The History and Development of Hyperbaric Oxygenation (HBO) in Thermal Burn Injury
Abstract
:1. Introduction
1.1. History of Hyperbaric Oxygenation
1.2. Principle and Mechanisms of Hyperbaric Oxygenation
1.3. HBO in Burn Injury
2. Materials and Methods
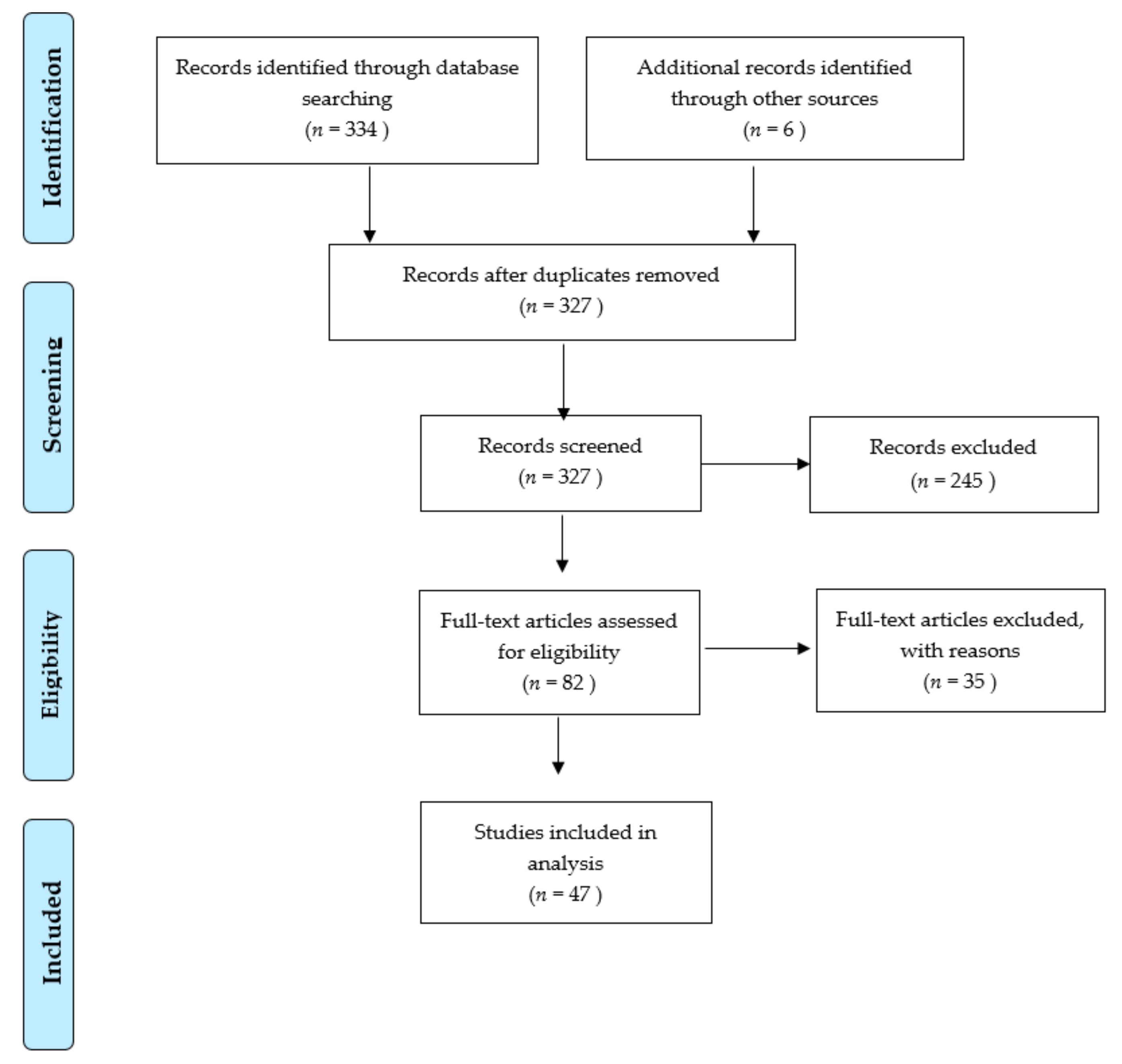
Literature Search and Evaluation
3. Results
3.1. General Considerations
3.2. Animal Studies
3.2.1. First Decade (1966–1977)
3.2.2. Last Two Decades before Turn of the Century (1978–1998)
3.2.3. New Millennium (2002–2019)
3.3. Human Volunteers
3.4. Clinical Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kindwall, E.P. A History of Hyperbaric Medicine. In Hyperbaric Medicine Practice, 2nd ed.; Best Publishing Company: Flagstaff, AZ, USA, 1995; pp. 2–15. [Google Scholar]
- Churchill-Davidson, I.; Sanger, C.; Thomlinson, R.H. High-pressure oxygen and radiotherapy. Lancet 1955, 268, 1091–1095. [Google Scholar] [CrossRef]
- Boerema, I. The use of hyperbaric oxygen. Am. Heart J. 1965, 69, 289–292. [Google Scholar] [CrossRef]
- Boerema, I.; Meyne, N.G.; Brummelkamp, W.H.; Bouma, S.; Mensch, M.H.; Kamermans, F.; Stern Hanf, M.; Alderen van, A. Life without blood. J. Cardiovasc. Surg. 1960, 1, 133–147. [Google Scholar]
- Cianci, P. Advances in the treatment of the diabetic foot: Is there a role for adjunctive hyperbaric oxygen therapy? Wound Repair Regen. 2004, 12, 2–10. [Google Scholar] [CrossRef]
- Thom, S.R. Hyperbaric oxygen: Its mechanisms and efficacy. Plast. Reconstr. Surg. 2011, 127, 131S–141S. [Google Scholar] [CrossRef] [Green Version]
- Rothfuss, A.; Speit, G. Investigations on the mechanism of hyperbaric oxygen (HBO)-induced adaptive protection against oxidative stress. Mutat. Res. Mol. Mech. Mutagen. 2002, 508, 157–165. [Google Scholar] [CrossRef]
- Camporesi, E.; Bosco, G. Mechanisms of action of hyperbaric oxygen therapy. Undersea Hyperb. Med. 2018, 41, 247–252. [Google Scholar]
- Domachevsky, L.; Pick, C.G.; Arieli, Y.; Krinsky, N.; Abramovich, A.; Eynan, M. Do hyperbaric oxygen-induced seizures cause brain damage? Epilepsy Res. 2012, 100, 37–41. [Google Scholar] [CrossRef]
- Wada, J.; Ikeda, T.; Kamata, K. Oxygen hyperbaric treatment for carbon monoxide poisoning and severe burn in coal mine gas explosion. Igakounoaymi 1965, 5, 53–57. [Google Scholar]
- Villanueva, E.; Bennett, M.H.; Wasiak, J.; Lehm, J.P. Hyperbaric oxygen therapy for thermal burns. Cochrane Database Syst. Rev. 2004, 2004, CD004727. [Google Scholar] [CrossRef]
- Cianci, P.; Lueders, H.W.; Lee, H.; Shaprio, R.L.; Sexton, J.; Williams, C.; Sato, R. Adjunctive Hyperbaric Oxygen Therapy Reduces Length of Hospitalization in Thermal Burns. J. Burn Care Rehab. 1989, 10, 432–435. [Google Scholar] [CrossRef]
- Niezgoda, J.A.; Cianci, P.; Folden, B.W.; Ortega, R.L.; Slade, J.B.; Storrow, A.B. The effect of hyperbaric oxygen therapy on a burn wound model in human volunteers. Plast. Reconstr. Surg. 1997, 99, 1620–1625. [Google Scholar] [CrossRef] [Green Version]
- Wasiak, J.; Bennett, M.; Cleland, H.J. Hyperbaric oxygen as adjuvant therapy in the management of burns: Can evidence guide clinical practice? Burns 2006, 32, 650–652. [Google Scholar] [CrossRef]
- Weitgasser, L.; Ihra, G.; Schäfer, B.; Markstaller, K.; Radtke, C. Update on hyperbaric oxygen therapy in burn treatment. Wien. Klin. Wochenschr. 2019, 1–7. [Google Scholar] [CrossRef]
- Kindwall, E.P.; Gottlieb, L.J.; Larson, D.L. Hyperbaric oxygen therapy in plastic surgery: A review article. Plast. Reconstr. Surg. 1991, 88, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Rogatsky, G.G.; Shifrin, E.G.; Mayevsky, A. Optimal dose as necessary condition for the efficacy of hyperbaric oxygen therapy in is-chemic stroke: A critical review. Neurol. Res. 2003, 25, 95–98. [Google Scholar] [CrossRef]
- Grossmann, A.R.; Grossmann, A.J. Update on hyperbaric oxygen and treatment of burns. Hyperb. Oxyg. Rev. 1982, 3, 51–59. [Google Scholar]
- Mathieu, D.; Marroni, A.; Kot, J. Tenth European Consensus Conference on Hyperbaric Medicine: Recommendations for acepted and non-accepted clinical indications and practic of hyperbaric oxygten treatment. Diving Hyperb. Med. J. 2017, 47, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.K.; Hopkins, R.O.; Chan, K.J.; Churchill, S.; Elliott, G.C.; Clemmer, T.P.; Orme, J.F., Jr.; Thomas, F.O.; Morris, A.H. Hyperbaric oxygen for acute carbon monoxide poisoning. New Engl. J. Med. 2002, 347, 1057–1067. [Google Scholar] [CrossRef]
- Attanasio, G.; Covelli, E.; Cagnoni, L.; Masci, E.; Ferraro, D.; Mancini, P.; Alessandri, E.; Cartocci, G.; Filipo, R.; Rocco, M. Does the addition of a second daily session of hyperbaric oxygen therapy to intratympanic steroid influence the outcomes of sudden hearing loss? Acta Otorhinolaryngol. Ital. 2015, 35, 272–276. [Google Scholar]
- McCormick, J.G.; Houle, T.T.; Saltzman, H.A.; Whaley, R.C.; Roy, R.C. Treatment of acute stroke with hyperbaric oxygen: Time window for efficacy. Undersea Hyperb. Med. 2011, 38, 321–334. [Google Scholar]
- Moher, D.; Liberati, A.; Tezlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses. The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Hart, G.B.; O’Reilly, R.R.; Broussard, N.D.; Cave, R.H.; Goodman, D.B.; Yanda, R.L. Treatment of burns with hyperbaric oxygen. Surg. Gynecol. Obstet. 1974, 139, 693–696. [Google Scholar]
- Chong, S.J.; Kan, E.M.; Song, C.; Soh, C.R.; Lu, J. Characterization of early thermal burns and the effects of hyperbaric oxygen treatment: A pilot study. Diving Hyperb. Med. J. 2013, 43, 157–161. [Google Scholar]
- Hammarlund, C.; Svedman, C.; Svedman, P. Hyperbaric oxygen treatment of healthy volunteers with u.v.-irradiated blister wounds. Burns 1991, 17, 296–301. [Google Scholar] [CrossRef]
- Rasmussen, V.M.; Borgen, A.E.; Jansen, E.C.; Rotboll Nielsen, P.H.; Werner, M.U. Hyperbaric oxygen therapy attenuates central sen-sitization induced by a thermal injury in humans. Acta Anaesthesiol. Scand. 2015, 59, 749–762. [Google Scholar] [CrossRef]
- Wahl, A.M.; Bidstrup, K.; Smidt-Nielsen, I.G.; Werner, M.U.; Hyldegaard, O.; Rotbøll-Nielsen, P. A single session of hyperbaric oxygen therapy demonstrates acute and long-lasting neuroplasticity effects in humans: A replicated, randomized controlled clinical trial. J. Pain Res. 2019, 12, 2337–2348. [Google Scholar] [CrossRef] [Green Version]
- Waisbren, B.A.; Schutz, D.; Collentine, G.; Banaszak, E.; Stern, M. Hyperbaric oxygen in severe burns. Burns 1982, 8, 176–179. [Google Scholar] [CrossRef]
- Cianci, P.; Williams, C.; Lueders, H.W.; Lee, H.; Shapiro, R.L.; Sexton, J.; Sato, R. Adjunctive hyperbaric oxygen in the treatment of thermal burns: An economic analysis. J. Burn Care Rehabil. 1990, 11, 140–143. [Google Scholar] [CrossRef]
- Brannen, A.L.; Still, J.; Haynes, M.; Orlet, H.; Rosenblum, F.; Law, E.; Thompson, W.O. A randomized prospective trial of hyperbaric oxygen in a referral burn center population. Am. Surg. 1997, 63, 205–208. [Google Scholar]
- Wiseman, D.H.; Grossman, A.R. Hyperbaric oxygen in the treatment of burns. Crit. Care Clin. Plast. Surg. 1978, 1, 163–171. [Google Scholar] [CrossRef]
- Niu, A.K.C.; Yang, C.; Lee, H.C. Burns treated with adjunctive hyperbaric oxygen therapy: A comparative study in humans. J. Hyperb. Med. 1987, 2, 75–85. [Google Scholar]
- Chen, K.-L.W.C.-J.; Tseng, W.-S.; Lee, H.-C.; Tsa, T.-P.; Huang, W.-S. Improvement of satisfaction in burn patients recieving adjuvant hyperbaric oxygen therapy. Formos. J. Surg. 2018, 51, 184–192. [Google Scholar]
- Lamy, M.L.; Hanquet, M.M. Application Opportunity for OHP in a General Hospital—A Two Years Experience with a Monoplace Hyperbaric Oxygen Chamber. In Proceedings of the 4th International Congress on Hyperbaric Medicine Sapporo, Japan, 2–4 September 1969; Igaku Shoin, Ltd.: Tokyo, Japan, 1970. [Google Scholar]
- Ikeda, K.; Ajiki, H.; Nagao, H.; Krino, K.; Sugh, S.; Iwa, T.; Wada, T. Experimental and Clinical Use of Hyperbaric Oxygen in Burns. In Proceedings of the 4th International Congress on Hyperbaric Medicine Sapporo, Japan, 2–4 September 1969; Igaku Shoin, Ltd.: Tokyo, Japan, 1970. [Google Scholar]
- Marchal, C.; Thibault, G.; Commebias, J.F.; Barlier, J.; Benichoux, R. Effects of OHP and THAM on Experimental Burns of Rats. In Proceedings of the 3rd International Congress on Hyperbaric Medicine Duke University, Durham, NC, USA, 17–20 November 1965; National Academy of Sciences Research Council: Washington, DC, USA, 1966. [Google Scholar]
- Nelson, B.S.; Stansell, G.B.; Kramer, J.G. Hyperbaric Oxygen in Experimental Burn. In Proceedings of the 3rd International Congress on Hyper-Baric Medicine Duke University, Durham, NC, USA, 17–20 November 1965; National Academy of Sciences Research Council: Washington, DC, USA, 1966. [Google Scholar]
- Ketchum, S.A.; Thomas, A.N.; Hall, A.D. Effect of hyperbaric oxygen on small first, second, and third degree burns. Surg. Forum 1967, 18, 65–67. [Google Scholar]
- Ketchum, S.A.; Thomas, A.N.; Hall, A.D. Angiographic Studies of the Effect of Hyperbaric Oxygen on Burn Wound Revascularization. In Proceedings of the 4th International Congress on Hyperbaric Medicine Sapporo, Japan, 2–4 September 1969 ; Wada, J., Iwa, T., Eds.; Igaku Shoin, Ltd.: Tokyo, Japan, 1970; pp. 388–394. [Google Scholar]
- Benichoux, R.; Marchal, C.; Thibaut, G.; Bertrand, J.P. Hyperbaric oxygen in treatment of severe experimental burns. J. Chir. 1968, 96, 445–452. [Google Scholar]
- Perrins, D.J.D. Failed Attempt to Limit Tissue Destruction in Scalds of Pig´s Skin with Hyperbaric Oxygen. In Proceedings of the 4th International Congress on Hyperbaric Medicine Sapporo, Japan, 2–4 September 1969; Igaku Shoin, Ltd.: Tokyo, Japan, 1970. [Google Scholar]
- Spinadel, L.; Výmola, F. Influence of hyperbaric oxygenation and antibiotics on burns in animal experiments. Zent. Chir. 1969, 94, 296–298. [Google Scholar]
- Gruber, R.P.; Brinkley, F.B.; Amato, J.J.; Mendelson, J.A. Hyperbaric Oxygen and Pedicle Flaps, Skin Grafts, and Burns. Plast. Reconstr. Surg. 1970, 45, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Bleser, F.; Sztuka, J.C.; Muller, J.-P. Effects of hyperbaric oxygen, THAM and antibiotics on experimental burns in rats. Changes in body fluid compartments, electrolytes and acid-base balance. Eur. Surg. Res. 1971, 3, 409–420. [Google Scholar] [CrossRef]
- Bleser, F.; Benichoux, R. Experimental surgery: The treatment of severe burns with hyperbaric oxygen. J. Chir. 1973, 106, 281–290. [Google Scholar]
- Hartwig, V.J.; Kirste, G. Experimentelle Untersuchungen über die Revaskularisierung von Verbrennungswunden unter Hyperbarer Sauerstofftherapie. Zent. Chir. 1974, 99, 1112–1117. [Google Scholar]
- Korn, H.N.; Wheeler, E.S.; Miller, T.A. Effect of Hyperbaric Oxygen on Second-Degree Burn Wound Healing. Arch. Surg. 1977, 112, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Niccole, M.W.; Thornton, J.W.; Danet, R.T.; Bartlett, R.H.; Tavis, M.J. Hyperbaric oxygen in burn management: A controlled study. Surg. Gynecol. Obstet. 1977, 82, 727–733. [Google Scholar]
- Wells, C.H.; Hilton, J.C. Effects of Hyperbaric Oxygen on Post-Burn Plasma Extravasation. In Hyperbaric Oxygen Therapy; Davis, J.C., Hunt, T.K., Eds.; Undersea Medical Society, Inc.: Bethesda, MD, USA, 1977; pp. 259–265. [Google Scholar]
- Kaiser, W.; Berger, A.; Von Der Lieth, H.; Heymann, H. Hyperbaric Oxygenation in Burns. Handchir. Mikrochir. Plast. Chir. 1985, 17, 326–330. [Google Scholar] [PubMed]
- Espinosa, C.; Mauvecin, G.; Lopez, C.; Brandon, J.; Ciampagna, H.; Pomar, M.; Lemmi, J. Study of edema regulation by hyperbaric oxygen in an experimental model of thermal burns. Prensa Med. Argent. 1995, 82, 235–240. [Google Scholar]
- Arzinger-Jonasch, H.; Sandner, J.K.; Bittner, H. Effect of hyperbaric oxygen on burns of various depths in anmial experiments. Z. Exp. Chir. 1978, 11, 6–10. [Google Scholar]
- Nylander, G.; Nordström, H.; Eriksson, E. Effects of hyperbaric oxygen on oedema formation after a scald burn. Burns 1984, 10, 193–196. [Google Scholar] [CrossRef]
- Kaiser, W.; Schnaidt, U.; Von Der Lieth, H. Effects of hyperbaric oxygen on fresh burn wounds. Handchir. Mikrochir. Plast. Chir. 1989, 21, 158–163. [Google Scholar]
- Stewart, R.J.; Yamaguchi, K.T.; Mason, S.W.; Rosideh, B.B.; Dabassi, N.I.; Ness, N.T. Tissue ATP levels in burn injured skin treated with hyperbaric oxygen. In Proceedings of the Annual Meeting of the Undersea and Hyperbaric Medical Society, Honolulu, Hawaii, USA, 6–11 June 1989. [Google Scholar]
- Saunders, P. Hyperbaric Oxygen Therapy in the Management of Carbon Monoxide Poisoning, Osteoradionecrosis, Burns, Skin Grafts, and Crush Injury. Int. J. Technol. Assess. Heal. Care 2003, 19, 521–525. [Google Scholar] [CrossRef]
- Tenenhaus, M.; Hansbrough, J.F.; Zapata-Sirvent, R.; Neumann, T. Treatment of Burned Mice with Hyperbaric Oxygen Reduces Mesenteric Bacteria but Not Pulmonary Neutrophil Deposition. Arch. Surg. 1994, 129, 1338–1342. [Google Scholar] [CrossRef]
- Hussmann, J.; Hebebrand, D.; Erdmann, D.; Roth, A.; Kucan, J.O.; Moticka, J. Lymphocyte subpopulations in spleen and blood following early burn wound excision and acute/chronic treatment with hyperbaric oxygen. Handchir. Mikrochir. Plast. Chir. 1996, 28, 103–107. [Google Scholar]
- Germonpre, P.; Reper, P.; Vanderkelen, A. Hyperbaric oxygen therapy and piracetam decrease the early extension of deep par-tial-thickness burns. Burns 1996, 22, 468–473. [Google Scholar] [CrossRef]
- Shoshani, O.; Shupak, A.; Barak, A.; Ullman, Y.; Ramon, Y.; Lindenbaum, E.; Peled, Y. Hyperbaric oxygen therapy for deep second degree burns: An experimental study in the guinea pig. Br. J. Plast. Surg. 1998, 51, 67–73. [Google Scholar] [CrossRef]
- Hatibie, M.J.; Islam, A.A.; Hatta, M.; Moenadjat, Y.; Susiolo, R.H.; Rendy, L. Hyperbaric Oxygen Therapy for Second-Degree Burn Healing: An Experimental Study in Rabbits. Adv. Skin Wound Care. 2019, 32, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Akin, M.L.; Gulluoglu, B.M.; Erenoglu, C.; Dundar, K.; Terzi, K.; Erdemoglu, A.; Celenk, T. Hyperbaric oxygen prevents bacterial translo-cation in thermally injured rats. J. Investig. Surg. 2002, 15, 303–310. [Google Scholar] [CrossRef]
- Bilic, I.; Petri, N.M.; Bezic, J.; Alfirevic, D.; Modun, D.; Capkun, V.; Bota, B. Effects of hyperbaric oxygen therapy on experimental burn wound healing in rats: A randomized controlled study. Undersea Hyperb. Med. 2005, 32, 1–9. [Google Scholar] [PubMed]
- Turkaslan, T.; Yogun, N.; Cimsit, M.; Solakoğlu, S.; Ozdemir, C.; Özsoy, Z. Is HBOT treatment effective in recovering zone of stasis? An experimental immunohistochemical study. Burns 2010, 36, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Selçuk, C.T.; Özalp, B.; Durgun, M.; Tekin, A.; Akkoç, M.F.; Alabalik, U.; Ilgezdi, S. The Effect of Hyperbaric Oxygen Treatment on the Healing of Burn Wounds in Nicotinized and Nonnicotinized Rats. J. Burn Care Res. 2013, 34, e237–e243. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Lo, J.-J.; Wu, S.-H.; Wang, C.-Z.; Chen, R.-F.; Lee, S.-S.; Chai, C.-Y.; Huang, S.-H. Early Hyperbaric Oxygen Treatment Attenuates Burn-Induced Neuroinflammation by Inhibiting the Galectin-3-Dependent Toll-Like Receptor-4 Pathway in a Rat Model. Int. J. Mol. Sci. 2018, 19, 2195. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.-S.; Wu, S.-H.; Lee, S.-S.; Lin, C.-H.; Chang, C.-H.; Lo, J.-J.; Chai, C.-Y.; Wu, C.-S.; Huang, S.-H. Dose-Dependent Effect of Hyperbaric Oxygen Treatment on Burn-Induced Neuropathic Pain in Rats. Int. J. Mol. Sci. 2019, 20, 1951. [Google Scholar] [CrossRef] [Green Version]
- Lang, T.C.; Zhao, R.; Kim, A.; Wijewardena, A.; Vandervord, J.; Xue, M.; Jackson, C.J. A Critical Update of the Assessment and Acute Management of Patients with Severe Burns. Adv. Wound Care 2019, 8, 607–633. [Google Scholar] [CrossRef] [Green Version]
- Barth, E.; Sullivan, T.; Berg, E. Animal Model for Evaluating Bone Repair with and without Adjunctive Hyperbaric Oxygen Therapy (HBO): Comparing Dose Schedules. J. Investig. Surg. 1990, 3, 387–392. [Google Scholar] [CrossRef]
- Cianci, P.; Slade, J.B.; Sato, R.M.; Faulkner, J. Adjunctive hyperbaric oxygen therapy in the treatment of thermal burns. Undersea Hyperb. Med. 2013, 40, 89–108. [Google Scholar] [CrossRef]
- Ratzenhofer-Komenda, B.; Offner, A.; Quehenberger, F.; Klemen, H.; Berger, J.; Fadai, J.H.; Spernbauer, P.; Prause, G.; Smolle-Jüttner, F.M. Hemodynamic and oxygenation profiles in the early period after hyperbaric oxygen therapy: An observational study of intensive-care patients. Acta Anaesthesiol. Scand. 2003, 47, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Davies, A.; Bland, S.; Brookes, S.; Blazeby, J.M. Systematic review of clinical outcome reporting in randomised controlled trials of burn care. BMJ Open 2019, 9, e025135. [Google Scholar] [CrossRef] [Green Version]
| Author/Year | Species | Nr. Individuals | Study Design | % TBSA | Depth of Burn | Local Treatment |
|---|---|---|---|---|---|---|
| Marchal/1966 | Rats | 187 | no burn/HBO: 10 | |||
| burn/untreated: 15; burn/HBO: 15 | 20 | FT | ||||
| 7 arm (at least 21 each;) burn/no treatment burn/HBO only burn/saline burn/saline, HBO burn/THAM burn/THAM, HBO (each day) burn/THAM, HBO (every other day) | 75 | PT | ||||
| Nelson/1966 | Dogs | 24 | 2 arm; burn/untreated: 12 burn/HBO: 12 | 75 | PT | |
| Ketchum/1967 | Rabbits | 26 | 2 arm; burn/untreated: 13; burn/HBO: 13 | 5 | FT, PT | |
| Ketchum/1970 | Rats | 30 | 2 arm; burn/untreated: 15 burn/HBO: 15 | 20 | FT | |
| Benichoux/1968 | Rats | 160 | 8 arm; no burn/HBO: 10 burn/no treatment: 25 burn/HBO only: 25 burn/saline: 25 burn/saline, HBO: 25 burn/THAM: 25 burn/THAM, HBO every day: 25 burn/THAM, HBO every second day: 25 | 75 | PT | |
| 200 | 8 arm; no burn/HBO; burn/THAM; burn/HBO every second day; burn/colmycine, penicilline; burn/THAM, HBO; burn/penicilline, colimycin, HBO; burn/THAM, penicilline, colimycine burn/THAM, penicillin, colimycine, HBO | 30 | PT | |||
| Perrins/1969 | Pigs | 8 | 2 arm; burn/untreated: 4 burn/HBO: 4 | 12 | FT | |
| 4 | 2 arm; burn/untreated: 2 burn/HBO: 2 | 8 | PT | |||
| Spinadel/1969 | Guinea pigs | 99 | 3 arm; burn/untreated: 33 burn/antibiotics 33 burn/HBO & antibiotics: 33 | 25 | PT | Gentamycin-powder |
| Hamsters | 75 | 3 arm; burn/untreated: 25 burn/antibiotics: 25 burn/HBO & antibiotics: 25 | 25 | PT | Gentamycin-powder | |
| Gruber/1970 | Rats | 24 | 3 arm; pedicled flap, replanted/HBO: 8 composite skin graft, replanted/HBO: 8; burn/HBO: 8 | 10 | FT | |
| Bleser/1971 | Rats | 520 | 3 arm; no burn/untreated: 20 burn/untreated: 250 burn/HBO, THAM, penicillin, colimycine: 250 | 32 | PT | |
| Bleser/1973 | Rats | 100 | 2 arm; burn/untreated: 50 burn/HBO: 50 | 5 | FT | |
| Härtwig/1974 | Rats | 100 | 2 arm; burn/untreated: 50 burn/HBO: 50 | 2 | FT | |
| Korn/1977 | Guinea pigs | 117 | Series I: 3 arm; burn/HBO: 52 burn/Hyperbaric normoxia: 27 burn/untreated: 38 | 5 | PT | open/pro-tected |
| 54 | Series II: 2 arm; burn/HBO: 30 burn/untreated: 24 | 5 | PT | open/pro-tected | ||
| 40 | Series III: 4 arm; no burn/control: 8 no burn/HBO: 8 burn/untreated: 12 burn/HBO: 12 | 5 | PT | open/pro-tected | ||
| Niccole/1977 | Rats | 80 | 4 arm; burn/untreated: 20 burn/HBO: 20 burn/sliver-sulfadiazine: 20 burn/HBO/silver-sulfadiazine: 20 | 20 | 40 PT 40 FT | sulfadiazine (removed before HBO); no dressings |
| Wells/1977 | Dogs | 24 | 3 arm; burn/no fluid: 8 burn/no fluid, NBO: 8 burn/fluid, HBO: 8 | 40 | FT | |
| Arzinger-Jonasch/1978 | Guinea pigs | 120 | 5 arm; burn/HBO: 10 | 15 | PT | |
| burn/HBO: 10 | FT | |||||
| burn/HBO: 20; necrectomy at various time points | FT | necrectomy | ||||
| burn/HBO: 20; necrectomy, full-thickness grafts at various time points | FT | necrectomy/skin graft | ||||
| burn/necrectomy, full-thickness grafts at various time time points: 60; | FT | necrectomy/skin graft | ||||
| Nylander/1984 | Mice | 54 | 2 arm; burn/untreated: 27 burn/HBO: 27 | 6 | PT | |
| Kaiser/1985 | Guinea pig | 102 | 5 arm: burn not infected/untreated: 21 burn not infected/HBO: 21 burn infected (pseudomonas)/untreated: 30 burn infected (pseudomonas)/primary HBO: 15 burn infected (pseudomonas)/secondary HBO: 15 | 5 | FT | |
| Kaiser/1988 | Guinea pigs | 75 | 2 arm; burn/untreated: 43 burn/HBO: 32 | 5 | PT | |
| Stewart/1989 | Rats | 90 | 15 arm; no burn/untreated: 6 | |||
| burn/untreated, biopsy at 12 h: 6 | 5 | PT | silver sulfadiazine | |||
| burn/1 HBO, biopsy at 12 h: 6 | PT | |||||
| burn/2 HBO, biopsy at 12 h: 6 | PT | |||||
| burn/1HBO biopsy at 36 h: 6 | PT | |||||
| burn/untreated, biopsy; 5 groups of 6 animals each at 36, 48, 72, 96 or 120 h | PT | |||||
| burn/2 HBO, biopsy; 5 groups of 6 animals each at 36, 48, 72, 96 or 120 h | PT | |||||
| Saunders/1989 | Guinea pigs | 30 | 2 arm; burn/untreated: 15 burn/HBO: 15 (3 different times of evaluation: 6, 24, 48 h) | PT | ||
| Tenenhaus/1994 | Mice | 125 | 5 arm; no burn/fluid, food: 22 burn/fluid, food: 32 burn/fluid, food, compressed air: 15 burn/, fluid, food, NBO: 24 burn/fluid, food, HBO: 32 | 32 | FT | |
| 139 | 4 arm; no burn/fluid, no food: 22 burn/fluid, no food: 51 burn/fluid, no food, HBO 2 × 120 min: 57 burn/fluid, no food, HBO 3 × 120 min: 9 | 32 | FT | |||
| Espinosa/1995 | Guinea pigs | 20 | 3 arm; burn/untreated: 6 burn/HBO: 7 burn/HBO, antibiotic: 7 | 10 | PT | |
| Hussmann/1996 | Rats | 74 | 11 arm; no burn/untreated: 4 | 10 | FT | |
| no burn/anaesthesia, untreated: 7 | ||||||
| burn/untreated: 7 | FT | |||||
| excision of 10% TBSA/suture: 7 | excision | |||||
| no burn/HBO (acute): 7 | ||||||
| no burn/HBO (chronical): 7 | ||||||
| burn/excision after 4 h: 7 | FT | excision | ||||
| burn/HBO (once): 7 | FT | |||||
| burn/HBO (repeated): 7 | FT | |||||
| burn/excision after 4 h and HBO (once): 7 | FT | excision | ||||
| burn/excision after 4 h and HBO (repeated): 7 | FT | excision | ||||
| Germonpré/1996 | Rats | 46 | 3 arm; burn/untreated: 10 burn/HBO: 17 burn/Piracetam: 19 | 5 | PT | mafenide gauze; Op-Site |
| Shoshani/1998 | Guinea pigs | 54 | 3 arm; burn/silversulfadiazine: 18 burn/NBO, silversulfadiazine: 18 burn/HBO, silversulfadiazine: 18 | 5 | PT | silver sulfadiazine |
| Akin/2002 | Rats | 54 | 7 arm; no burn/liquids: 6 no burn/liquids, HBO (short): 8 no burn/liquids, HBO (long): 8 burn/liquids: 16 burn/liquids, HBO (short): 8 burn/liquids, HBO long): 8 | 30 | PT | |
| Bilic/2005 | Rats | 70 | 2 arm randomized; burn/Hyperbaric normoxia: 35 burn/HBO: 35 | 20 | PT | silver sulfadiazine |
| Türkaslan/2010 | Rats | 20 | 4 arm; burn/untreated (evaluation at 24 h): 5 burn/HBO (evaluation at 24 h): 5 burn/untreated (evaluation on day 5): 5 burn/HBO (evaluation on day 5): 5 | 5 | PT | |
| Selcuk/2013 | Rats | 32 | 4 arm; burn/Nicotine, HBO: 8, burn/Nicotine: 8; burn/no nicotine/HBO: 8; burn/no Nicotine: 8 | 12 | PT, FT | |
| Wu/2018 | Rats | 36 | 6 arm; sham-burn/sham HBO: 6 sham burn/HBO: 6 burn/1 week sham HBO: 6 burn/2 week sham HBO:6 burn/1 week HBO: 6 burn/2 weeks HBO: 6 | 1 | FT | silver sulfadiazine |
| Wu/2019 | Rats | 30 | 5 arm; Sham-burn/Sham HBO: 6; sham burn/HBO: 6; burn/1 week sham HBO:6; burn/1 week HBO: 6; burn/2 weeks HBO: 6 | 1 | FT | silver sulfadiazine |
| Hatibie/2019 | Rabbits | 36 | 2 arm; burn/untreated; 18 burn/HBO: 18 | 1 | PT | vaseline |
| Ikeda/1967 | Patients | 43 | case series | >50 | PT, FT | silver nitrate 0,5% |
| Lamy/1970 | Patients | 27 | case series, historical comparator | 20 to >80 | PT, FT | |
| Hart/1974 | Patients | 191 | 2 arm double blind randomized; (included in observational 138 burn/HBO: 138 and burn/sham HBO: 53) | 10 to 50 | PT, FT | silver sulfadiazine |
| Group I (HBO: 2; sham HBO: 2) | >10 <20 | |||||
| Group II (HBO: 2; sham HBO: 2) | >20 <30 | |||||
| Group III (HBO: 2; sham HBO: 2) | >30 <40 | |||||
| Group IV (HBO: 2; sham HBO: 2) | <40 <50 | |||||
| Grossmann/1978 | Patients | 821 | 2 arm; nonrandomized controls; burn/routine treatment: 419; burn/routine treatment & HBO: 421 | >20 <80 | PT, FT | silver sulfadiazine |
| Waisbren/1982 | Patients | 72 | 2 arm: matched pairs; burn/routine treatment: 36; burn/routine treatment & HBO: 36 | about 50 | PT, FT | |
| Niu/1987 | Patients | 835 | 2 arm; nonrandomized comparator; burn/routine treatment: 609; burn/routine treatment & HBO: 226 | any; subgroup severe burns | PT, FT | |
| Cianci/1989 | Patients | 20 | 2 arm: nonrandomized controls burn/routine: 12 (had no access to HBO); burn/routine treatment & HBO: 8 | 18–39 | PT, FT | |
| Cianci/1990 | Patients | 21 | matched pairs burn/routine treatment: 11; burn/routine treatment & HBO: 10, | 19–50 (mean: 30) | PT, FT | |
| Hammar-lund/1991 | Volunteers | 8 | 2 arm cross-over at 10-day interval burn/untreated: 8 burn/HBO: 8 | <1 | PT | polyurethane film or hydrocolloid |
| Brannen/1997 | Patients | 125 | 2 arm matched pairs; burn/routine treatment: 62 burn/routine treatment & HBO: 63 | 20–50 (mean) | PT, FT | |
| Niezgoda/1997 | Volunteers | 12 | 2 arm randomized; burn/NBO: 6 burn/HBO: 6 | <1 | PT | hydrocolloid |
| Chong/2013 | Patients | 17 | 2 arm randomized; burn/routine treatment: 9 burn/routine treatment & HBO: 8; non-intubated | <35 (mean: 13) | PT, FT | bio-occlusive dressing |
| Rasmussen/2015 | Volunteers | 17 | 2 arm crossover: burn/HBO–NBO: 17 burn/NBO–HBO: 17 | 1 | S | |
| Chen/2018 | Patients | 35 | 2 arm retrospective case control; burn/routine treatment: 17 burn/routine treatment & HBO: 18 | <60 | PT, FT | |
| Wahl/2019 | Volunteers | 21 | 2 arm crossover; burn/HBO–NBO: 12 burn/NBO–HBO: 9 | 1 | S |
| Author/Year | Interval Burn—HBO (hours) | Pressure (ATA) | Duration min | HBO Frequency/Day | Total Number HBO Sessions | Results in Detail |
|---|---|---|---|---|---|---|
| Marchal/1966 | 3 | 60 | once a day | 21 | 1 rat died, one convulsed; no weight gain during treatment | |
| 3 | 60 | once a day | 28 | After day 12 better granulation, faster healing, less infection in HBO | ||
| 3 | 60 | once a day or every second day | 5 to 10 | mortality with daily HBO alone higher than in untreated controls. best survival in THAM with HBO every second day | ||
| Nelson/1966 | 0.1 | 2 | 60 | once | 1 | hematocrit drops less after HBO |
| Ketchum/1967 | 2 | 60 | four times a day with 1 h in between for 23 days | 92 | healing time reduced by 30%; reduction in positive cultures by 50%, purulent infection reduced | |
| Ketchum/1970 | 3 | 60 | four times a day with 1 h in between for 28 days | 112 | angiography: after day 28 extensive capillary proliferation underneath burn in HBO group; Histology: abundant capillary plexus | |
| Benichoux/1968 | 3 | 60 | once a day | up to 10 | positive effect on mortality in THAM and HBO | |
| 3 | 60 | every second day | up to 15 | HBO alone has no effect on burn, may even have adverse effect on survival; in THAM improved survival; longest survival in HBO&THAM& antibiotics | ||
| Perrins/1969 | 2 | 2 | 60 | four times a day | 12 | burns in HBO group healed slower than in controls |
| 2 | 2 | 60 | four times a day | 12 | no effect of HBO on healing process, no effect on depth of slough. | |
| Spinadel/1969 | 2 | 75 | HBO & antibiotics best results concerning healing; HBO alone and antibiotics alone equal but less good; untreated controls do markedly worse. | |||
| 3 | 120 | HBO & antibiotics best results concerning healing; HBO alone and antibiotics alone equal but less good; untreated controls do markedly worse. | ||||
| Gruber/1970 | 24 | 3 | 45 | once every week | 3 | return of pathologically low oxygen tensions to normal achieved by HBO in flaps, grafts or burns; oxygen levels returning to pretreatment values soon after discontinuing HBO |
| Bleser/1971 | 0.1 | 3 | 60 | every second day | 4 | rapid restoration of total body water; hematocrit, blood volume, plasma volumein HBO; accerlerated recovery in HBO |
| Bleser/1973 | 0.1 | 3 | 60 | once a day | 28 | first no effect, but soon better granulation, more rapid healing and less infection with HBO. |
| Härtwig/1974 | 0.3 | 2.5 | 60 | three times a day | 84 | hardly any edema or inflammation in HBO, hardly any loss of fluid; earlier shedding of eschar; microangiography: rapid revascularization in HBO |
| Korn/1977 | 0.5 | 2 | 90 | twice a day | 6, 8 or 10 | quicker epithelization, no full-thickness conversion in HBO |
| 0.5 | 2 | 90 | twice a day | 2, 4, 6, or 8 | earlier return of vascular patency in HBO | |
| 2 | 90 | twice a day | 8 | mitotic activity in epithelia of burnt controls not evaluable due to widespread necrosis | ||
| Niccole/1977 | 12 | 2.5 | 90 | twice a day | (75?) | no difference concerning edema for either FT or PT; no differences in treatement groups for time to epithelization in PT or to eschar separation in FT; less bacterial colonization in FT after HBO& sulfadiazine and in sulfadiazine alone |
| Wells/1977 | 0.5 | 2 | 60 | once | 1 | less reduction in plasma volume in HBO |
| 0.5 | 3 | 60 | twice | 2 | less reduction in plasma volume in HBO; less decline in postburn cardiac output in HBO | |
| Arzinger-Jonasch/1978 | 2 | 60 or 120 | once a day | 10 | Time until healing of partial or full-thickness burn shortened by 5 days in HBO. Quick reduction of edema, hardly any thromboses, collateral perfusion in HBO. Take of full-thickness skin graft shortened by 2 days in HBO. Positive effect unrelated to time of exposition. | |
| Nylander/1984 | <0.1 | 2.5 | 45 | once a day | 1 | less local and general edema formation at 2, 6 and 24 h after burn (fluid content of ear post HBO similar to untreated one). |
| Kaiser/1985 | 1 in primary HBO; 192 in secondary HBO | 3 | 60 | 3 times a day | up to 81 (until closure of wounds) | noninfected wounds in controls healed quicker than noninfected HBO treated wound; infected wounds treated with primary HBO healed quicker than infected controls; infected wounds treated with secondary HBO healed somewhat slower. |
| Kaiser/1988 | 1 | 3 | 60 | 3 times a day | Extent of burn increased in controls, not in HBO-group; Rapid reduction of wound surface and less edema only in HBO-group | |
| Stewart/1989 | ||||||
| consistently higher tissue ATP in HBO; 2 HBO/day better than one | ||||||
| 0.5 | 2.5 | 60 | once a day | 1 | ||
| 0.5 | 2.5 | 60 | twice a day | 2 | ||
| 0.5 | 2.5 | 60 | once a day | 1 | ||
| 0.5 | 2.5 | 60 | twice a day | 2 to 10 | 36 h post injury, with 2 HBO/day more than tenfold increase in tissue ATP compared to 36 h. controls | |
| Saunders/1989 | 2 | 2 | 60 | once a day | up to 4 | HBO improved microvascularity in all groups; perfusion of dermal und subdermal vessels beneath burn preserved; less permanent collagen denaturation |
| Tenenhaus/1994 | 0.5 | 2.4 | 90 or 120 | twice a day | 2 | mesenterial bacterial cultures are postburn sign. HBO: fewer mesenteric bacterial colonies; fewest colonies in fed, HBO treated mice; Villus length in HBO treated, fed mice as long as in nonburnt controls. |
| 120 | twice a day or three times a day | 3 | fasting produced more bacterial colonies. Three 120 min HBO per 24 h had detrimental effects (seizures); | |||
| Espinosa/1995 | 1 | 2.8 | 60 | twice a day | 8 | significant reduction of edema in HBO with or without antibiotic |
| Hussmann/1996 | ||||||
| 2.5 | 90 | once | 1 | |||
| 2.5 | 90 | twice a day | up to 14 | |||
| 4 | 2.5 | 90 | once | 1 | increase of cytotoxic cells unchanged | |
| 4 | 2.5 | 90 | twice a day | up to 14 | increase of cytotoxic cells unchanged | |
| 4 | 2.5 | 90 | once | 1 | only regimen to prevent increase of cytotoxic (OX8) T-cells on day 1 | |
| 4 | 2.5 | 90 | twice a day | up to 14 | only regimen to downregulate cytotoxic (OX8) T-cells to normal values on days 5 and 15 | |
| Germonpré/ 1996 | 4 | 2 | 60 | every 8 h first day, thereafter twice a day | 6 | Histology day 3: less subepidermal leucocyte infiltration, better preservation of basal membrane * and of skin appendages * after HBO; piracetam has effect only on basal membrane |
| Shoshani/1998 | up to 24 | 2 | 90 | once within first 24 h, twice a day thereafter | 29 | epithelization significantly slower with HBO |
| Akin/2002 | 2.5 | 90 | twice a day | 4 | day 3: HBO prevents intestinal bacterial overgrowth and translocation to lymph nodes, liver and spleen | |
| 14 | day 8: HBO prevents bacterial overgrowth and translocation to lymph nodes, liver and spleen | |||||
| Bilic/2005 | 2 | 2.5 | 60 | once a day for up to 21 days | 21 | Skin samples day 1, 2, 3, 5, 7, 15, 21: less edema, increased neoangiogenesis, higher number of regenatory follicles, earlier epithelization; no significant difference in necrosis staging or margination of leucocytes |
| Türkaslan/2010 | 0.5 | 2.5 | 90 | twice a day | 2 or 10 | no differences in the 24 h-groups; 5 day group HBO: Vital zones preserved; more cells in proliferative phase, more vital cells; prevents progression from zone of stasis to necrosis, less edema |
| 10 | augmented neovascularization, decreased edema in HBO; no secondary enlargement of burn area | |||||
| Selcuk/2013 | 1 | 2.5 | 90 | once a day | 7 | After 21 days no difference concerning microbiology; yet best epithlization, lowermost inflammatory cell response, fewest fibrosis in non-nicotine/HBO |
| Wu/2018 | 24 | 2.5 | 60 | once a day | 5 or 10 | early HBO inhibits Gal-3 dependent TLR-4 pathway; decreases proinflammatory cytokines and proteins in hind horn and paw; suppresses microglia/macrophage activation following burn injury; decreases mechanical withdrawal threshold; promotes wound healing; |
| Wu/2019 | 24 | 2.5 | 60 | once a day | 5 or 10 | more HBO sessions reduce burn—induced mechanical allodynia (upregulation: melatonin, opioid-receptors, downregulation: brain derived neurotropic factor, substance P, calcitonin gene related peptide) |
| Hatibie/2019 | 2.4 | 90 | once a day | 6 | day 14: fewer inflammatory cells and more epithelium in HBO; no difference in angiogenesis | |
| Chen/2018 | 2.5 | 120 | once a day | minimum 20 | postburn pain score lower in HBO | |
| Ikeda/1967 | 3 | once or twice a day | 5 to 10 | 6 patients died (those with 90–100% TBSA); no infection during HBO | ||
| Lamy/1970 | 3 | 60–90 | once or twice a day | HBO does not alter mortality in extensive burns; fewer infections, better granulation and healing | ||
| Hart/1974 | up to 24 | 2 | 90 | three times on first day, then twice a day until healed | various | healing time, morbidity and mortality decreased in HBO. Healing time related to percentage TBSA |
| mean healing time reduced | ||||||
| mean healing time in relation to %TBSA reduced | ||||||
| Grossmann/1978 | up to 4 | 2 | 90 | every 8 h during first 24 h, twice a day thereafter | fluid requirements, healing time 2nd degree, eschar separation time, donor graft harvesting time, length of hospital stay, complications, mortality all reduced compared to non-HBO group; no paralytic ileus in severe burns and HBO, reduction in cost | |
| Waisbren/1982 | worse renal function, lower rate of non-segmented polymorphonuclear leucocytes and higher rate of bacteriemia in HBO; better healing, 75% fewer grafts in HBO | |||||
| Niu/1987 | 2.5 | 90–120 | 2–3 during 1st 24 h; once a day thereafter | fluid loss reduced, earlier re-epithelization, overall mortality same as controls, less though in high-risk group | ||
| Cianci/1989 | about 12 | 2 | 90 | twice a day | duration of hospitalization and number of surgeries reduced in HBO | |
| Cianci/1990 | about 12 | 2 | 90 | twice a day | duration of hospitalization, cost of burn care and number of surgeries reduced in HBO | |
| Hammar-lund/1991 | 1.5; 10.5; or 21.5 | 2.8 | 60 | three times a day | 3 | at day 6: less exsudation, less hyperemia, wound size reduced in HBO; no significant effect on complete epithelization |
| Brannen/1997 | up to 24; mean: 11.5; one third within 8 | 2 | 90 | twice a day | minimum 10; maximal 1 per % TBSA | no difference in number of surgeries, duration of hospitalization or mortality; less fluid loss (mentioned in discussion); no data provided about the subgroup with early HBO application |
| Niezgoda/1997 | 2 | 2.4 | 90 | twice a day | 6 | exsudation, hyperemia, wound surface reduced in HBO; no effect on epithelization |
| Chong/2013 | max 15 | 2.4 | 90 | twice within 22 h | 2 | no effect of HBO on inflammatory markers IL Beta, 4, 6 and 10; significantly lower rate of positive bacterial cultures (staph aureus, pseudomonas). |
| Rasmussen/2015 | 0.1 | 2.4 | 90 | one (mean crossover interval 37 days) | 1 | HBO attenuates central sensitation by thermal injury (pin-prick test, thermal threshold, mechanical threshold; seondary hyperalgesia); no peripheral anti-inflammatory effect. |
| Wahl/2019 | 0.1 | 2.4 | 90 | one | 1 | after one single HBO long-lasting reduction of pain sensitivity surrounding injured area; immediate mitigating effect, long lasting preconditioning effect on hyperalgesia |
| Feature | Number of Experiments (Percentage)/Mean ± SD (Range) |
|---|---|
| species | |
| rat | 44 (57.9%) |
| guinea pig | 20 (26.3% |
| mouse | 4 (5.3%) |
| dog | 3 (4.0%) |
| pig | 2 (2.6%) |
| rabbit | 2 (2.6%) |
| hamster | 1 (1.3%) |
| animals per experiment | 19.1 ± 29.4 (2–250) |
| TBSA (percent) | 21.2 ± 22.8 (0–75) |
| burn thickness | |
| partial thickness | 43 (56.6%) |
| full thickness | 34 (44.7%) |
| superficial | 1 (1.3%) |
| not provided | 3 (3.9%) |
| hours since injury | 8.8 ± 29.6 (0.1–192) |
| ATA | 2.5 ± 0.39 (2.0–3.0) |
| duration of HBO session | 73.2 ± 20.2 (45–120) |
| HBO sessions per day | 1.5 ± 0.9 (0.5–4) |
| total number of HBO sessions | 17.1 ± 26.8 (0–138) |
| Feature | Number of Experiments (Percentage)/Mean ± SD (Range) |
|---|---|
| patients per clinical experimental group | 65.8 ± 111.9 (2–402) |
| study design | |
| controlled | 10 (66.7%) |
| randomized | 5 (33.3%) |
| TBSA (percent) | 35.2 ± 14.2 (13–65) |
| burn thickness | |
| partial thickness | 15 (100.0%) |
| full thickness | 15 (100.0%) |
| hours since injury | 18.7 ± 7.4 (4–24) |
| ATA | 2.2 ± 0.4 (2.0–3.0) |
| duration of HBO session | 92.7 ± 8.8 (85–120) |
| HBO sessions per day | 1.8 ± 0.4 (1–2) |
| total number of HBO sessions | 9.8 ± 7.6 (2–20) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolle, C.; Lindenmann, J.; Kamolz, L.; Smolle-Juettner, F.-M. The History and Development of Hyperbaric Oxygenation (HBO) in Thermal Burn Injury. Medicina 2021, 57, 49. https://doi.org/10.3390/medicina57010049
Smolle C, Lindenmann J, Kamolz L, Smolle-Juettner F-M. The History and Development of Hyperbaric Oxygenation (HBO) in Thermal Burn Injury. Medicina. 2021; 57(1):49. https://doi.org/10.3390/medicina57010049
Chicago/Turabian StyleSmolle, Christian, Joerg Lindenmann, Lars Kamolz, and Freyja-Maria Smolle-Juettner. 2021. "The History and Development of Hyperbaric Oxygenation (HBO) in Thermal Burn Injury" Medicina 57, no. 1: 49. https://doi.org/10.3390/medicina57010049





