Pathomechanisms of Non-Traumatic Acute Brain Injury in Critically Ill Patients
Abstract
1. Introduction
2. Hypoxia or Hyperoxia-Related Brain Injury
3. Neuroinflammatory Hypothesis
4. Neurotransmitter Disorders
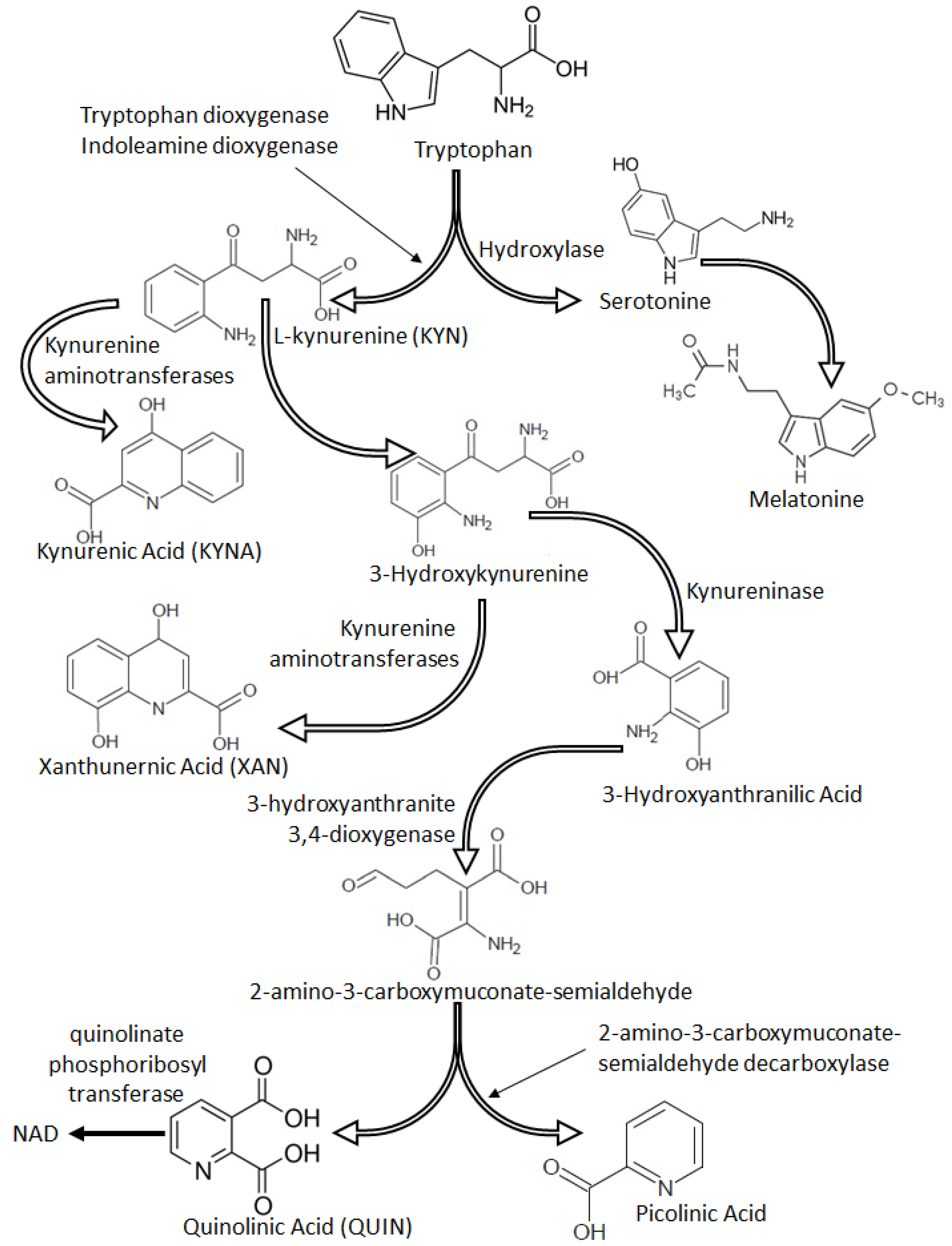
5. Tryptophan Metabolism and Kynurenine Pathway Dysregulation
6. Gut Microbiota Dysregulation
7. Treatment of Delirium
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Pub: Arlington, VA, USA, 2013. [Google Scholar]
- European Delirium Association; American Delirium Society. The DSM-5 criteria, level of arousal and delirium diagnosis: Inclusiveness is safer. BMC Med. 2014, 12, 141–148. [Google Scholar] [CrossRef]
- Shehabi, Y.; Riker, R.R.; Bokesch, P.M.; Wisemandle, W.; Shintani, A.; Ely, E.W.; SEDCOM Study Group. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit. Care Med. 2010, 38, 2311–2318. [Google Scholar] [CrossRef]
- Ely, E.W.; Shintani, A.; Truman, B.; Speroff, T.; Gordon, S.M.; Harrell, J.F.E.; Inouye, S.K.; Bernard, G.R.; Dittus, R.S. Delirium as a Predictor of Mortality in Mechanically Ventilated Patients in the Intensive Care Unit. JAMA 2004, 291, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Kotfis, K.; Marra, A.; Ely, E.W. ICU delirium—A diagnostic and therapeutic challenge in the intensive care unit. Anaesthesiol. Intensive Ther. 2018, 50, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Lindroth, H.; Khan, B.A.; Carpenter, J.S.; Gao, S.; Perkins, A.J.; Khan, S.H.; Wang, S.; Jones, R.N.; Boustani, M.A. Delirium Severity Trajectories and Outcomes in ICU Patients: Defining a Dynamic Symptom Phenotype. Ann. Am. Thorac. Soc. 2020, 17, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Hayhurst, C.J.; Marra, A.; Han, J.H.; Patel, M.B.; Brummel, N.E.; Thompson, J.L.; Jackson, J.C.; Chandrasekhar, R.; Ely, E.W.; Pandharipande, P.P.; et al. Association of Hypoactive and Hyperactive Delirium with Cognitive Function After Critical Illness. Crit. Care Med. 2020, 48, e480–e488. [Google Scholar] [CrossRef]
- Maldonado, J.R. Delirium pathophysiology: An updated hypothesis of the etiology of acute brain failure. Int. J. Geriatr. Psychiatry 2018, 33, 1428–1457. [Google Scholar] [CrossRef]
- Maldonado, J.R. Acute brain failure: Pathophysiology, diagnosis, management and sequelae of delirium. Crit. Care Clin. 2017, 33, 461–519. [Google Scholar] [CrossRef]
- Marra, A.; Kotfis, K.; Hosie, A.; MacLullich, A.M.J.; Pandharipande, P.; Ely, E.W.; Pun, B.T. Delirium Monitoring: Yes or No? That Is The Question. Am. J. Crit. Care 2019, 28, 127–135. [Google Scholar] [CrossRef]
- Vasilevskis, E.E.; Han, J.H.; Hughes, C.G.; Ely, E.W. Epidemiology and risk factors for delirium across hospital settings. Best Pr. Res. Clin. Anaesthesiol. 2012, 26, 277–287. [Google Scholar] [CrossRef]
- Sampath, H.; Jayaswal, A.K.; Soohinda, G.; Dutta, S. Delirium in medical intensive care units: Incidence, subtypes, risk factors, and outcome. Indian J. Psychiatry 2019, 61, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zacharias, E.; Hoff, P.; Tegtmeier, F. Ion channel involvement in anoxic depolarization induced by cardiac arrest un rat brain. J. Cereb. Blood Flow Metab. 1995, 15, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Nagayama, T.; Jin, K.; Stetler, R.A.; Zhu, R.L.; Graham, S.H.; Simon, R.P. Induction of Caspase-3-Like Protease May Mediate Delayed Neuronal Death in the Hippocampus after Transient Cerebral Ischemia. J. Neurosci. 1998, 18, 4914–4928. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.R.; Koerner, I.P.; Möller, T. Microglia in ischemic brain injury. Future Neurol. 2010, 5, 227–246. [Google Scholar] [CrossRef]
- Funk, D.J.; Kumar, A.; Klar, G. Decreases in cerebral saturation in patients with septic shock are associated with increased risk of death: A prospective observational single center study. J. Intensiv. Care 2016, 4, 42. [Google Scholar] [CrossRef]
- Mikkelsen, M.E.; Christie, J.D.; Lanken, P.N.; Biester, R.C.; Thompson, B.T.; Bellamy, S.L.; Localio, A.R.; Demissie, E.; Hopkins, R.O.; Angus, D.C.; et al. Faculty Opinions recommendation of The adult respiratory distress syndrome cognitive outcomes study: Long-term neuropsychological function in survivors of acute lung injury. Am. J. Respir. Crit. Care Med. 2012, 185, 1307–1315. [Google Scholar] [CrossRef]
- Hopkins, R.O.; Weaver, L.K.; Collingridge, D.; Parkinson, R.B.; Chan, K.J.; Orme, J.F. Two-Year Cognitive, Emotional, and Quality-of-Life Outcomes in Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2005, 171, 340–347. [Google Scholar] [CrossRef]
- Kupiec, A.; Adamik, B.; Forkasiewicz-Gardynik, K.; Gozdzik, W. Intra-operative hyperoxia and the risk of delirium in eldery patients after cardiac surgery. Aging 2020, 12, 7006–7014. [Google Scholar] [CrossRef]
- Mutch, W.A.C.; El-Gabalawy, R.; Ryner, L.; Puig, J.; Essig, M.; Kilborn, K.; Fidler, K.; Graham, M.R. Brain BOLD MRI O2 and CO2 stress testing: Implications for perioperative neurocognitive disorder following surgery. Crit. Care 2020, 24, 1–13. [Google Scholar] [CrossRef]
- Damiani, E.; Adrario, E.; Girardis, M.; Romano, R.; Pelaia, P.; Singer, M.; Donati, A. Arterial hyperoxia and mortality in critically ill patients: A systematic review and meta-analysis. Crit. Care 2014, 18, 711. [Google Scholar] [CrossRef]
- Terraneo, L.; Samaja, M. Comparative Response of Brain to Chronic Hypoxia and Hyperoxia. Int. J. Mol. Sci. 2017, 18, 1914. [Google Scholar] [CrossRef] [PubMed]
- Felderhoff-Mueser, U.; Bittigau, P.; Sifringer, M.; Jarosz, B.; Korobowicz, E.; Mahler, L.; Piening, T.; Moysich, A.; Grune, T.; Thor, F.; et al. Oxygen causes cell death in the developing brain. Neurobiol. Dis. 2004, 17, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.G.; Pandharipande, P.; Morse, J.; Shotwell, M.S.; Milne, G.; Pretorius, M.; Shaw, A.D.S.; Roberts, L.J.; Billings, F.T. Intraoperative cerebral oxygenation, oxidative injury, and delirium following cardiac surgery. Free Radic. Biol. Med. 2017, 103, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Roberts II, L.J.; Gobeil, F.; Taber, D.; Kanai, K.; Abran, D.; Brault, S.; Checchin, D.; Sennlaub, F.; Lachapelle, P.; et al. Isomer-specific contractile effects of a series of synthetic F2-isoprostanes on retinal and cerebral microvasculature. Free Radic. Biol. Med. 2004, 36, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Kotfis, K.; Biernawska, J.; Zegan-Barańska, M.; Żukowski, M. Peripheral Blood Lymphocyte Subsets (CD4+, CD8+ T Cells, NK Cells) in Patients with Cardiovascular and Neurological Complications after Carotid Endarterectomy. Int. J. Mol. Sci. 2015, 16, 10077–10094. [Google Scholar] [CrossRef]
- Song, T.-T.; Bi, Y.-H.; Gao, Y.-Q.; Huang, R.; Hao, K.; Xu, G.; Tang, J.-W.; Ma, Z.-Q.; Kong, F.-P.; Coote, J.H.; et al. Systemic pro-inflammatory response facilitates the development of cerebral edema during short hypoxia. J. Neuroinflamm. 2016, 13, 63. [Google Scholar] [CrossRef]
- Matt, S.M.; Johnson, R.W. Neuro-immune dysfunction during brain aging: New insights in microglial cell regulation. Curr. Opin. Pharmacol. 2016, 26, 96–101. [Google Scholar] [CrossRef][Green Version]
- Zhang, S.; Dong, H.; Zhang, X.; Li, N.; Sun, J.; Qian, Y.-N. Cerebral mast cells contribute to postoperative cognitive dysfunction by promoting blood brain barrier disruption. Behav. Brain Res. 2016, 298, 158–166. [Google Scholar] [CrossRef]
- Skelly, D.T.; Hennessy, E.; Dansereau, M.-A.; Cunningham, C. A Systematic Analysis of the Peripheral and CNS Effects of Systemic LPS, IL-1Β, TNF-α and IL-6 Challenges in C57BL/6 Mice. PLoS ONE 2013, 8, e69123. [Google Scholar] [CrossRef]
- Minagar, A.; Long, A.; Ma, T.; Jackson, T.H.; Kelley, R.E.; Ostanin, D.V.; Sasaki, M.; Warren, A.C.; Jawahar, A.; Cappell, B.; et al. Interferon (IFN)-beta 1a and IFN-beta 1b block IFN-gamma disinterration of endothelial junction integrity and barrier. Endothelium 2003, 10, 299–307. [Google Scholar] [CrossRef]
- Rahman, M.T.; Ghosh, C.; Hossain, M.; Linfield, D.; Rezaee, F.; Janigro, D.; Marchi, N.; Van Boxel-Dezaire, A.H. IFN-γ, IL-17A, or zonulin rapidly increase the permeability of the blood-brain and small intestinal epithelial barriers: Relevance for neuro-inflammatory diseases. Biochem. Biophys. Res. Commun. 2018, 507, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.-S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.C.; Carrier, M.; Tremblay, M.-È. Morphology of Microglia across Contexts of Health and Disease. Methods Mol. Biol. 2019, 2034, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H.; Holmes, C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 2014, 10, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Velagapudi, R.; Subramaniyan, S.; Xiong, C.; Porkka, F.; Rodriguiz, R.M.; Wetsel, W.C.; Terrando, N. Orthopedic Surgery Triggers Attention Deficits in a Delirium-Like Mouse Model. Front. Immunol. 2019, 10, 2675. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.A.; Ochani, M.; Gallowitsch-Puerta, M.; Ochani, K.; Huston, J.M.; Czura, C.J.; Al-Abed, Y.; Tracey, K.J. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc. Natl. Acad. Sci. USA 2006, 103, 5219–5223. [Google Scholar] [CrossRef] [PubMed]
- Mariscalco, G.; Mariani, S.; Biancari, F.; Banach, M. Effects of statins on delirium following cardiac surgery—Vidence from literature. Psychiatr. Pol. 2015, 49, 1359–1370. [Google Scholar] [CrossRef]
- Marra, A.; McGrane, T.J.; Henson, C.P.; Pandharipande, P. Melatonin in Critical Care. Crit. Care Clin. 2019, 35, 329–340. [Google Scholar] [CrossRef]
- Fracassi, A.; Marangoni, M.; Rosso, P.; Pallottini, V.; Fioramonti, M.; Siteni, S.; Segatto, M. Statins and the Brain: More than Lipid Lowering Agents? Curr. Neuropharmacol. 2019, 17, 59–83. [Google Scholar] [CrossRef]
- Van Munster, B.C.; Korevaar, J.C.; Zwinderman, A.H.; Levi, M.; Wiersinga, W.J.; De Rooij, S.E. Time-Course of Cytokines during Delirium in Elderly Patients with Hip Fractures. J. Am. Geriatr. Soc. 2008, 56, 1704–1709. [Google Scholar] [CrossRef]
- Cerejeira, J.; Firmino, H.; Vaz-Serra, A.; Mukaetova-Ladinska, E.B. The neuroinflammatory hypothesis of delirium. Acta Neuropathol. 2010, 119, 737–754. [Google Scholar] [CrossRef] [PubMed]
- Umholtz, M.; Nader, N.D. Anesthetic Immunomodulation of the Neuroinflammation in Postoperative Cognitive Dysfunction. Immunol. Investig. 2017, 46, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Jardine, K.H.; Wideman, C.E.; MacGregor, C.; Sgarbossa, C.; Orr, D.; Mitchnick, K.A.; Winters, B.D. Activation of cortical M1 muscarinic receptors and related intracellular signaling is necessary for reactivation-induced object memory updating. Sci. Rep. 2020, 10, 9209. [Google Scholar] [CrossRef] [PubMed]
- Field, R.H.; Gossen, A.; Cunningham, C. Prior pathology in the basal forebrain cholinergic system predisposes to inflammation-induced working memory deficits: Reconciling inflammatory and cholinergic hypotheses of delirium. J. Neurosci. 2012, 32, 6288–6294. [Google Scholar] [CrossRef]
- Taepavarapruk, P.; Song, C. Reductions of acetylcholine release and nerve growth factor expression are correlated with memory impairment induced by interleukin-1β administrations: Effects of omega-3 fatty acid EPA treatment. J. Neurochem. 2010, 112, 1054–1064. [Google Scholar] [CrossRef]
- Adam, E.H.; Haas, V.; Lindau, S.; Zacharowski, K.; Scheller, B. Cholinesterase alterations in delirium after cardiosurgery: A German monocentric prospective study. BMJ Open 2020, 10, e031212. [Google Scholar] [CrossRef]
- John, M.; Ely, E.W.; Halfkann, D.; Schoen, J.; Sedemund-Adib, B.; Klotz, S.; Radtke, F.; Stehr, S.; Hueppe, M. Acetylcholinesterase and butyrylcholinesterase in cardiosurgical patients with postoperative delirium. J. Intensiv. Care 2017, 5, 29. [Google Scholar] [CrossRef]
- Jackson, D.M.; Westlind-Danielsson, A. Dopamine receptors: Molecular biology, biochemistry and behalioural aspects. Pharmacol. Ther. 1994, 64, 291–369. [Google Scholar] [CrossRef]
- Sokoloff, P.; Schwartz, J.-C. Novel dopamine receptors half a decade later. Trends Pharmacol. Sci. 1995, 16, 270–275. [Google Scholar] [CrossRef]
- Pedrosa, R.; Soares-da-Silva, P. Oxidative and non-oxidative mechanisms of neuronal cel death and apoptosis by 1-3,4-dihydroxyphenylalanine (L-dopa) and dopamine. Br. J. Pharmacol. 2002, 137, 1305–1313. [Google Scholar] [CrossRef]
- Ebrahimi-Ghiri, M.; Nasehi, M.; Zarrindast, M.-R. The modulatory role of accumbens and hippocampus D2 receptors in anxiety and memory. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.; Fadel, M.; Sarter, M.; Bruno, J.P. Role of accumbens and cortical dopamine receptors in the regulation of cortical acetycholine release. Neuroscience 1999, 88, 811–822. [Google Scholar] [CrossRef]
- Yilmaz, S.; Aksoy, E.; Diken, A.I.; Yalçınkaya, A.; Erol, M.E.; Cagli, K. Dopamine Administration is a Risk Factor for Delirium in Patients Undergoing Coronary Artery Bypass Surgery. Heart Lung Circ. 2016, 25, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Masood, B.; Lepping, P.; Romanov, D.; Poole, R. Treatment of alcohol-induced psychotic disorder (alcoholic hallucinosis)—A systematic review. Alcohol Alcohol. 2018, 53, 259–267. [Google Scholar] [CrossRef]
- Jembrek, M.; Vlainić, J. GABA Receptors: Pharmacological Potential and Pitfalls. Curr. Pharm. Des. 2015, 21, 4943–4959. [Google Scholar] [CrossRef]
- Yoshitaka, S.; Egi, M.; Kanazawa, T.; Toda, Y.; Kiyoshi, M. The association of plasma gamma-aminobutyric acid concentration with postoperative delirium in critically ill patients. Crit. Care Resusc. 2014, 16, 269–273. [Google Scholar]
- Wisden, W.; Yu, X.; Franks, N.P. GABA Receptors and the Pharmacology of Sleep. Handb. Exp. Pharmacol. 2019, 253, 279–304. [Google Scholar] [CrossRef]
- Tilleux, S.; Hermans, E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J. Neurosci. Res. 2007, 85, 2059–2070. [Google Scholar] [CrossRef]
- Carvalho, A.L.; Caldeira, M.V.; Santos, S.D.; Duarte, C.B. Role of the brain-derived neutrophic factor at glutamateric synapses. Br. J. Pharmacol. 2008, 153, S310–S324. [Google Scholar] [CrossRef]
- Wyrobek, J.; LaFlam, A.; Max, L.; Tian, J.; Neufeld, K.; Kebaish, K.; Walston, J.; Hogue, C.; Riley, L.; Everett, A.; et al. Association of intraoperative changes in brain-derived neurotrophic factor and postoperative delirium in older adults. Br. J. Anaesth. 2017, 119, 324–332. [Google Scholar] [CrossRef]
- Lipton, J.O.; Sahin, M. The neurology of mTOR. Neuron 2014, 84, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Meneses, A. Neural activity, memory, and dementias: Serotonergic markers. Behav. Pharmacol. 2017, 28, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Rebholz, H.; Friedman, E.; Castello, J. Alterations of Expression of the Serotonin 5-HT4 Receptor in Brain Disorders. Int. J. Mol. Sci. 2018, 19, 3581. [Google Scholar] [CrossRef] [PubMed]
- Terry, A.V.; Buccafusco, J.J.; Wilson, C. Cognitive dysfunction in neuropsychiatric disorders: Selected serotonin receptor subtypes as therapeutic targets. Behav. Brain Res. 2008, 195, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Sternbach, H. The serotonin syndrome. Am. J. Psychiatry 1991, 148, 705–713. [Google Scholar]
- Madsen, K.; Haahr, M.T.; Marner, L.; Keller, S.H.; Baaré, W.F.; Svarer, C.; Hasselbalch, S.G.; Knudsen, G.M. Age and sex effects on 5-HT4receptors in the human brain: A [11C]SB207145 PET study. J. Cereb. Blood Flow Metab. 2011, 31, 1475–1481. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Jeon, S.W. Neuroinflammation and the Immune-Kynurenine Pathway in Anxiety Disorders. Curr. Neuropharmacol. 2018, 16, 574–582. [Google Scholar] [CrossRef]
- Felger, J.C. Imaging the Role of Inflammation in Mood and Anxiety-related Disorders. Curr. Neuropharmacol. 2018, 16, 533–558. [Google Scholar] [CrossRef]
- Stone, T.W.; Forrest, C.M.; Mackay, G.M.; Stoy, N.; Darlington, L.G. Tryptophan, adenosine, neurodegeneration and neuroprotection. Metab. Brain Dis. 2007, 22, 337–352. [Google Scholar] [CrossRef]
- Schurr, A. Neuroprotection against ischemic/hypoxic brain damage: Blockers of ionotropic glutamate receptor and voltage sensitive calcium channels. Curr. Drug Targets 2004, 5, 603–618. [Google Scholar] [CrossRef]
- Dobelis, P.; Staley, K.J.; Cooper, D.C. Lack of modulation of nicotinic acetylcholine alpha-7 receptor currents by kynurenic acid in adult hippocampal interneurons. PLoS ONE 2012, 7, e41108. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, W.; Kwiecień, J.M.; Rola, R.; Klapec, M.; Stanisz, G.J.; Kotlińska-Hasiec, E.; Oakden, W.; Janik, R.; Coote, M.; Frey, B.N.; et al. Prolonged Subdural Infusion of Kynurenic Acid Is Associated with Dose-Dependent Myelin Damage in the Rat Spinal Cord. PLoS ONE 2015, 10, e0142598. [Google Scholar] [CrossRef] [PubMed]
- Vohra, M.; Lemieux, G.A.; Lin, L.; Ashrafi, K. Kynurenic acid accumulation underlies learning and memory impairment associated with aging. Genes Dev. 2018, 32, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Kozak, R.; Campbell, B.M.; Strick, C.A.; Horner, W.; Hoffmann, W.E.; Kiss, T.; Chapin, D.S.; McGinnis, D.; Abbott, A.L.; Roberts, B.M.; et al. Reduction of Brain Kynurenic Acid Improves Cognitive Function. J. Neurosci. 2014, 34, 10592–10602. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.M.; Croteau, D.; Ellis, R.J.; Rosario, D.; Potter, M.; Guillemin, G.J.; Brew, B.J.; Woods, S.P.; Letendre, S.L. HIV, prospective memory, and cerebrospinal fluid concentrations of quinolinic acid and phosphorylated Tau. J. Neuroimmunol. 2018, 319, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Valle, M.; Price, R.W.; Nilsson, A.; Heyes, M.; Verotta, D. CSF quinolinic acid levels are determined by local HIV infection: Cross-sectional analysis and modelling of dynamics following antiretroviral therapy. Brain 2004, 127, 1047–1060. [Google Scholar] [CrossRef]
- Gulaj, E.; Pawlak, K.; Bien, B.; Pawlak, D. Kynurenine and its metabolites in Alzheimer’s sidease patients. Adv. Med. Sci. 2010, 55, 204–211. [Google Scholar] [CrossRef]
- Solvang, S.-E.H.; Nordrehaug, J.E.; Aarsland, D.; Lange, J.; Ueland, P.M.; McCann, A.; Midttun, Ø.; Tell, G.S.; Giil, L.M. Kynurenines, Neuropsychiatric Symptoms, and Cognitive Prognosis in Patients with Mild Dementia. Int. J. Tryptophan Res. 2019, 12, 1178646919877883. [Google Scholar] [CrossRef]
- Voils, S.A.; Shoulders, B.R.; Singh, S.; Solberg, L.M.; Garrett, T.L.; Frye, R.F. Intensive care unit delirium in surgical patients is associated with upreguloation in tryptophan metabolism. Pharmacotherapy 2020, 40, 500–506. [Google Scholar] [CrossRef]
- Leonard, B.E. Inflammation and depression: A causal or coincidental link to the pathophysiology? Acta Neuropsychiatr. 2018, 30, 1–16. [Google Scholar] [CrossRef]
- Taylor, M.W.; Feng, G.S. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991, 5, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Stone, T.W.; Smith, R. Neurotoxicity of tryptophan metabolites. Biochem. Soc. Trans. 2007, 35, 1287–1289. [Google Scholar] [CrossRef] [PubMed]
- Kwidzinski, E.; Bechmann, I. IDO expression in the brain: A double-edged sword. J. Mol. Med. 2007, 85, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Ouma, B.J.; Ssenkusu, J.M.; Shabani, E.; Datta, D.; Opoka, R.O.; Idro, R.; Bangirana, P.; Park, G.; Joloba, M.L.; Kain, K.C.; et al. Endothelial Activation, Acute Kidney Injury, and Cognitive Impairment in Pediatric Severe Malaria. Crit. Care Med. 2020, 48, e734–e743. [Google Scholar] [CrossRef]
- Tao, X.; Yan, M.; Wang, L.; Zhou, Y.; Wang, Z.; Xia, T.; Liu, X.-M.; Pan, R.-L.; Chang, Q. Homeostasis Imbalance of Microglia and Astrocytes Leads to Alteration in the Metabolites of the Kynurenine Pathway in LPS-Induced Depressive-Like Mice. Int. J. Mol. Sci. 2020, 21, 1460. [Google Scholar] [CrossRef]
- Ridaura, V.; Belkaid, Y. Gut microbiota: The link to your second brain. Cell 2015, 161, 193–194. [Google Scholar] [CrossRef]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef]
- Schmidt, C. Mental Health: Thinking from the Gut. Nature 2015, 518, S12–S15. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Clément, K. The Effects of Gastrointestinal Surgery on Gut Microbiota: Potential Contribution to Improved Insulin Sensitivity. Curr. Atheroscler. Rep. 2014, 16, 454. [Google Scholar] [CrossRef]
- Lapthorne, S.; Bines, J.E.; Fouhy, F.; Dellios, N.L.; Wilson, G.; Thomas, S.L.; Scurr, M.; Stanton, C.; Cotter, P.D.; Pereira-Fantini, P. Changes in the colon microbiota and intestinal cytokine gene expression following minimal intestinal surgery. World J. Gastroenterol. 2015, 21, 4150–4158. [Google Scholar] [CrossRef]
- Lederer, A.-K.; Pisarski, P.; Kousoulas, L.; Fichtner-Feigl, S.; Hess, C.; Huber, R. Postoperative changes of the microbiome: Are surgical complications related to the gut flora? A systematic review. BMC Surg. 2017, 17, 125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bi, J.-J.; Guo, G.-J.; Yang, L.; Zhu, B.; Zhan, G.-F.; Li, S.; Huang, N.-N.; Hashimoto, K.; Yang, C.; et al. Abnormal composition of gut microbiota contributes to delirium-like behaviors after abdominal surgery in mice. CNS Neurosci. Ther. 2019, 25, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Liufu, N.; Liu, L.; Shen, S.; Jiang, Z.; Dong, Y.; Wang, Y.; Culley, D.; Crosby, G.; Cao, M.; Shen, Y.; et al. Anesthesia and surgery induce age-dependent changes in behaviors and microbiota. Aging 2020, 12, 1965–1986. [Google Scholar] [CrossRef] [PubMed]
- Kitsios, G.; Fair, K.; Xie, M.; Shah, F.; Fitch, A.; Rapport, S.; Huwe, J.; Alexander, S.; Morris, A.; Girard, T.D.; et al. Gut microbiome dysbiosis and delirium in mechanically ventilated adults patients: A prospective cohort study. In Critical Care: Microbiome, Genetics, and Other Biomarkers in Acute Critical Illness; American Thoracic Society: New York, NY, USA, 2018; p. A2777. [Google Scholar]
- Liskiewicz, P.; Kaczmarczyk, M.; Misiak, B.; Wronski, M.; Baba-Kubis, A.; Skonieczna-Zydecka, K.; Marlicz, W.; Bienkowski, P.; Misera, A.; Pelka-Wysiecka, J.; et al. Analysis of gut microbiota and intestinal integrity markers of inpatients with major depressive disorder. Prog. Neuropharmacol. Biol. Psychiatry 2020, 19, 110076. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, X.; Li, Z.; Shen, Y.; Shi, X.; Wang, L.; Li, G.; Yuan, Y.; Wang, J.; Zhang, Y.; et al. Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 3329–3337. [Google Scholar] [CrossRef]
- Inouye, S.K.; Bogardus, S.T., Jr.; Charpentier, P.A.; Leo-Summers, L.; Acampora, D.; Holford, T.R.; Cooney, L.M., Jr. A multicomponent intervention to prevent delirium in hospitalized ilder patients. N. Engl. J. Med. 1999, 340, 669–676. [Google Scholar] [CrossRef]
- Barr, J.; Fraser, G.L.; Puntillo, K.; Ely, E.W.; Gélinas, C.; Dasta, J.F.; Davidson, J.E.; Devlin, J.W.; Kress, J.P.; Joffe, A.M.; et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit. Care Med. 2013, 41, 263–306. [Google Scholar] [CrossRef]
- Morandi, A.; Piva, S.; Ely, E.W.; Myatra, S.N.; Salluh, J.I.F.; Amare, D.; Azoulay, E.; Bellelli, G.; Csomos, A.; Fan, E.; et al. Worldwide survey of the “Assessing pain, both spontaneous awakening and breathing trialsm choice of drugs, delirium monitoring/management, early exercise/mobility, and family empowerment” (ABCDEF) bundle reply. Crit. Care Med. 2017, 45, e1111–e1122. [Google Scholar] [CrossRef]
- Banerjee, A.; Girard, T.D.; Pandharipande, P. The complex interplay between delirium, sedation, and early mobility during critical illness: Applications in the trauma unit. Curr. Opin. Anaesthesiol. 2011, 24, 195–201. [Google Scholar] [CrossRef]
- Mailhot, T.; Cossette, S.; Lambert, J.; Cournoyer, A.; Denault, A. Cerebral oximetry as a biomarker of postoperative delirium in cardiac surgery patients. J. Crit. Care 2016, 34, 17–23. [Google Scholar] [CrossRef]
- Lei, L.; Katznelson, R.; Fedorko, L.; Carroll, J.; Poonawala, H.; Machina, M.; Styra, R.; Rao, V.; Djaiani, G. Cerebral oximetry and postoperative delirium after cardiac surgery: A randomised, controlled trial. Anaesthesia 2017, 72, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla-Vaca, A.; Healy, R.; Grant, M.C.; Joshi, B.; Rivera-Lara, L.; Brown, C.; Mirski, M.A. Intraoperative cerebral oximetry-based management for optimizing perioperative outcomes: A meta-analysis of randomized controlled trials. Can. J. Anesth. 2018, 65, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.F.; Wood, M.D.; Maslove, D.M.; Muscedere, J.; Boyd, J.G. Dysfunctional cerebral autoregulation is associated with delirium in critically ill adults. J. Cereb. Blood Flow Metab. 2019, 39, 2512–2520. [Google Scholar] [CrossRef] [PubMed]
- Scheeren, T.W.L.; Belda, F.J.; Perel, A. Correction to: The oxygen reserve index (ORI): A new tool to monitor oxygen therapy. J. Clin. Monit. 2018, 32, 379–389. [Google Scholar] [CrossRef]
- Chen, S.-T.; Min, S. Oxygen reserve index, a new method of monitoring oxygenation status: What do we need to know? Chin. Med. J. 2020, 133, 229–234. [Google Scholar] [CrossRef]
- Skrobik, Y.; Duprey, M.S.; Hill, N.S.; Devlin, J.W. Low-dose nocturnal dexmedetomidyne prevents ICU delirium. A randomized, placebo-controlles trial. Am. J. Respir. Crit. Care Med. 2018, 197, 1147–1156. [Google Scholar] [CrossRef]
- Dessap, A.M.; Roche-Campo, F.; Launay, J.-M.; Charles-Nelson, A.; Katsahian, S.; Brun-Buisson, C.; Brochard, L. Delirium and Circadian Rhythm of Melatonin During Weaning From Mechanical Ventilation: An ancillary study of a weaning trial. Chest 2015, 148, 1231–1241. [Google Scholar] [CrossRef]
- Olofsson, K.; Alling, C.; Lundberg, D.; Malmros, C. Abolished circadian rhythm of melatonin secretion in sedated and artificially ventilated intensive care patients. Acta Anaesthesiol. Scand. 2004, 48, 679–684. [Google Scholar] [CrossRef]
- Baumgartner, L.; Lam, K.; Lai, J.; Barnett, M.; Thompson, A.; Gross, K.; Morris, A. Effectiveness of Melatonin for the Prevention of Intensive Care Unit Delirium. J. Hum. Pharmacol. Drug Ther. 2019, 39, 280–287. [Google Scholar] [CrossRef]
- Weitzman, E.D.; Weinberg, U.; D’Eletto, R.; Lynch, H.; Wurtman, R.J.; Czeisler, C.; Erlich, S. Studies of the 24 h rhythm of melatonin in man. J. Neural Transm. Suppl. 1978, 13, 325–337. [Google Scholar]
- Rajaratnam, S.M.; Dijk, D.J.; Middleton, B.; Stone, B.M.; Arendt, J. Melatonin phase-shifts humen circadiad rhythms with no evidence of changes in the duration of endogenous melatonin secretion or the 24-h production of reproductive hormones. J. Clin. Endocrinol. Metab. 2003, 88, 4303–4309. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Venkatesh, B.; Cohen, J.; Hickman, I.J.; Nisbet, J.; Thomas, P.; Ward, G.; Hall, J.; Prins, J.; Prins, J.B. Evidence of altered cortisol metabolism in critically ill patients: A prospective study. Intensiv. Care Med. 2007, 33, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sheng, H.; Tang, Z.; Lu, J.; Ni, X. Inflammation and increased IDO in hippocampus contribute to depression-like behaviour induced by estrogen deficiency. Behav. Brain Res. 2015, 288, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Catena-Dell’Osso, M.; Rotella, F.; Dell’Osso, A.; Fagiolini, A.; Marazziti, D. Inflammation, serotonin and major depression. Curr. Drug Targets 2013, 14, 571–577. [Google Scholar] [CrossRef]
- Zhao, X.; Cao, F.; Liu, Q.; Li, X.; Xu, G.; Liu, G.; Zhang, Y.; Yang, X.; Yi, S.; Xu, F.; et al. Behavioral, inflammatory and neurochemical disturbances in LPS and UCMS-induced mouse models of depression. Behav. Brain Res. 2019, 364, 494–502. [Google Scholar] [CrossRef]
- Rioli, G.; Tassi, S.; Mattei, G.; Ferrari, S.; Galeazzi, G.M.; Mancini, S.; Alboni, S.; Roncucci, L. The Association Between Symptoms of Anxiety, Depression, and Cardiovascular Risk Factors: Rresults from an Italian cross-sectional study. J. Nerv. Ment. Dis. 2019, 207, 340–347. [Google Scholar] [CrossRef]
- Kotfis, K.; Bott-Olejnik, M.; Szylińska, A.; Listewnik, M.; Rotter, I. Characteristic, risk factor and outcome of early-onset delirium in elderly patients with first ever acute ischemic stroke—A prospective observational cohort study. Clin. Interv. Aging 2019, 14, 1771–1782. [Google Scholar] [CrossRef]
- Li, L.-Q.; Wang, C.; Fang, M.-D.; Xu, H.-Y.; Lu, H.-L.; Zhang, H.-Z. Effects of Dexamethasone on Post-Operative Cognitive Dysfunction and Delirium in Adults Following General Anaesthesia: A Meta-Analysis of Randomised Controlled Trials. BMC Anesthesiol. 2019, 19, 113. [Google Scholar] [CrossRef]
- Lally, L.; McCarthy, G.M.; Meehan, K. Hyperactive delirium following administration of intra-articular corticosteroid. BMJ Case Rep. 2017, 2017, 2014217483. [Google Scholar] [CrossRef]
- Reis, P.A.; Alexandre, P.C.; D’Avila, J.C.; Siqueira, L.D.; Antunes, B.; Estato, V.; Tibiriça, E.V.; Verdonk, F.; Sharshar, T.; Chrétien, F.; et al. Statins prevent cognitive impairment after sepsis by reverting neuroinflammation, and microcirculatory/endothelial dysfunction. Brain Behav. Immun. 2017, 60, 293–303. [Google Scholar] [CrossRef]
- Alam, A.; Hana, Z.; Jin, Z.; Suen, K.C.; Ma, D. Surgery, neuroinflammation and cognitive impairment. EBioMedicine 2018, 37, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.W.; Shiue, Y.L.; Liao, J.C.; Wee, H.Y.; Wang, C.C.; Chio, C.C.; Chang, C.H.; Hu, C.Y.; Kuo, J.R. Simvastatin therapy in the acute stage of traumatic brain injury attenuates brain trauma-induced depression-like behaviour in rats by reducing neuroinflammation in the hippocampus. Neurocrit. Care 2017, 26, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Morandi, A.; Hughes, C.G.; Thompson, J.L.; Pandharipande, P.; Shintani, A.K.; Vasilevskis, E.E.; Han, J.H.; Jackson, J.C.; Laskowitz, D.T.; Bernard, G.R.; et al. Statins and Delirium During Critical Illness. Crit. Care Med. 2014, 42, 1899–1909. [Google Scholar] [CrossRef] [PubMed]
- Salluh, J.I.F.; Latronico, N. Does this critically ill patient with delirium require any drug treatment? Intensiv. Care Med. 2019, 45, 501–504. [Google Scholar] [CrossRef]
- Al-Qadheeb, N.S.; Skrobik, Y.; Schumaker, G.; Pacheco, M.N.; Roberts, R.J.; Ruthazer, R.R.; Devlin, J.W. Preventing ICU Subsyndromal Delirium Conversion to Delirium With Low-Dose IV Haloperidol: A double-blind, placebo-controlles pilot study. Crit. Care Med. 2016, 44, 583–591. [Google Scholar] [CrossRef]
- Felton, M.A.; Jarrett, J.B.; Hoffmaster, R.; D’Amico, F.J.; Sakely, H.; Proskowski, J. Comparison of haloperidol, non-haloperidol antipsychotics, and no pharnmacotherapy for the management of delirium in an inpatient geriatric palliative care population. J. Pain Palliat. Care Pharmacother. 2018, 32, 141–148. [Google Scholar] [CrossRef]
- Sher, Y.; Cramer, A.C.M.; Ament, A.; Lolak, S.; Maldonado, J.R. Valproic Acid for Treatment of Hyperactive or Mixed Delirium: Rationale and Literature Review. Psychosomatics 2015, 56, 615–625. [Google Scholar] [CrossRef]
- Devlin, J.W.; Skrobik, Y.; Gélinas, C.; Needham, D.M.; Slooter, A.J.C.; Pandharipande, P.P.; Watson, P.L.; Weinhouse, G.L.; Nunnally, M.E.; Rochwerg, B.; et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit. Care Med. 2018, 46, e825–e873. [Google Scholar] [CrossRef]
- Girard, T.D.; Exline, M.C.; Carson, S.S.; Hough, C.L.; Rock, P.; Gong, M.N.; Douglas, I.S.; Malhotra, A.; Owens, R.L.; Feinstein, D.J.; et al. Haloperidol and Ziprasidone for Treatment of Delirium in Critical Illness. N. Engl. J. Med. 2018, 379, 2506–2516. [Google Scholar] [CrossRef]
- Shah, A.A.; Aftab, A.; Coverdale, J. QTc Prolongation with Antipsychotics: Is routine ECG monitoring recommended? J. Psychiatr. Pract. 2014, 20, 196–206. [Google Scholar] [CrossRef]
- Sher, Y.; Miller, A.C.; Lolak, S.; Ament, A.; Maldonado, J.R. Adjunctive Valproic Acid in Management-Refractory Hyperactive Delirium: A Case Series and Rationale. J. Neuropsychiatry Clin. Neurosci. 2015, 27, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Cutuli, D.; De Guevara-Miranda, D.L.; Castilla-Ortega, E.; Santín, L.; Sampedro-Piquero, P. Highlighting the Role of Cognitive and Brain Reserve in the Substance use Disorder Field. Curr. Neuropharmacol. 2019, 17, 1056–1070. [Google Scholar] [CrossRef] [PubMed]
| Pathomechanisms | Authors and Reference Number | Study Design | Number of Patients | Results |
|---|---|---|---|---|
| Hypoxia | Funk et al. [16] | Prospective controlled clinical study | 15 septic shock patients | Decrease in cerebral saturation corresponds to the incidence of delirium |
| Mikkelsen et al. [17] | Prospective, multicentre cohort clinical study | 406 adult patients treated for ARDS | Low PaO2 was associated with cognitive impairment | |
| Hopkins et al. [18] | Prospective controlled clinical study | 120 adult patients treated for ARDS | Hypoxia assessed as SaO2 < 90% is associated with long-term neurocognitive disorders | |
| Hyperoxia | Kupiec et al. [19] | Retrospective clinical study | 93 cardiac surgery patients | Hyperoxia defined as PaO2 > 120 mmHg is associated with the occurrence of postoperative delirium |
| Mutch et al. [20] | Prospective clinical study | 12 healthy volunteers | Disturbance in cerebral blood flow following hyperoxia corresponds with postoperative neuropsychological disorders | |
| Lopez et al. [24] | Prospective controlled clinical study | 310 cardiac surgery patients | Hyperoxia defined as any intraoperative cerebral oxygenation greater than baseline | |
| Neuroinflammation | Velagapudi et al. [36] | Experimental, behavioural and histological study | 61 animals undergoing orthopaedic surgery | Orthopaedic surgery leads to microglial activation, astrogliosis and brain blood-barrier disruption |
| Disorders in neurotransmitters | Adam et al. [47] | Prospective observational study | 114 cardiac surgery patients | Decrease in acetylcholine hydrolysing enzyme activity increases risk for delirium |
| John et al. [48] | Prospective observational study | 251 cardiac surgery patients | There are no correlations between acetylcholine hydrolysing enzyme activity and risk of delirium | |
| Yilmaz et al. [54] | Prospective observational study | 137 cardiac surgery patients | Dopamine infusion is an independent risk factor for delirium | |
| Yoshitaka et al. [57] | Prospective observational study | 40 critically ill patients | Plasma GABA activity is associated with delirium | |
| Wyrobek et al. [61] | Prospective observational study | 77 elderly patients undergoing spinal surgery | Decrease in the brain-derived neurotrophic factor is associated with delirium | |
| Madsen et al. [67] | Prospective observational study | 30 healthy volunteers | Disorders in 5-HT4 receptor correlate with impaired memory and risk for neuropsychiatric disorders | |
| Tryptophan metabolism and kynurenine pathway dysregulation | Kozak et al. [75] | Experimental, behavioural and histological study | Animal study | Elevated brain kynurenic acid impairs cognitive function |
| Valle et al. [77] | Prospective observational study | 62 HIV-infected patients | Elevated quinolinic acid is a risk factor for neurocognitive disorders | |
| Gulaj et al. [78] | Prospective observational study | 34 patients with Alzheimer dementia | Plasma kynurenic acid and quinolinic acid correlate with impaired cognitive function | |
| Solvang et al. [79] | Prospective observational study | 155 patients with dementia | Kynurenine had a nonlinear quadratic relationship with cognitive disorders | |
| Gut microbiota dysregulation | Zhang et al. [93] | Experimental, behavioural study | 11 pigs | Gut microbiota disorders induce delirium |
| Liufu et al. [94] | Experimental, behavioural study | 10 mice | Gut microbiota disorders induce delirium | |
| Liskiewicz et al. [96] | Prospective observational study | 16 patients with major depression | Disorders in gut microbiota are associated with the severity of depression | |
| Huang et al. [97] | Prospective observational study | 54 patients with major depression | Defects of the Firmicutes (gut bacteria) increase a risk for depression |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabrowski, W.; Siwicka-Gieroba, D.; Gasinska-Blotniak, M.; Zaid, S.; Jezierska, M.; Pakulski, C.; Williams Roberson, S.; Wesley Ely, E.; Kotfis, K. Pathomechanisms of Non-Traumatic Acute Brain Injury in Critically Ill Patients. Medicina 2020, 56, 469. https://doi.org/10.3390/medicina56090469
Dabrowski W, Siwicka-Gieroba D, Gasinska-Blotniak M, Zaid S, Jezierska M, Pakulski C, Williams Roberson S, Wesley Ely E, Kotfis K. Pathomechanisms of Non-Traumatic Acute Brain Injury in Critically Ill Patients. Medicina. 2020; 56(9):469. https://doi.org/10.3390/medicina56090469
Chicago/Turabian StyleDabrowski, Wojciech, Dorota Siwicka-Gieroba, Malgorzata Gasinska-Blotniak, Sami Zaid, Maja Jezierska, Cezary Pakulski, Shawniqua Williams Roberson, Eugene Wesley Ely, and Katarzyna Kotfis. 2020. "Pathomechanisms of Non-Traumatic Acute Brain Injury in Critically Ill Patients" Medicina 56, no. 9: 469. https://doi.org/10.3390/medicina56090469
APA StyleDabrowski, W., Siwicka-Gieroba, D., Gasinska-Blotniak, M., Zaid, S., Jezierska, M., Pakulski, C., Williams Roberson, S., Wesley Ely, E., & Kotfis, K. (2020). Pathomechanisms of Non-Traumatic Acute Brain Injury in Critically Ill Patients. Medicina, 56(9), 469. https://doi.org/10.3390/medicina56090469






