Influences of Successive Exposure to Bleaching and Fluoride Preparations on the Surface Hardness and Roughness of the Aged Resin Composite Restoratives
Abstract
:1. Introduction
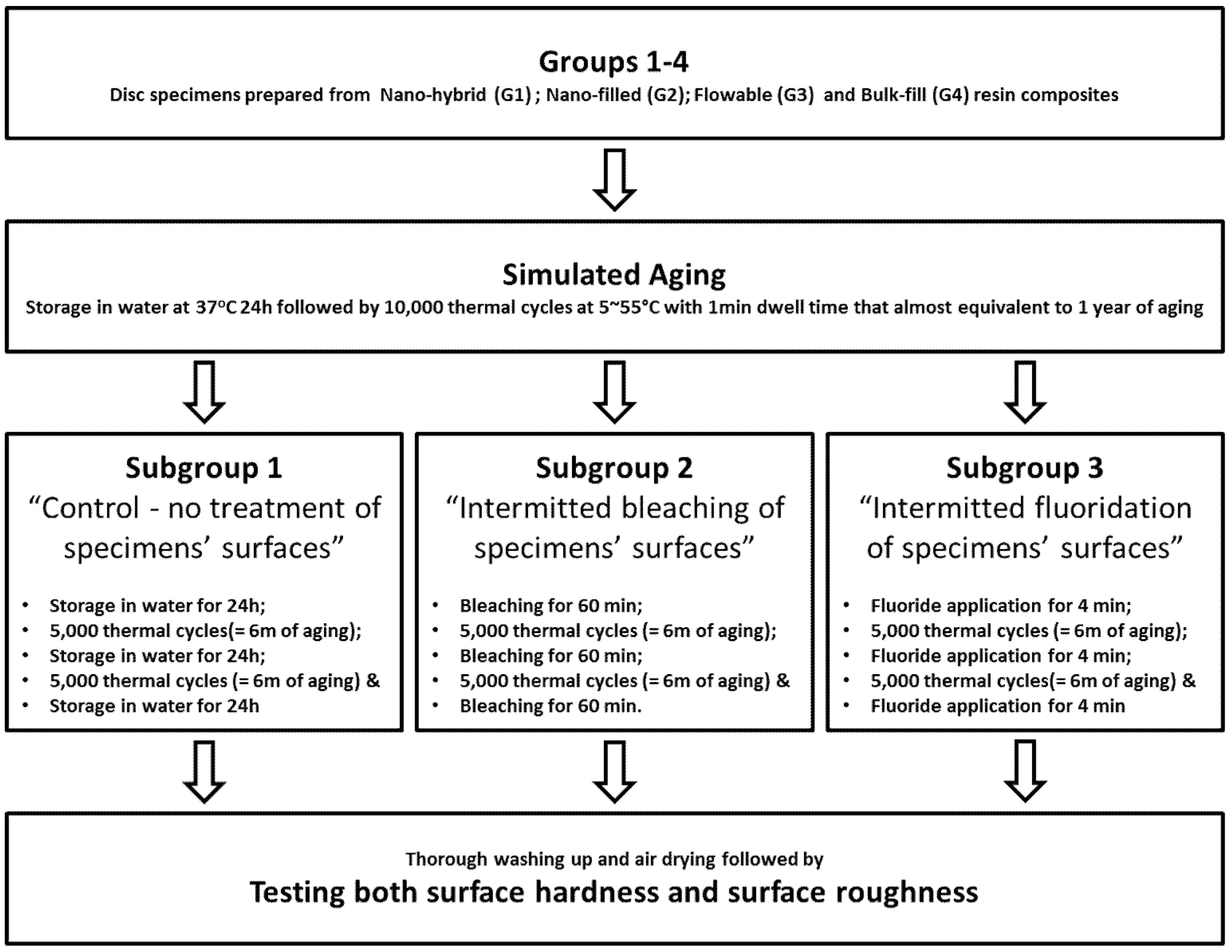
2. Materials and Methods
3. Results
3.1. Surface Hardness
3.2. Surface Roughness
4. Discussion
5. Conclusions
- Aged resin composite restorative materials can provide minimal surface alterations on successive bleaching and fluoride application; however, the flowable type of resin composites is the most affected by both clinical procedures.
- Although successive fluoride applications are deteriorating to the surfaces of the tested resin composites, repeated bleaching seems less lethal for the more viscous types.
- Following up of the existing restorations is advised following the bleaching and fluoride application procedures to determine the necessity of replacing the stained or worn restorations.
Author Contributions
Funding
Conflicts of Interest
References
- Davies, R.A.; Ardalan, S.; Mu, W.; Tian, K.; Farsaikiya, F.; Darvell, B.W.; Chass, G.A. Geometric, electronic and elastic properties of dental silver amalgam g-(Ag3Sn), g1-(Ag2Hg3), g2-(Sn8Hg) phases, comparison of experiment and theory. Intermetallics 2010, 18, 756–760. [Google Scholar] [CrossRef]
- Sharanbir, K.; Sidhu, S.K.; Nicholson, J.W. A review of glass-ionomer cements for clinical dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, X.; Li, M.; Peng, X.; Wang, S.; Zhou, X.; Cheng, L. Development and status of resin composite as dental restorative materials. J. Appl. Polym. Sci. 2019, 136, 48180. [Google Scholar] [CrossRef] [Green Version]
- Bayne, S.C.; Thompson, J.Y.; Swift, E.J., Jr.; Stamatiades, P.; Wilkerson, M. A characterization of first generation of flowable composite. J. Am. Dent. Assoc. 1998, 129, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Rashidian, A.; Saghiri, M.A.; Bigloo, S.M.; Afsharianzadeh, M. Effect of fluoride gel on microhardness of flowable composites: An in vitro study. J. Dent. Sch. 2014, 32, 16–22. [Google Scholar]
- Gupta, R.; Tomer, A.K.; Kumari, A.; Mullick, S.; Dubey, S. Bulkfill flowable composite resins—A review. Int. J. Appl. Dent. Sci. 2017, 3, 38–40. [Google Scholar]
- Mitra, S.B.; Wu, D.; Holmes, B.N. Holmes an Application of nanotechnology in advanced dental materials. J. Am. Dent. 2003, 134, 1382–1390. [Google Scholar]
- Schulze, K.A.; Marshall, S.J.; Gansky, S.A.; Marshall, G.W. Color stability and hardness in dental composites after accelerated aging. Dent. Mater. 2003, 19, 612–619. [Google Scholar]
- Khurshid, Z.; Zafar, M.; Qasim, S.; Shahab, S.; Naseem, M.; AbuReqaiba, A. Advances in nanotechology for restorative dentistry. Materials 2015, 8, 717–731. [Google Scholar] [CrossRef] [Green Version]
- Fortin, D.; Vargas, M.A. The spectrum of composites: New techniques and materials. J. Am. Dent. Assoc. 2000, 131, 26–30. [Google Scholar]
- Bashetty, K.; Joshi, S. The effect of one-step and multi-step polishing systems on surface texture of two different resin composites. J. Conserv. Dent. 2010, 13, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Wilson, F.; Heath, J.R.; Watts, D.C. Finishing composite restorative materials. J. Oral Rehabil. 1990, 17, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Kumari, C.M.; Bhat, K.M.; Bansal, R. Evaluation of surface roughness of different restorative composites after polishing using atomic force microscopy. J. Conserv. Dent. 2016, 19, 56–62. [Google Scholar] [CrossRef]
- Zuryati, A.G.; Qian, O.Q.; Dasmawati, M. Effects of home bleaching on surface hardness and surface roughness of an experimental nanocomposite. J. Conserv. Dent. 2013, 16, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Carretero-Pelaez, M.A.; Esparza-Gomez, G.C.; Figuero-Ruiz, E.; Cerero-Lapiedra, R. Alcohol-containing mouthwashes and oral cancer. Critical analysis of literature. Med. Oral. 2004, 9, 120–123. [Google Scholar] [PubMed]
- Dadoun, M.P.; Bartlett, D.W. Safety issues when using carbamide peroxide to bleach vital teeth—A review of the literature. Eur. J. Prosthodont. Restorat. Dent. 2003, 11, 9–13. [Google Scholar]
- Heithersay, G.S.; Dahlstrom, S.W.; Marin, P.D. Incidence of invasive cervical resorption in bleached root-filled teeth. Aust. Dent. J. 1994, 39, 82–87. [Google Scholar] [CrossRef]
- Attin, T.; Hannig, C.; Weigand, A.; Attin, R. Effect of bleaching on restorative materials and restorations—A systematic review. Dent. Mater. 2004, 20, 852–861. [Google Scholar] [CrossRef]
- Turker, S.B.; Biskin, T. Effect of three bleaching agents on the surface properties of three different esthetic restorative materials. J. Prosthet. Dent. 2003, 89, 466–473. [Google Scholar] [CrossRef]
- Turker, S.B.; Biskin, T. The effect of bleaching agents on the microhardness of dental aesthetic restorative materials. J. Oral Rehabil. 2002, 29, 657–661. [Google Scholar] [CrossRef]
- Yap, A.U.; Mok, B.Y. Effects of professionally applied topical fluorides on surface hardness of composite- based restoratives. Operat. Dent. 2002, 27, 576–581. [Google Scholar]
- Abate, P.F.; Bertacchini, S.M.; Garcia-Godoy, F.; Macchi, R.L. Barcoll hardness of dental materials treated with an APF foam. J. Clin. Pediatr. Dent. 2001, 25, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Hafez, R.; Ahmed, D.; Yousry, M.; El-Badrawy, W.; El-Mowafy, O. Effect of in-office bleaching on color and surface roughness of composite restoratives. Eur. J. Dent. 2010, 4, 118–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dionysopoulos, D.; Koliniotou-Koumpia, E. Effect of acidulated phosphate fluoride gel on the surface of dental nanocomposite restorative materials. J. Nano Res. 2018, 51, 1–12. [Google Scholar]
- Hamza, T.A.; Alameldin, A.A.; Elkouedi, A.Y.; Wee, A.G. Effect of artificial accelerated aging on surface roughness and color stability of different ceramic restorations. Stomatol. Dis. Sci. 2017, 1, 8–13. [Google Scholar]
- Melo, M.; de Veiga, A.; Ribeiro, M.M.; dos Santos, S.G.; Alcântara, C.A.P.; Ribeiro Rabelo, J.C. Effects of different surface treatments and accelerated artificial aging on the bond strength of composite resin repairs. Braz. Oral Res. 2011, 25, 485–491. [Google Scholar]
- Ghavami-Lahiji, M.; Firouzmanesh, M.; Bagheri, H.; Kashi, T.S.J.; Razazpour, F.; Behroozibakhsh, M. The effect of thermocycling on the degree of conversion and mechanical properties of a microhybrid dental resin composite. Restorat. Dent. Endodont. 2018, 43, e26. [Google Scholar]
- Galea, M.S.; Darvellref, B.W. Thermal cycling procedures for laboratory testing of dental restorations. J. Dent. 1999, 27, 89–99. [Google Scholar]
- Ozcan, M.; Barbosa, S.H.; Melo, R.M.; Galhano, G.A.; Bottino, M.A. Effect of surface conditioning methods on the microtensile bond strength of resin composite to composite after aging conditions. Dent. Mater. 2007, 23, 1276–1282. [Google Scholar]
- Braxton, A.; Garrett, L.; Versluis-Tantbirojn, D.; Versluis, A. Does fluoride gel/foam application time affect enamel demineralization? J. Tenn. Dent. Assoc. 2014, 94, 28–31. [Google Scholar]
- Fiorillo, L.; Laino, L.; De Stefano, R.; D’Amico, C.; Bocchieri, S.; Amoroso, G.; Isola, G.; Cervino, G. Dental whitening gels: Strengths and weaknesses of an increasingly used method. Gels 2019, 5, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richard, B.T.; Price, R.B.T.; Sedarous, M.; Hiltz, G.S. The pH of tooth-whitening products. J. Can. Dent. Assoc. 2000, 66, 421–426. [Google Scholar]
- ADA Oral Health Topics. Topical and Systemic Supplements. Available online: https://www.ada.org/en/member-center/oral-health-topics/fluoride-topical-and-systemic-supplements (accessed on 12 July 2020).
- Botta, A.C.; Mollica, F.B.; Ribeiro, C.F.; De Araujo, M.A.M.; Di Nicoló, R.; Balducci, I. Influence of topical acidulated phosphate fluoride on surface roughness of human enamel and different restorative materials. Rev. Odonto Ciênc. 2010, 25, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Fiorillo, L.; Cervino, G.; Herford, A.S.; Laino, L.; Cicciù, M. Stannous fluoride effects on enamel: A systematic review. Biomimetics 2020, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.H.Y.; Chik, F.F. Fluoride retention following topical fluoride foam and gel application. Pediatr. Dent. 1990, 12, 368–374. [Google Scholar]
- Bharti, R.; Wadhwani, K.K.; Tikku, A.P.; Chandra, A. Dental amalgam: An update. J. Conserv. Dent. 2010, 13, 204–208. [Google Scholar] [CrossRef]
- Ramoglu, S.I.; Usumez, S.; Buyukyilmaz, T. Accelerated aging effects on surface hardness and roughness of lingual retainer adhesives. Angle Orthodont. 2008, 78, 140–144. [Google Scholar] [CrossRef]
- Francis, G.; Pradeep, K.; Ginjupalli, K.; Saraswathi, V. Effects of bleaching agents on the microhardness and surface roughness of bulk fill composites. World J. Dent. 2017, 8, 196–201. [Google Scholar]
- Bahannan, S.A. Effects of different bleaching agent concentrations on surface roughness and microhardness of esthetic restorative materials. Saudi J. Dent. Res. 2015, 6, 124–128. [Google Scholar] [CrossRef] [Green Version]
- El-Murr, J.; Ruel, D.; St-Georges, A.J. Effects of external bleaching on restorative materials: A review. J. Can. Dent. Assoc. 2011, 77, b59. [Google Scholar]
- Bicer, C.O.; Oz, F.D.; Attar, N. Effects of two different bleaching agents on surface roughness and microhardness of different novel nano-restorative materials. Eur. J. Gen. Dent. 2017, 6, 86–91. [Google Scholar]
- Leal, A.; Paula, A.; Ramalho, A.; Esteves, M.; Ferreira, M.M.; Carrilho, E. Roughness and microhardness of composites after different bleaching techniques. J. Appl. Biomater. Funct. Mater. 2015, 13, e381–e388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, Y.H.; Shin, D.H.; Yun, D.I.; Heo, Y.J.; Seol, H.J.; Kim, H.I. Effect of hydrogen peroxide on microhardness and color change of resin nanocomposites. Am. J. Dent. 2010, 23, 19–22. [Google Scholar] [PubMed]
- Mujeeb, A.; Mansouri, S.; Hussain, S.A.; Ramaswamy, K. In vitro evaluation of topical fluoride pH and their effect on surface hardness of composite resin-based restorative materials. J. Contemp. Dent. Pract. 2014, 15, 190–194. [Google Scholar]
- Yeh, S.T.; Wang, H.T.; Liao, H.Y.; Su, S.L.; Chang, C.C.; Kao, H.C.; Lee, B.S. The roughness, microhrdness and surface analysis of nanocomposites after application of topical fluoride gels. Dent. Mater. 2011, 27, 187–196. [Google Scholar] [CrossRef]
- Mazaheri, R.; Pishevar, L.; Keyhanifard, N.; Ghasemi, E. Comparing the effect of topical acidulated phosphate fluoride on micro-hardness of two fissure sealants and one flowable composite. J. Dent. Sch. 2014, 32, 103–110. [Google Scholar]
- Diab, M.; Zaazou, M.H.; Mubarak, E.H.; Fahmy, O.M.I. Effect of five commercial mouthrinses on the microhardness and color stability of two resin composite restorative materials. Aust. J. Basic Appl. Sci. 2007, 1, 667–674. [Google Scholar]
- Yikilgan, İ.; Kamak, H.; Akgul, S.; Ozcan, S.; Bala, O. Effects of three different bleaching agents on microhardness and roughness of composite sample surfaces finished with different polishing techniques. J. Clin. Exp. Dent. 2017, 9, e460–e465. [Google Scholar] [CrossRef] [Green Version]
- Taib, F.M.; Ghani, Z.A.B.; Mohamad, D. Effect of home bleaching agents on the hardness and surface roughness of resin composites. Arch. Orofac. Sci. 2013, 8, 34–40. [Google Scholar]
- Giannini, M.; Di Francescantonio, M.; Pacheco, R.R.; Cidreira Boaro, L.C.; Braga, R.R. Characterization of water sorption, solubility, and roughness of silorane- and methacrylate-based composite resins. Operat. Dent. 2014, 39, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, D.; Mor, C.; Dogan, H.; Zacks, B.; Rotstein, I. Effect of salivary biofilm on the adherence of oral bacteria to bleached and non-bleached restorative material. Dent. Mater. 1999, 15, 14–20. [Google Scholar]
- Gurgan, S.; Yalcin, F. The effect of 2 different bleaching regimens on the surface roughness and hardness of tooth-colored restorative materials. Quintessence Int. 2007, 38, e83–e87. [Google Scholar]
- Dogan, A.; Ozcelik, S.; Dogan, O.M.; Hubbezoglu, I.; Cakmak, M.; Bolayir, G. Effect of bleaching on roughness of dental composite resins. J. Adhes. 2008, 84, 897–914. [Google Scholar]
- Nica, I.; Stoleriu, S.; Iovan, G.; Pancu, G.; Ursu, L.; Georgescu, A.; Andrian, S. Qualitative assessment of surface characteristics of flowable composite submitted to acidic challenges. Inter. J. Med. Dent. 2018, 22, 358–367. [Google Scholar]
- Poggio, C.; Dagna, A.; Chiesa, M.; Colombo, M.; Scribante, A. Surface roughness of flowable resin composites eroded by acidic and alcoholic drinks. J. Conserv. Dent. 2012, 15, 137–140. [Google Scholar]
- Jafarzadeh, M.; Malekafzali, B.; Tadayon, N.; Fallahi, S. Retention of a flowable composite resin in comparison to a conventional resin-based sealant: Oneyear follow-up. J. Dent. 2010, 7, 1–5. [Google Scholar]
- Nica, I.; Iovan, G.; Ghiorghe, A.; Stoleriu, S.; Pancu, G.; Andrian, S. Comparative study regarding the chemical corrosion of different types of composite resins in artificial saliva. Rom. J. Oral Rehabil. 2015, 7, 37–42. [Google Scholar]
- Han, L.; Okamoto, A.; Fukushima, M.; Okiji, T. Evaluation of flowable resins composite surface eroded by acidic and alcoholic drinks. Dent. Mater. J. 2008, 27, 455–465. [Google Scholar]
| Product | Description | Composition | Manufacturer |
|---|---|---|---|
| Filtek Z250 | Micro-hybrid universal composite restorative | Matrix: bisGMA, UDMA, TEGDMA, Bis-EMA | 3M ESPE St. Paul, MN |
| Filler (78 wt%/60 vol%): silica/zirconia. The filler particle size distribution is 0.01 µm to 3.5 µm with an average particle size of 0.6 µm. | |||
| Filtek 350 XT | Nano-filled visible light-activated universal composite restorative | Matrix: bis-GMA, UDMA, TEGDMA, bis-EMA, PEGDMA resins. | 3M ESPE St. Paul, MN |
| Fillers (72.5 wt%/55.6 vol%): Combination of 20 nm non-agglomerated/non-aggregated silica filler; 4–11 nm non-agglomerated/non-aggregated zirconia filler, and 0.6–10 µm surface modified aggregated zirconia (4–11 nm)/silica (20 nm) clusters. | |||
| Filtek 350 XT Flowable | Nano-filled visible light-activated flowable composite restorative | Matrix: bis-GMA, TEGDMA, procrylate resin | 3M ESPE St. Paul, MN |
| Fillers (65 wt%/46 vol%): Combination of 1–5 µm yetterbium triflioride fillers; 20 and 75 nm non-agglomerated, non-aggregated silica fillers and 0.6–10 µm surface modified aggregated zirconia (4–11 nm)/silica (20 nm) clusters. | |||
| Filtek Bulk-fill | Nano-filled visible light-activated posterior composite restorative. | Matrix: AUDMA, UDMA and 1, 12-dodecane-DMA. | 3M ESPE St. Paul, MN |
| Filler (76.5 wt%/58.4 vol%): combination of a non-agglomerated/non-aggregated 20 nm silica filler, a non-agglomerated/non-aggregated 4 to 11 nm zirconia filler, aggregated zirconia/silica cluster filler (comprised of 20 nm silica and 4 to 11 nm zirconia particles) and a ytterbium trifluoride filler consisting of agglomerate 100 nm particles. | |||
| Opalescence Boost PF | Chemically-activated neutral (pH 7) in-office bleaching agent | Barrel 1: 1.1% sodium fluoride and 3% potassium nitrate, along with a unique chemical activator. | Ultradent Products Inc. South Jordan, UT |
| Barrel 2: Hydrogen peroxide. | |||
| After mixing the final hydrogen peroxide concentration is 40%. | |||
| Gelato APF gel | Acidulated phosphate fluoride gel | Active ingredients: 2.09% Sodium fluoride and Hydrofluoric acid providing 1.23% fluoride ions. | Deepak Inc. Miami, FL |
| Inactive ingredients: Flavor, phosphoric acid, sodium saccharin, xylitol, citric acid, sodium benzoate, water, titanium dioxide polysorbate 20, xanthan gum, magnesium aluminum silicate, FD&C red # 40. |
| Surface Treatment | Resin Composites | |||
|---|---|---|---|---|
| Conventional | Bulk-Fill (G4) | |||
| Micro-Hybrid (G1) | Nano-Filled (G2) | Flowable (G3) | ||
| No-Treatment (SG1) | 68.85 ± 3.54 A,1 | 55.26 ± 4.05 B,1 | 49.83 ± 9.48 B,1 | 48.77 ± 5.29 B,1 |
| Bleaching (SG2) | 68.97 ± 4.59 A,1 | 57.05 ± 7.12 B,1 | 41.11 ± 8.19 C,1,2 | 41.88 ± 7.76 C,1 |
| Fluoride (SG3) | 53.90 ± 5.69 A,2 | 51.31 ± 4.56 A,1 | 38.66 ± 3.64 B,2 | 39.96 ± 2.55 B,1 |
| Surface Treatment | Resin Composites | |||
|---|---|---|---|---|
| Conventional | Bulk-Fill (G4) | |||
| Micro-Hybrid (G1) | Nano-Filled (G2) | Flowable (G3) | ||
| Non-Treatment (SG1) | 17.34 ± 2.90 A,1 | 14.64 ± 3.15 A,1 | 8.32 ± 1.26 A,1 | 10.18 ± 2.69 A,1 |
| Bleaching (SG2) | 18.68 ± 1.47 A,1 | 19.81 ± 2.18 A,1 | 45.36 ± 6.86 B,2 | 21.50 ± 2.68 A,1 |
| Fluoride (SG3) | 39.90 ± 4.36 A,2 | 58.20 ± 10.55 B,2 | 76.94 ± 4.97 C,3 | 63.24 ± 7.44 B,2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelaziz, K.M.; Mir, S.; Khateeb, S.U.; Baba, S.M.; Alshahrani, S.S.; Alshahrani, E.A.; Alsafi, Z.A. Influences of Successive Exposure to Bleaching and Fluoride Preparations on the Surface Hardness and Roughness of the Aged Resin Composite Restoratives. Medicina 2020, 56, 476. https://doi.org/10.3390/medicina56090476
Abdelaziz KM, Mir S, Khateeb SU, Baba SM, Alshahrani SS, Alshahrani EA, Alsafi ZA. Influences of Successive Exposure to Bleaching and Fluoride Preparations on the Surface Hardness and Roughness of the Aged Resin Composite Restoratives. Medicina. 2020; 56(9):476. https://doi.org/10.3390/medicina56090476
Chicago/Turabian StyleAbdelaziz, Khalid M., Shugufta Mir, Shafait Ullah Khateeb, Suheel M. Baba, Saud S. Alshahrani, Eman A. Alshahrani, and Zahra A. Alsafi. 2020. "Influences of Successive Exposure to Bleaching and Fluoride Preparations on the Surface Hardness and Roughness of the Aged Resin Composite Restoratives" Medicina 56, no. 9: 476. https://doi.org/10.3390/medicina56090476





