Low-Dose Pre-Operative Botulinum Toxin A Effectively Facilitates Complex Ventral Hernia Repair: A Case Report and Review of the Literature
Abstract
:1. Introduction
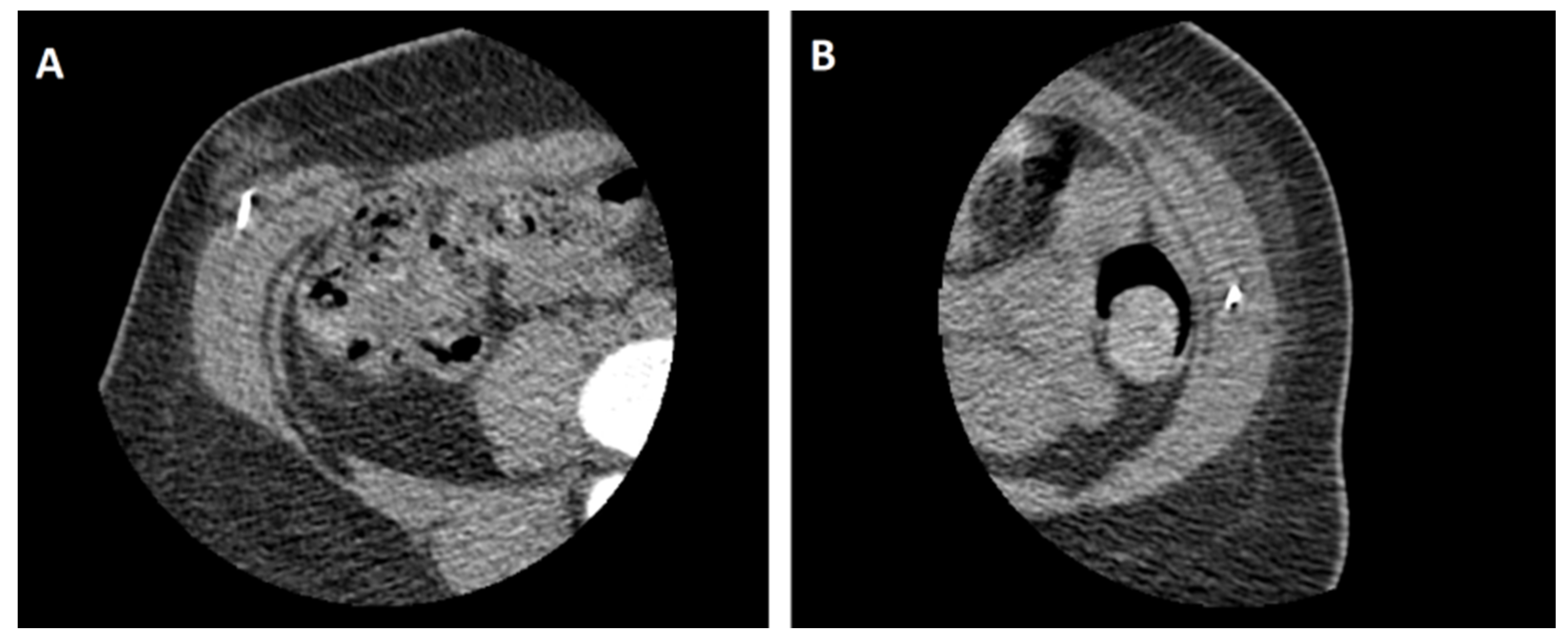
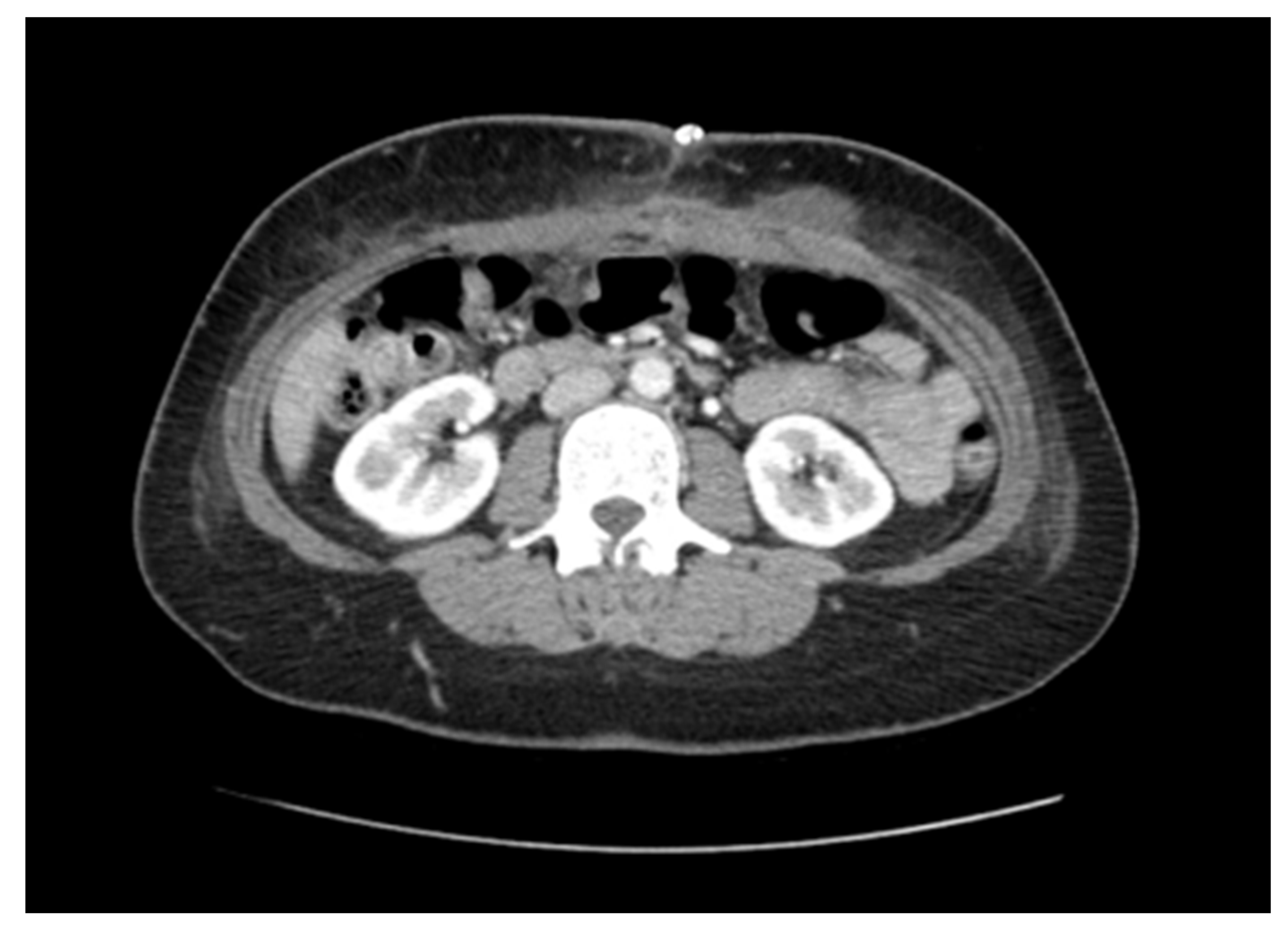
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ramshorst, G.H.; Eker, H.H.; Hop, W.C.; Jeekel, J.; Lange, J.F. Impact of incisional hernia on health-related quality of life and body image: A prospective cohort study. Am. J. Surg. 2012, 204, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Vries, R.T.S.; Goor, H.; Rosman, C.; Bemelmans, M.H.; Jong, D.; Nieuwenhoven, E.J.; Engelend, M.I.v.A.; Bleichrodt, R.P. "Components separation technique" for the repair of large abdominal wall hernias. J. Am. Coll. Surg. 2003, 196, 32–37. [Google Scholar]
- Scheuerlein, H.; Settmacher, U.; Lenschow, M.; Rauchfuß, F. Complex Incisional Hernias. Arch Clin Gastroenterol. 2016, 2, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Slater, N.J.; Montgomery, A.; Berrevoet, F.; Carbonell, A.M.; Chang, A.; Franklin, M.; Kercher, K.W.; Lammers, B.J.; Parra-Davilla, E.; Towfigh, S.; et al. Criteria for definition of a complex abdominal wall hernia. Hernia. J. Hernias Abdom. Wall Surg. 2014, 18, 7–17. [Google Scholar] [CrossRef]
- Ramirez, O.M.; Ruas, E.; Dellon, A.L. “Components Separation” Method for Closure of Abdominal-Wall Defects: An Anatomic and Clinical Study. Plast. Reconstr. Surg. 1990, 86, 519–526. [Google Scholar] [CrossRef]
- Heller, L.; McNichols, C.H.; Ramirez, O.M. Component separations. Semin Plast Surg. 2012, 26, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Verheyden, J.; Blitzer, A.; Brin, M.F. Other noncosmetic uses of BOTOX. Semin. Cutan. Med. Surg. 2001, 20, 121–126. [Google Scholar] [CrossRef]
- Dressler, D. Clinical applications of botulinum toxin. Curr. Opin. Microbiol. 2012, 15, 325–336. [Google Scholar] [CrossRef]
- Köhler, G. Preoperative conditioning and surgical strategies for treatment of complex abdominal wall hernias. Chirurg 2020, 91, 134–142. [Google Scholar] [CrossRef]
- Ibarra, H.T.R.; Nuño, G.C.M.; Echeagaray, H.J.E.; Robles, V.E.; Jesús, G.J.J. Use of botulinum toxin type a before abdominal wall hernia reconstruction. World J. Surg. 2009, 33, 2553–2556. [Google Scholar] [CrossRef]
- Ibarra, H.T.R.; Nuno, G.C.M.; Miranda, D.A.G.; Troyo, S.R.; Navarro, I.R.; Bravo, C.L. Effect of botulinum toxin type A in lateral abdominal wall muscles thickness and length of patients with midline incisional hernia secondary to open abdomen management. Hernia. J. Hernias Abdom. Wall Surg. 2014, 18, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Farooque, F.; Jacombs, A.S.; Roussos, E.; Read, J.W.; Dardano, A.N.; Edye, M.; Ibrahim, N. Preoperative abdominal muscle elongation with botulinum toxin A for complex incisional ventral hernia repair. ANZ J. Surg. 2016, 86, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Elstner, K.E.; Read, J.W.; Rodriguez, A.O.; Cosman, P.H.; Dardano, A.N.; Jacombs, A.S.; Edye, M.; Zea, A.; Boesel, T.; Mikami, D.J.; et al. Preoperative chemical component relaxation using Botulinum toxin A: Enabling laparoscopic repair of complex ventral hernia. Surg. Endosc. 2017, 31, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.O.; Elstner, K.E.; Jacombs, A.S.W.; Read, J.W.; Martins, R.T.; Arduini, F.; Wehrhahm, M.; Craft, C.; Cosman, P.H.; Dardano, A.N.; et al. Preoperative Botulinum toxin A enabling defect closure and laparoscopic repair of complex ventral hernia. Surg. Endosc. 2018, 32, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Christy, M.R.; Apostolides, J.; Rodriguez, E.D.; Manson, P.N.; Gens, D.; Scalea, T. The component separation index: A standardized biometric identity in abdominal wall reconstruction. Eplasty 2012, 12, 169–176. [Google Scholar]
- Blair, L.J.; Ross, S.W.; Huntington, C.R.; Watkins, J.D.; Prasad, T.; Lincourt, A.E.; Augenstein, V.A.; Heniford, B.T. Computed tomographic measurements predict component separation in ventral hernia repair. J. Surg. Res. 2015, 199, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Satriyasa, B.K. Botulinum toxin (Botox) A for reducing the appearance of facial wrinkles: A literature review of clinical use and pharmacological aspect. Clin. Cosmet. Investig. Dermatol. 2019, 12, 223–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wohlfarth, K.; Sycha, T.; Ranoux, D.; Naver, H.; Caird, D. Dose equivalence of two commercial preparations of botulinum neurotoxin type A: Time for a reassessment? Curr. Med. Res. Opin. 2009, 25, 1573–1584. [Google Scholar] [CrossRef]
- Tamboli, M.; Kitamura, R.; Ma, W.; Kumar, G.; Harrison, T.K.; Wang, R.R.; Mariano, E.R.; Leng, J.C. Preoperative Ultrasound-Guided Botulinum Toxin A Injection Facilitates Closure of a Complex Abdominal Wall Hernia: A Case Report. A&A Pract. 2019, 13, 193–196. [Google Scholar]
- Hsu, T.S.; Dover, J.S.; Arndt, K.A. Effect of volume and concentration on the diffusion of botulinum exotoxin A. Arch. Dermatol. 2004, 140, 1351–1354. [Google Scholar] [CrossRef]
- Cura, J.L. Ultrasound-guided therapeutic procedures in the musculoskeletal system. Curr. Probl. Diagn. Radiol. 2008, 37, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Bueno, L.J.; Carreño, S.O.; Torregrosa, G.A.; Pous, S.S. Preoperative Botulinum Toxin and Progressive Pneumoperitoneum in Loss of Domain Hernias—Our First 100 Cases. Front. Surg. 2020, 7, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zendejas, B.; Khasawneh, M.A.; Srvantstyan, B.; Jenkins, D.H.; Schiller, H.J.; Zielinski, M.D. Outcomes of chemical component paralysis using botulinum toxin for incisional hernia repairs. World J. Surg. 2013, 37, 2830–2837. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourad, A.P.; De Robles, M.S.; Winn, R.D. Low-Dose Pre-Operative Botulinum Toxin A Effectively Facilitates Complex Ventral Hernia Repair: A Case Report and Review of the Literature. Medicina 2021, 57, 14. https://doi.org/10.3390/medicina57010014
Mourad AP, De Robles MS, Winn RD. Low-Dose Pre-Operative Botulinum Toxin A Effectively Facilitates Complex Ventral Hernia Repair: A Case Report and Review of the Literature. Medicina. 2021; 57(1):14. https://doi.org/10.3390/medicina57010014
Chicago/Turabian StyleMourad, Ali P., Marie Shella De Robles, and Robert D. Winn. 2021. "Low-Dose Pre-Operative Botulinum Toxin A Effectively Facilitates Complex Ventral Hernia Repair: A Case Report and Review of the Literature" Medicina 57, no. 1: 14. https://doi.org/10.3390/medicina57010014




