Evaluation of the Effects of Cuminum cyminum on Cellular Viability, Osteogenic Differentiation and Mineralization of Human Bone Marrow-Derived Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Plant Materials
2.2. Study Design Using Bone Marrow Mesenchymal Stem Cells (BMSCs)
2.3. Evaluation of Cell Morphology
2.4. Evaluation of Cellular Viability
2.5. Quantitative Assay of Alkaline Phosphatase Activity and Quantitative Detection of Alizarin Red S Staining
2.6. Total RNA Extraction and Quantification of RUNX2, BSP, OCN and COL2A1 mRNA by Real-Time Polymerase Chain Reaction (PCR)
2.7. Statistical Analysis
3. Results
3.1. Evaluation of Cell Morphology
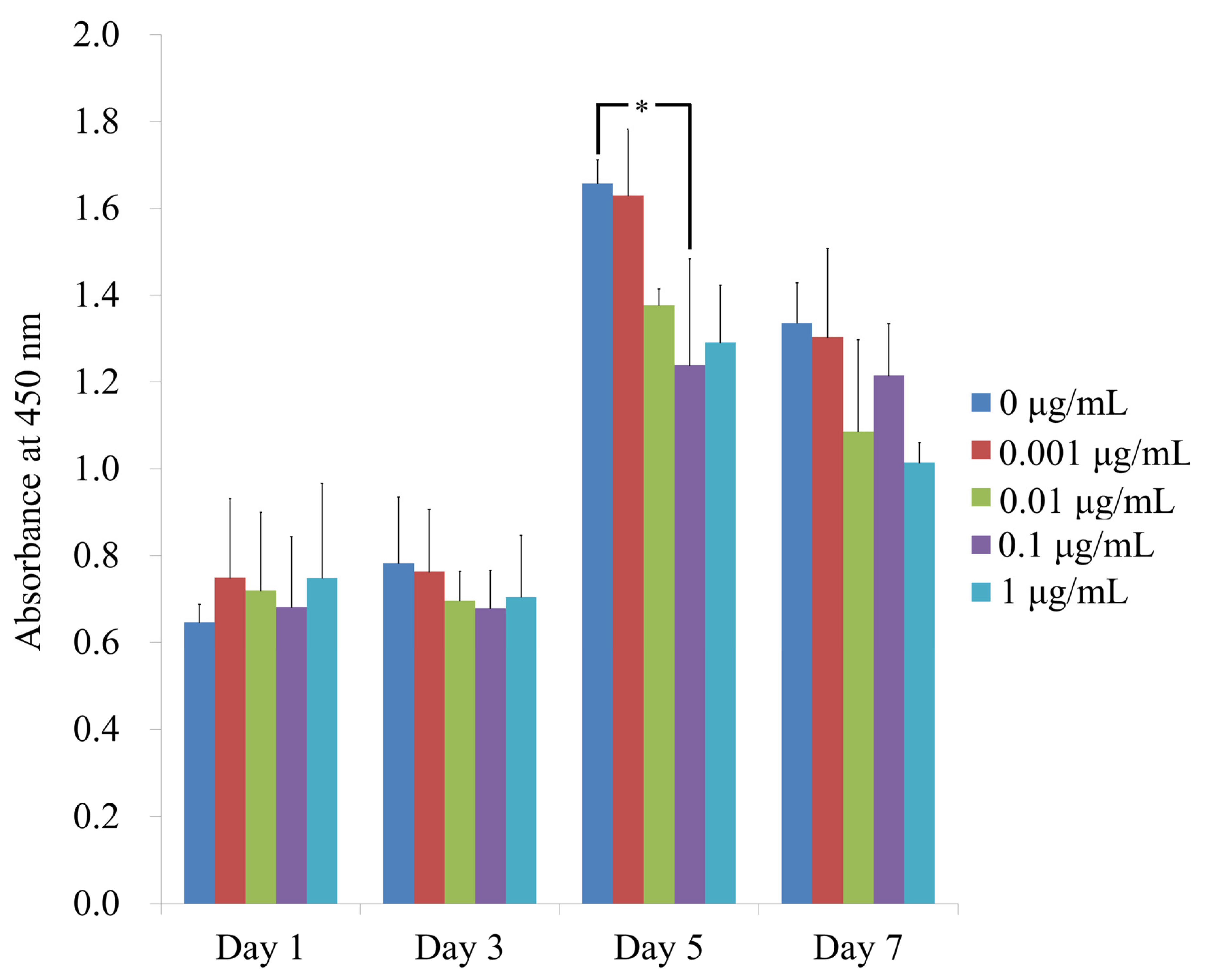
3.2. Cellular Viability
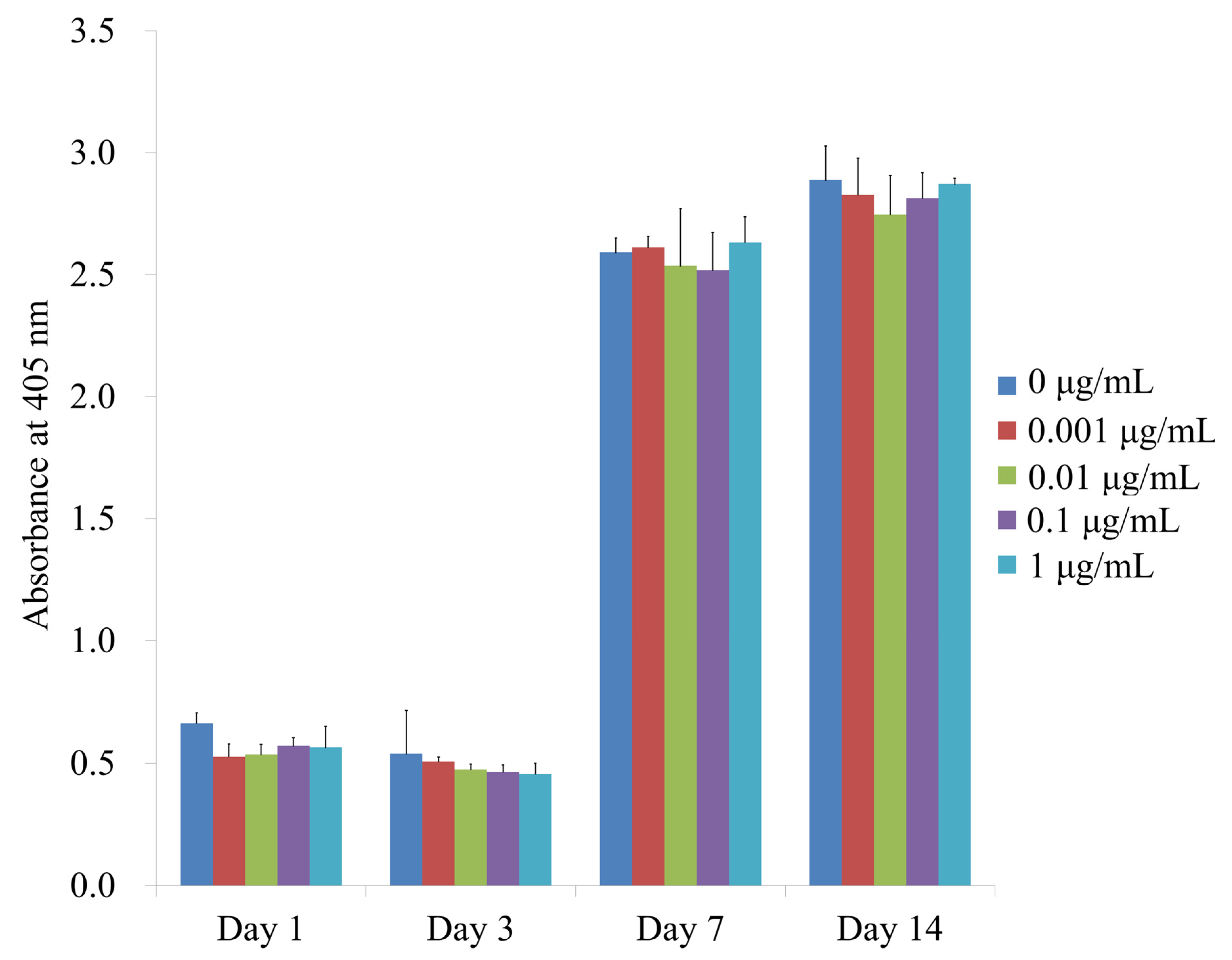
3.3. Alkaline Phosphatase Activity Assays
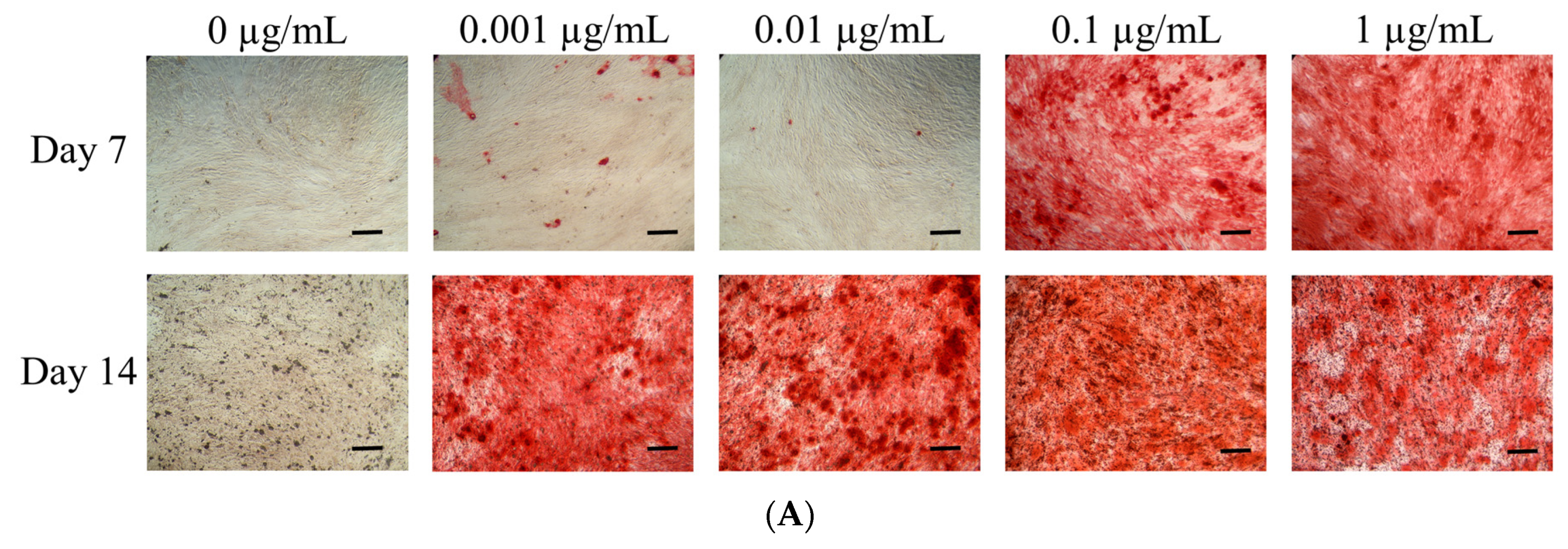
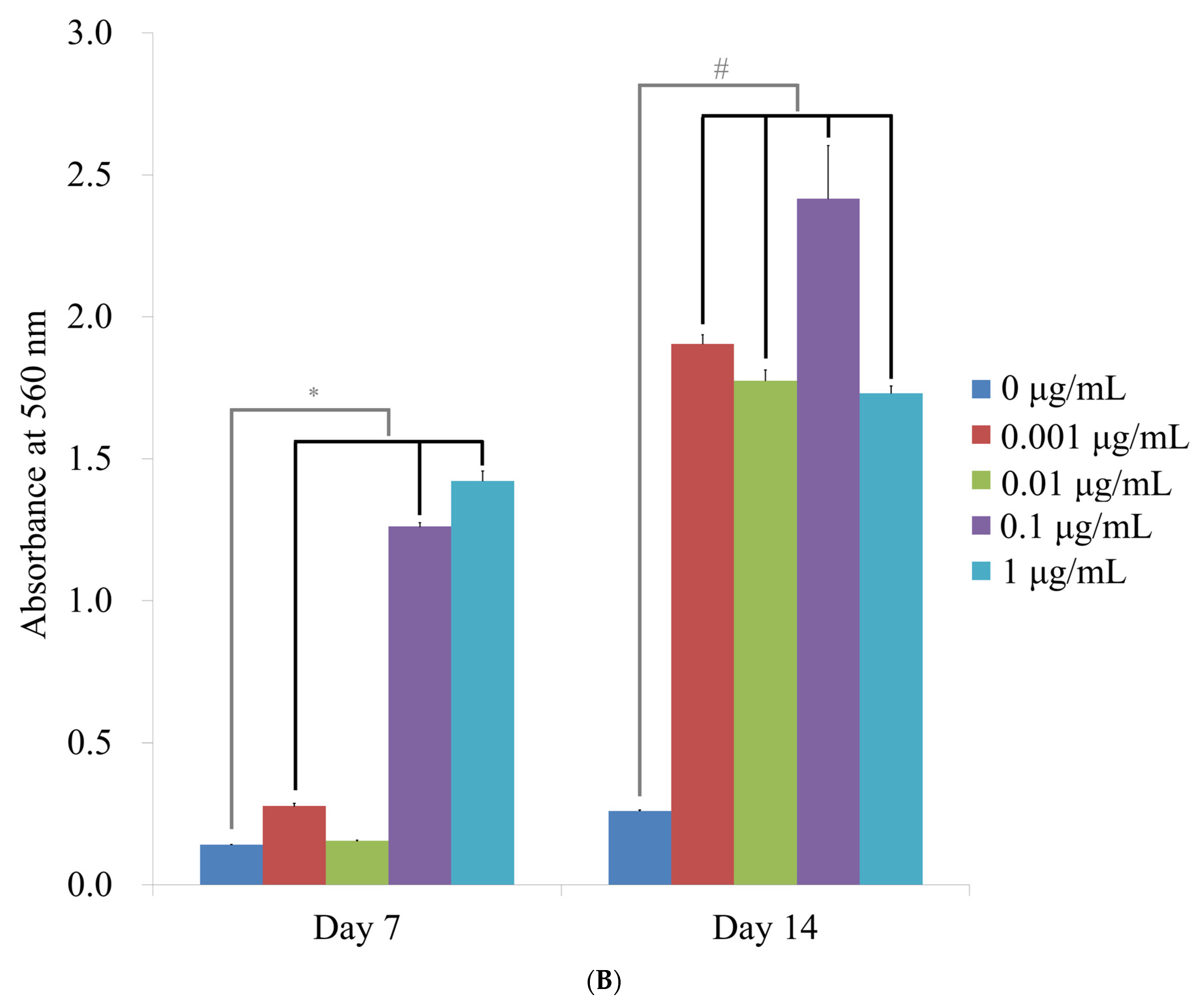
3.4. Mineralization Assay
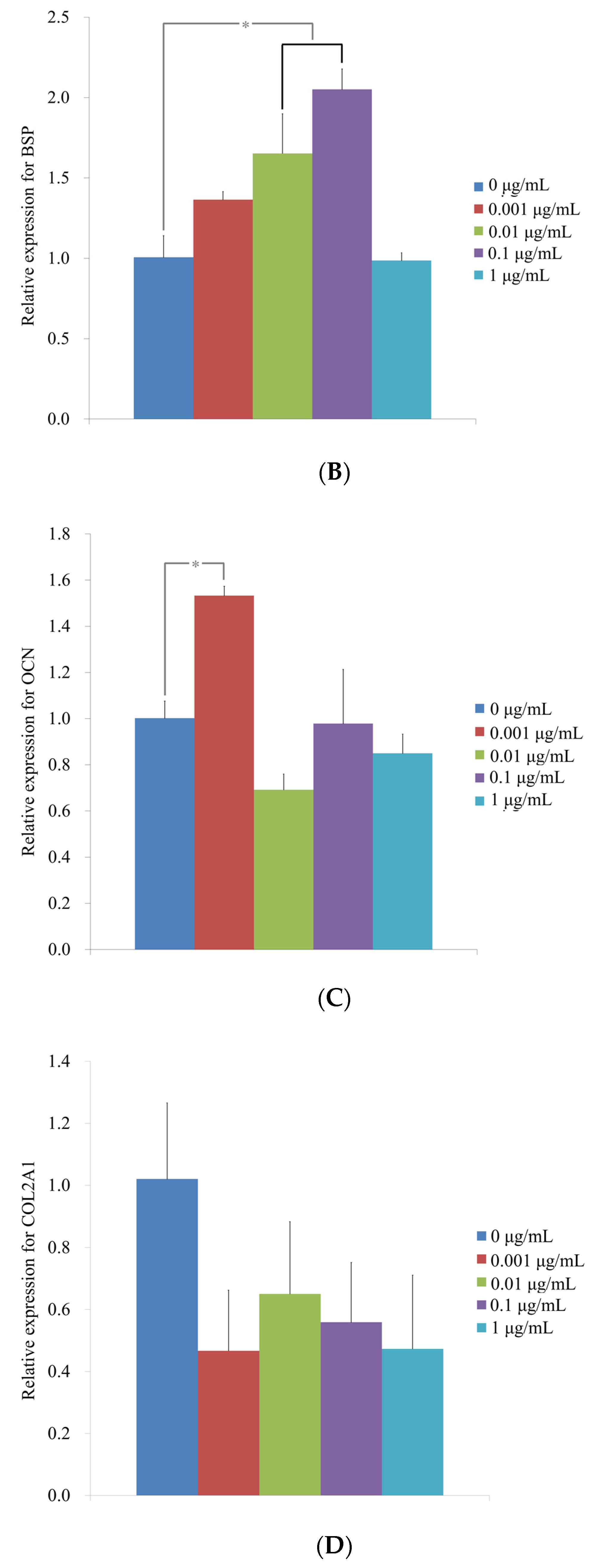
3.5. Evaluation of RUNX2, BSP, OCN and COL2A1 mRNA by Quantitative Real-Time PCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johri, R.K. Cuminum cyminum and Carum carvi: An update. Pharmacogn. Rev. 2011, 5, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Kermani, M.; Azizi, P.; Haghparast, A. The role of nitric oxide in the effects of cumin (Cuminum Cyminum L.) fruit essential oil on the acquisition of morphine-induced conditioned place preference in adult male mice. Chin. J. Integr. Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Effect of the Cumin cyminum L. Intake on Weight Loss, Metabolic Profiles and Biomarkers of Oxidative Stress in Overweight Subjects: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Ann. Nutr. Metab. 2015, 66, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Morovati, A.; Pourghassem Gargari, B.; Sarbakhsh, P. Effects of cumin (Cuminum cyminum L.) essential oil supplementation on metabolic syndrome components: A randomized, triple-blind, placebo-controlled clinical trial. Phytother. Res. 2019, 33, 3261–3269. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.; Mohammadi, H.; Hadi, Z.; Roshanravan, N.; Kafeshani, M. Cumin (Cuminum cyminum L.) is a safe approach for management of lipid parameters: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2018, 32, 2146–2154. [Google Scholar] [CrossRef]
- Sowbhagya, H.B. Chemistry, technology, and nutraceutical functions of cumin (Cuminum cyminum L): An overview. Crit. Rev. Food Sci. Nutr. 2013, 53, 1–10. [Google Scholar] [CrossRef]
- Mnif, S.; Aifa, S. Cumin (Cuminum cyminum L.) from traditional uses to potential biomedical applications. Chem. Biodivers. 2015, 12, 733–742. [Google Scholar] [CrossRef]
- Katiraee, F.; Ahmadi Afshar, S.; Rahimi Pirmahalleh, S.F.; Shokri, H. In vitro antifungal activity of essential oils extracted from plants against fluconazole-susceptible and -resistant Candida albicans. Curr. Med. Mycol. 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Wongkattiya, N.; Sanguansermsri, P.; Fraser, I.H.; Sanguansermsri, D. Antibacterial activity of cuminaldehyde on food-borne pathogens, the bioactive component of essential oil from Cuminum cyminum L. collected in Thailand. J. Complement. Integr. Med. 2019, 16. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Tae, J.Y.; Ko, Y.; Park, J.B. Evaluation of fibroblast growth factor-2 on the proliferation of osteogenic potential and protein expression of stem cell spheroids composed of stem cells derived from bone marrow. Exp. Ther. Med. 2019, 18, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Baraniak, P.R.; McDevitt, T.C. Stem cell paracrine actions and tissue regeneration. Regen. Med. 2010, 5, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, S.I.; Ko, Y.; Park, J.B. Evaluation of the secretion and release of vascular endothelial growth factor from two-dimensional culture and three-dimensional cell spheroids formed with stem cells and osteoprecursor cells. Adv. Clin. Exp. Med. 2018, 27, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Park, J.B.; Kim, I.; Lee, W.; Kim, H. Assessment of stem cell viability in the initial healing period in rabbits with a cranial bone defect according to the type and form of scaffold. J. Periodontal. Implant. Sci. 2019, 49, 258–267. [Google Scholar] [CrossRef]
- Probst, F.A.; Fliefel, R.; Burian, E.; Probst, M.; Eddicks, M.; Cornelsen, M.; Riedl, C.; Seitz, H.; Aszódi, A.; Schieker, M.; et al. Bone regeneration of minipig mandibular defect by adipose derived mesenchymal stem cells seeded tri-calcium phosphate- poly(D,L-lactide-co-glycolide) scaffolds. Sci. Rep. 2020, 10, 2062. [Google Scholar] [CrossRef]
- Udalamaththa, V.L.; Jayasinghe, C.D.; Udagama, P.V. Potential role of herbal remedies in stem cell therapy: Proliferation and differentiation of human mesenchymal stromal cells. Stem Cell Res. Ther. 2016, 7, 110. [Google Scholar] [CrossRef]
- Lee, H.; Uddin, M.S.; Lee, S.W.; Choi, S.; Park, J.B. Effects of Bambusa tulda on the proliferation of human stem cells. Exp. Ther. Med. 2017, 14, 5696–5702. [Google Scholar]
- Lee, J.E.; Kim, B.B.; Ko, Y.; Jeong, S.H.; Park, J.B. Effects of Cimicifugae Rhizoma on the osteogenic and adipogenic differentiation of stem cells. Exp. Ther. Med. 2017, 13, 443–448. [Google Scholar] [CrossRef]
- Thomas, A.B.; Kadam, A.S.; Jiwane, R.M.; Nanda, R.K.; Kothapalli, L.P. Biodegradable macromolecule based topical gels containing natural oils in the management of burn wounds. Pharm. Drug Dev. Ther. 2017, 2, 1–11. [Google Scholar] [CrossRef]
- Patil, D.N.; Kulkarni, A.R.; Shahapurkar, A.A.; Hatappakki, B.C. Natural cumin seeds for wound healing activity in albino rats. Int. J. Biol. Chem. 2009, 3, 148–152. [Google Scholar] [CrossRef]
- Jeong, C.H.; Kim, S.M.; Lim, J.Y.; Ryu, C.H.; Jun, J.A.; Jeun, S.S. Mesenchymal stem cells expressing brain-derived neurotrophic factor enhance endogenous neurogenesis in an ischemic stroke model. Biomed. Res. Int. 2014, 2014, 129145. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.H.; Yong, C.S.; Kim, J.O.; Jeong, J.H.; Park, J.B. Effects of tacrolimus on morphology, proliferation and differentiation of mesenchymal stem cells derived from gingiva tissue. Mol. Med. Rep. 2016, 14, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, H.; Na, C.B.; Park, J.B. The effects of simvastatin on cellular viability, stemness and osteogenic differentiation using 3-dimensional cultures of stem cells and osteoblast-like cells. Adv. Clin. Exp. Med. 2019, 28, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Min, S.K.; Oh, J.; Park, J.B. The Effects of Morinda citrifolia (Noni) on the Cellular Viability and Osteogenesis of Stem Cell Spheroids. Medicina 2020, 56, 389. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B. The use of enamel matrix derivative for the treatment of the apically involved tooth: A case report. Medicine (Baltimore) 2019, 98, e18115. [Google Scholar] [CrossRef] [PubMed]
- Rutkovskiy, A.; Stensløkken, K.O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef]
- Lee, H.; Son, J.; Yi, G.; Koo, H.; Park, J.B. Cellular viability and osteogenic differentiation potential of human gingiva-derived stem cells in 2D culture following treatment with anionic, cationic, and neutral liposomes containing doxorubicin. Exp. Ther. Med. 2018, 16, 4457–4462. [Google Scholar] [CrossRef]
- Tae, J.Y.; Lee, H.; Lee, H.; Ko, Y.; Park, J.B. Osteogenic potential of cell spheroids composed of varying ratios of gingiva-derived and bone marrow stem cells using concave microwells. Exp. Ther. Med. 2018, 16, 2287–2294. [Google Scholar] [CrossRef]
- Min, S.K.; Kim, M.; Park, J.B. Bone morphogenetic protein 2-enhanced osteogenic differentiation of stem cell spheres by regulation of Runx2 expression. Exp. Ther. Med. 2020, 20, 79. [Google Scholar] [CrossRef]
- Bouet, G.; Bouleftour, W.; Juignet, L.; Linossier, M.T.; Thomas, M.; Vanden-Bossche, A.; Aubin, J.E.; Vico, L.; Marchat, D.; Malaval, L. The impairment of osteogenesis in bone sialoprotein (BSP) knockout calvaria cell cultures is cell density dependent. PLoS ONE 2015, 10, e0117402. [Google Scholar] [CrossRef]
- Bianco, P.; Riminucci, M.; Bonucci, E.; Termine, J.D.; Robey, P.G. Bone sialoprotein (BSP) secretion and osteoblast differentiation: Relationship to bromodeoxyuridine incorporation, alkaline phosphatase, and matrix deposition. J. Histochem. Cytochem. 1993, 41, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Tamaddon, M.; Burrows, M.; Ferreira, S.A.; Dazzi, F.; Apperley, J.F.; Bradshaw, A.; Brand, D.D.; Czernuszka, J.; Gentleman, E. Monomeric, porous type II collagen scaffolds promote chondrogenic differentiation of human bone marrow mesenchymal stem cells in vitro. Sci. Rep. 2017, 7, 43519. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Son, J.; Min, S.K.; Na, C.B.; Yi, G.; Koo, H.; Park, J.B. A Study of the Effects of Doxorubicin-Containing Liposomes on Osteogenesis of 3D Stem Cell Spheroids Derived from Gingiva. Materials 2019, 12, 2693. [Google Scholar] [CrossRef] [PubMed]
- Hada, M.; Nishi, K.; Ishida, M.; Onda, H.; Nishimoto, S.; Sugahara, T. Inhibitory effect of aqueous extract of Cuminum cyminum L. seed on degranulation of RBL-2H3 cells and passive cutaneous anaphylaxis reaction in mice. Cytotechnology 2019, 71, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.; Ostad, S.N.; Asemi, Z.; Mahboubi, M.; Hejazi, S.; Sharafati-Chaleshtori, R.; Rashidi, A.; Akbari, H.; Sharifi, N. Sub-chronic oral toxicity of Cuminum cyminum L.’s essential oil in female Wistar rats. Regul. Toxicol. Pharmacol. 2017, 88, 138–143. [Google Scholar] [CrossRef]
- Mahmood, R.; Ikram, R.; Rizwani, G.H.; Khatoon, H. Comparison of neuropharmacological activities of methanolic extracts of Cuminum nigrum (Linn.) and Centratherum anthelminticum (Linn.) in mice. Pak. J. Pharm. Sci. 2019, 32, 81–87. [Google Scholar]
- Mahalakshmi, R.; Priyanga, J.; Vedha Hari, B.N.; Bhakta-Guha, D.; Guha, G. Hexavalent chromium-induced autophagic death of WRL-68 cells is mitigated by aqueous extract of Cuminum cyminum L. seeds. 3 Biotech 2020, 10, 191. [Google Scholar] [CrossRef]
- Nirmala, M.J.; Durai, L.; Rao, K.A.; Nagarajan, R. Ultrasonic Nanoemulsification of Cuminum cyminum Essential Oil and Its Applications in Medicine. Int. J. Nanomed. 2020, 15, 795–807. [Google Scholar] [CrossRef]
- Ho, S.C.; Tsai, T.H.; Tsai, P.J.; Lin, C.C. Protective capacities of certain spices against peroxynitrite-mediated biomolecular damage. Food Chem. Toxicol. 2008, 46, 920–928. [Google Scholar] [CrossRef]
- Tabarsa, M.; You, S.; Yelithao, K.; Palanisamy, S.; Prabhu, N.M.; Nan, M. Isolation, structural elucidation and immuno-stimulatory properties of polysaccharides from Cuminum cyminum. Carbohydr. Polym. 2020, 230, 115636. [Google Scholar] [CrossRef]
- Srivsatava, R.; Srivastava, S.P.; Jaiswal, N.; Mishra, A.; Maurya, R.; Srivastava, A.K. Antidiabetic and antidyslipidemic activities of Cuminum cyminum L. in validated animal models. Med. Chem. Res. 2011, 20, 1656–1666. [Google Scholar] [CrossRef]
- Dinparvar, S.; Bagirova, M.; Allahverdiyev, A.M.; Abamor, E.S.; Safarov, T.; Aydogdu, M.; Aktas, D. A nanotechnology-based new approach in the treatment of breast cancer: Biosynthesized silver nanoparticles using Cuminum cyminum L. seed extract. J. Photochem. Photobiol. B 2020, 208, 111902. [Google Scholar] [CrossRef]
- Koohsari, S.; Sheikholeslami, M.A.; Parvardeh, S.; Ghafghazi, S.; Samadi, S.; Poul, Y.K.; Pouriran, R.; Amiri, S. Antinociceptive and antineuropathic effects of cuminaldehyde, the major constituent of Cuminum cyminum seeds: Possible mechanisms of action. J. Ethnopharmacol. 2020, 255, 112786. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Yuan, R.; Huang, L.; Liu, Z.; Huang, D.; Huang, L.; Gao, H.; Liu, Y.; Xu, Q.M.; Yang, S. Atypical Nitrogen-Containing Flavonoid in the Fruits of Cumin (Cuminum cyminum L.) with Anti-inflammatory Activity. J. Agric. Food Chem. 2019, 67, 8339–8347. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Song, Y.; Park, Y.-H.; Uddin, M.S.; Park, J.-B. Evaluation of the Effects of Cuminum cyminum on Cellular Viability, Osteogenic Differentiation and Mineralization of Human Bone Marrow-Derived Stem Cells. Medicina 2021, 57, 38. https://doi.org/10.3390/medicina57010038
Lee H, Song Y, Park Y-H, Uddin MS, Park J-B. Evaluation of the Effects of Cuminum cyminum on Cellular Viability, Osteogenic Differentiation and Mineralization of Human Bone Marrow-Derived Stem Cells. Medicina. 2021; 57(1):38. https://doi.org/10.3390/medicina57010038
Chicago/Turabian StyleLee, Hyunjin, Youngmin Song, Yoon-Hee Park, Md. Salah Uddin, and Jun-Beom Park. 2021. "Evaluation of the Effects of Cuminum cyminum on Cellular Viability, Osteogenic Differentiation and Mineralization of Human Bone Marrow-Derived Stem Cells" Medicina 57, no. 1: 38. https://doi.org/10.3390/medicina57010038
APA StyleLee, H., Song, Y., Park, Y.-H., Uddin, M. S., & Park, J.-B. (2021). Evaluation of the Effects of Cuminum cyminum on Cellular Viability, Osteogenic Differentiation and Mineralization of Human Bone Marrow-Derived Stem Cells. Medicina, 57(1), 38. https://doi.org/10.3390/medicina57010038






