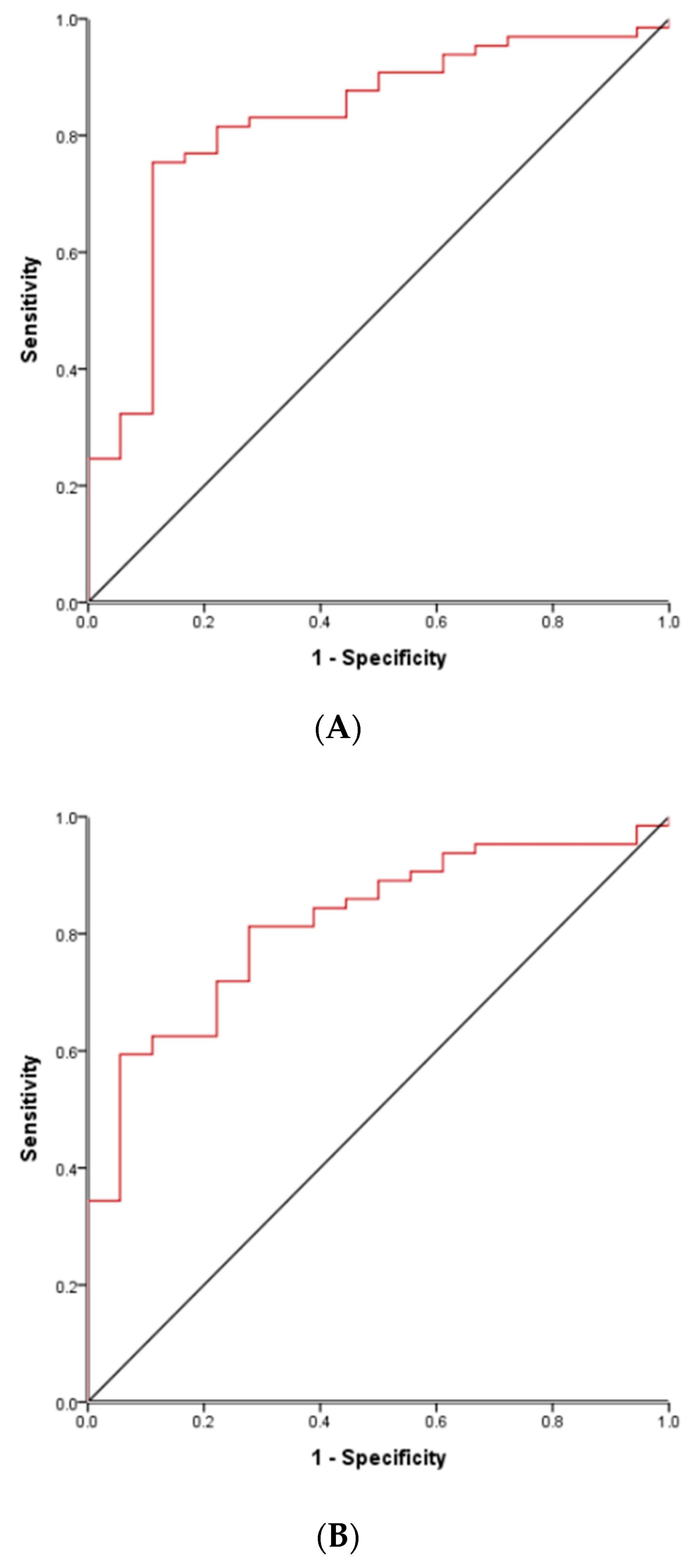
3.5. ROC Curve to Differentiate True Complete KD Groups and FC Groups in Acute Phase
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rosenfeld, E.A.; Corydon, K.E.; Shulman, S.T. Kawasaki disease in infants less than one year of age. J. Pediatr. 1995, 126, 524–529. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A scientific statement for health professionals from the American Heart Association. Circulation 2017, 135, 927–999. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.C.; Khodadadi, H.; Malik, A.; Davidson, B.; Salles, É.D.S.L.; Bhatia, J.; Hale, V.L.; Baban, B. Innate immunity of neonates and infants. Front. Immunol. 2018, 9, 1759. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Hong, J.H.; Han, J.W.; Lee, J.S.; Lee, B.C.; Burgner, D. Features of Kawasaki disease at the extremes of age. J. Paediatr. Child. Health 2006, 42, 423–427. [Google Scholar] [CrossRef]
- Kwak, J.H.; Lee, J.; Ha, K.S. Significance of differential characteristics in infantile Kawasaki disease. Korean Circ. J. 2019, 49, 755–765. [Google Scholar] [CrossRef]
- Tremoulet, A.H.; Jain, S.; Jaggi, P.; Jimenez-Fernandez, S.; Pancheri, J.M.; Pancheri, J.M.; Sun, X.; Kanegaye, J.T.; Kovalchin, J.P.; Printz, B.F.; et al. Infliximab for intensification of primary therapy for Kawasaki disease: A phase 3 randomised, double-blind, placebo-controlled trial. Lancet 2014, 383, 1731–1738. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Jennifer, S.L.; Minich, L.L.; Colan, S.D.; Atz, A.M.; Takahashi, M.; Vetter, V.L.; Gersony, W.M.; Mitchell, P.D.; Newburger, J.W. Coronary Artery Involvement in Children with Kawasaki Disease: Risk Factors From Analysis of Serial Normalized Measurements. Circulation 2007, 10, 174–179. [Google Scholar] [CrossRef]
- Taylor, M.R.; Holland, C.V.; Spencer, R.; Jackson, J.F.; O’Connor, G.I.; O’Donnell, J.R. Haematological reference ranges for schoolchildren. Clin. Lab. Haematol. 1997, 19, 1–15. [Google Scholar] [CrossRef]
- Bellamy, G.J.; Hinchliffe, R.F.; Crawshaw, K.C.; Finn, A.; Bell, F. Total and differential leucocyte counts in infants at 2, 5 and 13 months of age. Clin. Lab. Haematol. 2000, 22, 81–87. [Google Scholar] [CrossRef]
- Kwak, J.H.; Lee, J.; Ha, K.S. Erratum: Significance of differential characteristics in infantile Kawasaki disease. Korean Circ. J. 2020, 50, 641–643. [Google Scholar] [CrossRef]
- Lanzkowsky, P.; Lipton, J.; Fish, J. Lanzkowsky’s Manual of Pediatric Hematology and Oncology, Hematological Reference Values, 6th ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2016; pp. 712, 719. [Google Scholar]
- Ha, K.S.; Lee, J.; Jang, G.Y.; Lee, J.; Lee, K.C.; Son, C.S.; Lee, J.W. Value of neutrophil-lymphocyte ratio in predicting outcomes in Kawasaki disease. Am. J. Cardiol. 2015, 116, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.C.; Lo, C.W.; Hwang, B.; Lee, P.C. Clinical manifestations vary with different age spectrums in infants with Kawasaki disease. Sci. World J. 2012, 2012, 210382. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.A.; Carr, M.; Pahl, E.; DeMarais, N.; Shulman, S.T.; Rowley, A.H. Coronary artery aneurysms are more severe in infants than in older children with Kawasaki disease. Arch. Dis. Child. 2019, 104, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Diesner, S.C.; Forster-Waldl, E.; Olivera, A.; Pollak, A.; Jensen-Jarolim, E.; Untersmayr, E. Perspectives on immunomodulation early in life. Pediatr. Allergy Immunol. 2012, 23, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Oharaseki, T.; Yokouchi, Y. Histopathological aspects of cardiovascular lesions in Kawasaki disease. Int. J. Rheum. Dis. 2018, 21, 31–35. [Google Scholar] [CrossRef]
- Takahashi, K.; Oharaseki, T.; Naoe, S.; Wakayama, M.; Yokouchi, Y. Neutrophilic involvement in the damage to coronary arteries in acute stage of Kawasaki disease. Pediatr. Int. 2005, 47, 305–310. [Google Scholar] [CrossRef]
- Gru, A.A.; O’Malley, D.P. Autoimmune and medication-induced lymphadenopathies. Semin. Diagn. Pathol. 2018, 35, 34–43. [Google Scholar] [CrossRef]
- Sugitani, Y.; Furuno, K.; Sueishi, K.; Hara, T. Macrophages and cytotoxic T cells infiltrate the destructed mitral tissue in Kawasaki disease. BMJ Case Rep. 2018, 2, 2018. [Google Scholar] [CrossRef]
- Hua, W.; Ma, F.; Wang, Y.; Fu, S.; Wang, W.; Xie, C.; Zhang, Y.; Gong, F. A new scoring system to predict Kawasaki disease with coronary artery lesions. Clin. Rheumatol. 2019, 38, 1099–1107. [Google Scholar] [CrossRef]
- Tremoulet, A.H.; Jain, S.; Chandrasekar, D.; Sun, X.; Sato, Y.; Burns, J.C. Evolution of laboratory values in patients with Kawasaki disease. Pediatr. Infect. Dis. J. 2011, 30, 1022–1026. [Google Scholar] [CrossRef]
- Huang, Y.H.; Kuo, H.C. Anemia in Kawasaki disease: Hepcidin as a potential biomarker. Int. J. Mol. Sci. 2017, 18, 820. [Google Scholar] [CrossRef] [PubMed]
- Kling, P.J.; Schmidt, R.L.; Roberts, R.A.; Widness, J.A. Serum erythropoietin levels during infancy: Associations with erythropoiesis. J. Pediatr. 1996, 128, 791–796. [Google Scholar] [CrossRef]
- Tremoulet, A.H.; Best, B.M.; Song, S.; Wang, S.; Corinaldesi, E.; Eichenfield, J.R.; Martin, D.D.; Newburger, J.W.; Burns, J.C. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J. Pediatr. 2008, 153, 117–211. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.S.; Jang, G.Y.; Lee, J.; Lee, K.C.; Son, C.S. Laboratory markers in incomplete Kawasaki disease according to coronary artery outcome. Korean Circ. J. 2018, 48, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hua, Y.; Zhang, C.; Chen, S.; Zhang, Q.; Liao, Y.; Yan, H.; Wang, Y.; Liu, P.; Qi, J.; et al. Neutrophil-to-lymphocyte ratio predicts intravenous immunoglobulin-resistance in infants under 12-months old with Kawasaki disease. Front. Pediatr. 2019, 19, 81. [Google Scholar] [CrossRef]
- Demir, F.; Karadeniz, C.; Ozdemir, R.; Yozgat, Y.; Çelegen, K.; Karaaslan, U.; Mustafa Demirol, M.; Meşe, T.; Ünal, N. Usefulness of neutrophil to lymphocyte ratio in prediction of coronary artery lesions in patients with Kawasaki disease. Balkan Med. J. 2015, 32, 371–376. [Google Scholar] [CrossRef]
- Zahorec, R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl. Lek. Listy 2001, 102, 5–14. [Google Scholar]
- Abe, J.; Ebata, R.; Jibiki, T.; Yasukawa, K.; Saito, H.; Terai, T. Elevated granulocyte colony-stimulating factor levels predict treatment failure in patients with Kawasaki disease. J. Allergy Clin. Immunol. 2008, 122, 1008–1013. [Google Scholar] [CrossRef]
- Dionne, A.; Le, C.; Poupart, S.; Autmizguine, J.; Meloche-Dumas, L.; Turgeon, J.; Fournier, A.; Dahdah, N. Profile of resistance to IVIG treatment in patients with Kawasaki disease and concomitant infection. PLoS ONE 2018, 13, e0206001. [Google Scholar] [CrossRef]
- Rigante, D.; Valentini, P.; Rizzo, D.; Leo, A.; De Rosa, G.; Onesimo, R.; De Nisco, A.; Angelone, D.F.; Compagnone, A.; Delogu, A.B. Responsiveness to intravenous immunoglobulins and occurrence of coronary artery abnormalities in a single-center cohort of Italian patients with Kawasaki syndrome. Rheumatol. Int. 2010, 30, 841–846. [Google Scholar] [CrossRef]
| Clinical | Infantile KD (n = 223) | Non-Infantile KD (n = 681) | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| Features | Extreme Young (EY) (Age ≤ 6 Months) | Typical Young (TY) (Age 7–12 Months) | Typical Old (TO) (Age 13–59 Months) | Extreme Old (EO) (Age ≥ 5 Years) | Kruskal-Wallis Test | EY vs. TY | TY vs. TO | TO vs. EO |
| Numbers | 82 | 141 | 574 | 107 | - | - | - | - |
| Age (months) | 3 (4, 5) | 9 (8, 10) | 32 (23, 44) | 71 (64, 90) | <0.001 | <0.001 | <0.001 | <0.001 |
| M:F | 48:34 | 77:64 | 333:241 | 61:46 | 0.728 | 0.936 | 0.506 | 0.280 |
| iKD (incomplete KD) (%) | 80/223 (36%) | 145/681 (21%) | p value between infants vs. non-infants: <0.001 | |||||
| 29/82 (35%) | 51/141 (36%) | 121/574 (21%) | 24/107 (22%) | <0.001 | 0.687 | 0.007 | 0.498 | |
| Symptoms | 4 (3, 4) | 4 (3, 4) | 4 (4, 5) | 4 (4, 4) | <0.001 | 0.750 | <0.001 | 0.125 |
| a Total fever | 6 (5–7) | 6 (7–8) | p value between infants vs. non-infants: <0.001 | |||||
| 6 (5, 7) | 6 (5, 8) | 7 (6, 8) | 7 (6, 8) | <0.001 | 0.077 | 0.118 | 0.271 | |
| Pre-IVIG (intravenous immunoglobulin) | 5 (4, 5) | 5 (4, 6) | 5 (4, 6) | 5 (4, 7) | 0.057 | 0.542 | 0.843 | 0.011 |
| Post-IVIG | 1 (0, 2) | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | <0.001 | 0.004 | 0.751 | 0.112 |
| IVIG rest (%) | 22/82 (27%) | 60/141 (43%) | 207/574 (36%) | 34/107 (32%) | 0.101 | 0.019 | 0.374 | 0.395 |
| CAL (coronary artery lesion) (%) | 40/223 (18%) | 70/681 (10%) | p value between infants vs. non-infants: 0.004 | |||||
| 16/82 (20%) | 24/141 (17%) | 63/574 (11%) | 7/107 (7%) | <0.001 | 0.640 | 0.061 | 0.166 | |
| CAD (coronary artery dilatation) (%) | 11/82 (13%) | 18/141 (13%) | 46/574 (8%) | 2/107 (2%) | 0.002 | 0.890 | 0.098 | 0.023 |
| CAA (coronary artery aneurysm) (%) | 5/82 (6%) | 6/141 (4%) | 17/574 (3%) | 5/107 (5%) | 0.326 | 0.540 | 0.427 | 0.670 |
| A. | ||||
| Subjects | Risk Factors for CAL | OR | 95% CI | p |
| Total patients | Ages | 0.984 | 0.969–0.999 | 0.041 |
| Incomplete KD (iKD) | 2.079 | 1.257–3.440 | 0.004 | |
| a (risk factor of ages for iKD) | (0.991) | (0.984–0.998) | (0.013) | |
| Post-IVIG fever | 1.413 | 1.274–1.566 | <0.001 | |
| IVIG resistance | 3.063 | 1.964–5.034 | <0.001 | |
| Convalescent Z-eosinophil | 1.288 | 1.063–1.561 | 0.010 | |
| Subacute platelet | 1.003 | 1.002–1.005 | <0.001 | |
| EY subgroup | IVIG resistance | 8.433 | 2.695–26.388 | <0.001 |
| Acute Z-neutrophil | 2.636 | 1.299–5.350 | 0.007 | |
| Subacute Z-neutrophil | 0.127 | 0.027–0.598 | 0.009 | |
| Subacute NLR (neutrophil to lymphocyte ratio) | 23.26 | 2.19–247.43 | 0.009 | |
| Subacute platelet | 1.012 | 1.005–1.020 | 0.002 | |
| TY subgroup | Acute Z-hemoglobin | 0.323 | 0.169–0.618 | 0.001 |
| Convalescent Z-hemoglobin | 0.223 | 0.051–0.977 | 0.047 | |
| TO subgroup | iKD | 2.870 | 1.371–6.004 | 0.005 |
| Post-IVIG fever | 1.374 | 1.207–1.565 | <0.001 | |
| IVIG resistance | 3.139 | 1.495–6.591 | 0.003 | |
| Subacute platelet | 1.004 | 1.001–1.006 | 0.002 | |
| Subacute Z-monocyte | 1.297 | 1.028–1.636 | 0.028 | |
| B. | ||||
| CAL | Cutoff Values | Sensitivity/Specificity | AUC (95% CI) | p Value |
| Subacute platelet | 506 k | 0.635/0.630 | 0.744 (0.642–0.846) | <0.001 |
| Subacute platelet in TO | 505 k | 0.688/0.646 | 0.749 (0.663–0.834) | <0.001 |
| Acute Z-hemoglobin in TY | −2.6 | 0.735/0.647 | 0.766 (0.641–0.892) | 0.001 |
| Acute Variables | Extreme Young (EY) (Age ≤ 6 Months) | p Value | Typical Young (TY) (Age 7–12 Months) | p Value | Typical Old (TO) (Age 13–59 Months) | p Value | Extreme Old (EO) (Age ≥ 5 Years) | p Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KD (n = 82) | FC (n = 21) | KD vs. FC | KD (n = 141) | FC (n = 23) | KD vs. FC | KD (n = 574) | FC (n = 96) | KD vs. FC | KD (n =107) | FC (n = 36) | KD vs. FC | |
| Z-Leukocyte | 0.7 (−0.2, 2.2) | 0.9 (−1.0, 2.2) | 0.805 | 0.9 (−0.1, 2.0) | −0.6 (−2.3, 1.9) | 0.001 | 1.4 (0.2, 2.7) | −0.6 (−1.5, 1.2) | <0.001 | 2.3 (0.6, 4.5) | −0.1 (−0.6, 1.7) | <0.001 |
| Z-Neutrophil | 2.4 (1.1, 3.9) | 1.1 (−0.3, 2.5) | 0.049 | 2.6 (1.2, 3.8) | 0.2 (−1.3, 1.8) | <0.001 | 3.3 (1.7, 5.2) | 0.4 (−0.8, 2.4) | <0.001 | 3.8 (1.7, 6.8) | 0.9 (−0.7, 2.4) | <0.001 |
| Z-Lymphocyte | −1.1 (−1.6, −3.3) | −0.3 (−0.7, 0.1) | 0.058 | −1.4 (−0.6, −2.1) | −1.6 (−2.6, 0.3) | 0.827 | −1.7 (−2.3, −1.1) | −2.0 (−2.5, −1.2) | 0.162 | −1.4 (−1.8, −0.9) | −1.1 (−1.4, −0.4) | 0.018 |
| Z-Eosinophil | −0.1 (−0.9, 1.1) | −0.7 (−1.5, −0.0) | 0.162 | 0.0 (−1.0, 1.9) | −1.2 (−1.4, 0.6) | 0.061 | −0.5 (−1.3, 0.8) | −1.3 (−2.0, −0.4) | <0.001 | −0.5 (−0.8, 0.5) | −0.8 (−0.9, −0.2) | 0.006 |
| Z-Monocyte | 1.2 (−0.2, 3.1) | 2.8 (0.8, 4.4) | 0.119 | 0.8 (−0.4, 1.8) | 1.3 (0.2, 2.2) | 0.288 | 0.7 (−0.4, 2.0) | 1.2 (−0.1, 3.3) | 0.045 | 1.0 (−0.3, 2.4) | 1.1 (0.2, 2.8) | 0.299 |
| NLR | 2 (1.3, 3.3) | 1.2 (0.8, 1.7) | 0.009 | 1.8 (1.3, 3.0) | 0.8 (0.4, 1.2) | <0.001 | 3.2 (2.0, 5.4) | 1.6 (0.9, 3.7) | <0.001 | 6.4 (3.7, 13.6) | 2.9 (1.3, 4.8) | <0.001 |
| Z-Hemoglobin | −0.9 (−1.8, −0.4) | −1.3 (−1.9, 0.3) | 0.850 | −2 (−2.9, −1.1) | −1.2 (−2.0, −0.8) | 0.044 | −1.5 (−2.1, −0.7) | −0.9 (−1.6, −0.3) | <0.001 | −1.7 (−2.4, −0.9) | −1.1 (−1.9, −0.5) | 0.038 |
| Platelet (/1000) | 383 (332, 461) | 433 (224, 540) | 0.985 | 384 (312, 471) | 304 (176, 379) | 0.001 | 329 (277, 402) | 240 (201, 331) | <0.001 | 322 (272, 393) | 278 (169, 309) | <0.001 |
| CRP (mg/L) | 71 (33, 124) | 29 (11, 75) | 0.026 | 78 (41, 110) | 22 (2, 51) | <0.001 | 77 (46, 128) | 34 (7, 52) | <0.001 | 89 (56, 135) | 39 (23, 80) | <0.001 |
| ESR | 56 (41, 73) | 68 (29, 79) | 0.803 | 74 (46, 104) | 24 (9, 65) | <0.001 | 80 (58, 99) | 46 (26, 72) | <0.001 | 79 (56, 100) | 39 (24, 60) | <0.001 |
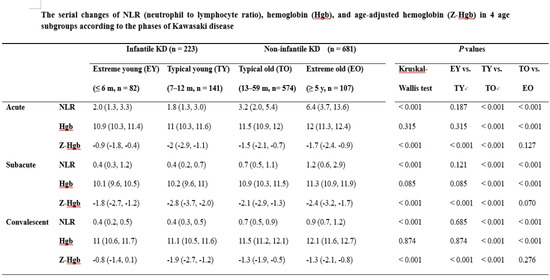
| Variables | Phases | Infantile KD (n = 223) | Non-Infantile KD (n = 681) | p Values | |||||
|---|---|---|---|---|---|---|---|---|---|
| Extreme Young (EY) (≤6 m, n = 82) | Typical Young (TY) (7–12 m, n = 141) | Typical Old (TO) (13–59 m, n= 574) | Extreme Old (EO) (≥5 y, n = 107) | Kruskal–Wallis Test | EY vs. TY | TY vs. TO | TO vs. EO | ||
| Z-Leukocyte | Acute | 0.7 (−0.2, 2.2) | 0.9 (−0.1, 2.0) | 1.4 (0.2. 2.7) | 2.3 (0.6, 4.5) | <0.001 | 0.899 | 0.026 | <0.001 |
| Subacute | −0.5 (−1.2, 1.0) | −1.3 (−1.9. −0.2) | −1.0 (−1.5, −0.2) | −0.7 (−1.2, 0.7) | <0.001 | 0.001 | 0.033 | 0.004 | |
| Convalescent | −0.8 (−1.4, −0.3) | −1.0 (−1.3, −0.4) | −0.7 (−1.2, −0.2) | −0.7 (−1.1, −0.2) | 0.186 | 0.734 | 0.165 | 0.818 | |
| Z-Neutrophil | Acute | 2.4 (1.1, 3.9) | 2.6 (1.2, 3.8) | 3.3 (1.7, 5.2) | 3.8 (1.7, 6.8) | <0.001 | 0.830 | 0.003 | 0.119 |
| Subacute | −0.7 (−1.2, 1.4) | −0.9 (−1.4, −0.3) | −0.6 (−1.1, 0.3) | −0.7 (−1.5, 0.6) | 0.023 | 0.043 | 0.002 | 0.367 | |
| Convalescent | −1.0 (−1.4, −0.5) | −0.9 (−1.1, −0.5) | −0.5 (−0.9, 0.1) | −0.9 (−1.3, −0.4) | <0.001 | 0.192 | <0.001 | <0.001 | |
| Z-Lymphocyte | Acute | −1.1 (−1.6, −0.3) | −1.4 (−2.1, −0.6) | −1.7 (−2.3, −1.1) | −1.4 (−1.8, −0.9) | <0.001 | 0.012 | 0.007 | 0.001 |
| Subacute | −0.7 (−1.2, −0.2) | −1.5 (−2.0, −0.4) | −1.3 (−1.8, −0.7) | −0.7 (−1.2, −0.2) | <0.001 | <0.001 | 0.469 | <0.001 | |
| Convalescent | −0.6 (1.1, −0.2) | −0.8 (−1.4, −0.3) | −0.9 (−1.5, −0.3) | −0.1 (−0.4, 0.3) | <0.001 | 0.292 | 0.696 | <0.001 | |
| Z-Eosinophil | Acute | −0.1 (−0.9, 1.1) | −0.0 (−1.0, 1.9) | −0.5 (−1.3, 0.8) | −0.5 (−0.8, 0.5) | <0.001 | 0.665 | 0.002 | 0.014 |
| Subacute | 1.0 (0.1, 2.1) | 0.8 (−0.4, 2.0) | 0.5 (−0.4, 1.4) | 0.4 (−2.6, 1.1) | 0.019 | 0.199 | 0.209 | 0.963 | |
| Convalescent | 0.6 (−0.2, 2.0) | 0.5 (−0.2, 2.0) | −0.3 (−0.8, 0.7) | 0.1 (−0.3, 0.7) | <0.001 | 0.463 | 0.002 | 0.003 | |
| Z-Monocyte | Acute | 1.2 (−0.2, 3.1) | 0.8 (−0.4, 1.8) | 0.7 (−0.4, 2.1) | 1.0 (−0.3, 2.4) | 0.041 | 0.083 | 0.853 | 0.081 |
| Subacute | 0.6 (−0.3, 2.4) | 0.2 (−0.6, 1.2) | 0.4 (−0.4, 1.3) | 0.6 (−0.3, 1.2) | 0.116 | 0.019 | 0.292 | 0.482 | |
| Convalescent | 0.4 (0.3, 0.7) | 0.2 (−0.6, 0.7) | 0.1 (−0.4, 0.8) | 0.1 (−0.3, 0.8) | 0.433 | 0.541 | 0.652 | 0.607 | |
| NLR | Acute | 2.0 (1.3, 3.3) | 1.8 (1.3, 3.0) | 3.2 (2.0, 5.4) | 6.4 (3.7, 13.6) | <0.001 | 0.187 | <0.001 | <0.001 |
| Subacute | 0.4 (0.3, 1.2) | 0.4 (0.2, 0.7) | 0.7 (0.5, 1.1) | 1.2 (0.6, 2.9) | <0.001 | 0.121 | <0.001 | <0.001 | |
| Convalescent | 0.4 (0.2, 0.5) | 0.4 (0.3, 0.5) | 0.7 (0.5, 0.9) | 0.9 (0.7, 1.2) | <0.001 | 0.685 | <0.001 | <0.001 | |
| Hemoglobin | Acute | 10.9 (10.3, 11.4) | 11 (10.3, 11.6) | 11.5 (10.9, 12) | 12 (11.3, 12.4) | <0.001 | 0.315 | <0.001 | <0.001 |
| Subacute | 10.1 (9.6, 10.5) | 10.2 (9.6, 11) | 10.9 (10.3, 11.5) | 11.3 (10.9, 11.9) | <0.001 | 0.085 | <0.001 | <0.001 | |
| Convalescent | 11 (10.6, 11.7) | 11.1 (10.5, 11.6) | 11.5 (11.2, 12.1) | 12.1 (11.6, 12.7) | <0.001 | 0.874 | <0.001 | <0.001 | |
| Z-Hemoglobin | Acute | −0.9 (−1.8, −0.4) | −2 (−2.9, −1.1) | −1.5 (−2.1, −0.7) | −1.7 (−2.4. −0.9) | <0.001 | <0.001 | <0.001 | 0.127 |
| Subacute | −1.8 (−2.7, −1.2) | −2.8 (−3.7, −2.0) | −2.1 (−2.9, −1.3) | −2.4 (−3.2, −1.7) | <0.001 | <0.001 | <0.001 | 0.070 | |
| Convalescent | −0.8 (−1.4, 0.1) | −1.9 (−2.7, −1.2) | −1.3 (−1.9, −0.5) | −1.3 (−2.1, −0.8) | <0.001 | <0.001 | <0.001 | 0.276 | |
| Platelet (/1000) | Acute | 383 (332, 461) | 384 (312, 471) | 329 (277, 402) | 322 (272, 393) | <0.001 | 0.988 | <0.001 | 0.443 |
| Subacute | 507 (412, 612) | 490 (390, 638) | 461 (357, 553) | 443 (344, 523) | 0.002 | 0.550 | 0.026 | 0.325 | |
| Convalescent | 436 (331, 520) | 357 (299, 493) | 331 (277, 409) | 343 (287, 405) | <0.001 | 0.182 | 0.028 | 0.544 | |
| CRP (mg/L) | Acute | 71 (33, 124) | 78 (41, 110) | 77 (46, 128) | 89 (56, 135) | 0.177 | 0.823 | 0.354 | 0.179 |
| Subacute | 27 (10, 50) | 16 (7, 33) | 17 (8, 32) | 25 (10, 54) | 0.001 | 0.012 | 0.702 | 0.004 | |
| Convalescent | 0.4 (0.2, 1.5) | 0.3 (0.2, 1.1) | 0.5 (0.3, 2.0) | 0.4 (0.3, 1.5) | 0.010 | 0.402 | 0.002 | 0.400 | |
| ESR | Acute | 56 (41, 73) | 74 (46, 104) | 80 (58, 99) | 79 (56, 100) | <0.001 | <0.001 | 0.289 | 0.608 |
| Subacute | 50 (39, 75) | 84 (60, 115) | 87 (63, 112) | 86 (69, 111) | <0.001 | <0.001 | 0.540 | 0.909 | |
| Convalescent | 10 (5, 20) | 16 (8, 30) | 23 (14, 41) | 28 (12, 53) | <0.001 | 0.064 | 0.013 | 0.569 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.J.; Kim, W.Y.; Park, S.M.; Lee, J.H.; Shin, H.J.; Jang, G.Y.; Ha, K.S. The Risk Prediction of Coronary Artery Lesions through the Novel Hematological Z-Values in 4 Chronological Age Subgroups of Kawasaki Disease. Medicina 2020, 56, 466. https://doi.org/10.3390/medicina56090466
Cho HJ, Kim WY, Park SM, Lee JH, Shin HJ, Jang GY, Ha KS. The Risk Prediction of Coronary Artery Lesions through the Novel Hematological Z-Values in 4 Chronological Age Subgroups of Kawasaki Disease. Medicina. 2020; 56(9):466. https://doi.org/10.3390/medicina56090466
Chicago/Turabian StyleCho, Hwa Jin, Woo Young Kim, Sung Man Park, Jung Hwa Lee, Hong Ju Shin, Gi Young Jang, and Kee Soo Ha. 2020. "The Risk Prediction of Coronary Artery Lesions through the Novel Hematological Z-Values in 4 Chronological Age Subgroups of Kawasaki Disease" Medicina 56, no. 9: 466. https://doi.org/10.3390/medicina56090466
APA StyleCho, H. J., Kim, W. Y., Park, S. M., Lee, J. H., Shin, H. J., Jang, G. Y., & Ha, K. S. (2020). The Risk Prediction of Coronary Artery Lesions through the Novel Hematological Z-Values in 4 Chronological Age Subgroups of Kawasaki Disease. Medicina, 56(9), 466. https://doi.org/10.3390/medicina56090466