Impact of Newborn Screening on Clinical Presentation of Congenital Adrenal Hyperplasia
Abstract
1. Introduction
2. Materials and Methods
2.1. Newborn Screening
2.2. Biochemical Diagnosis of Salt-Wasting CAH
2.3. Molecular Diagnostics
2.4. Subjects
- (1)
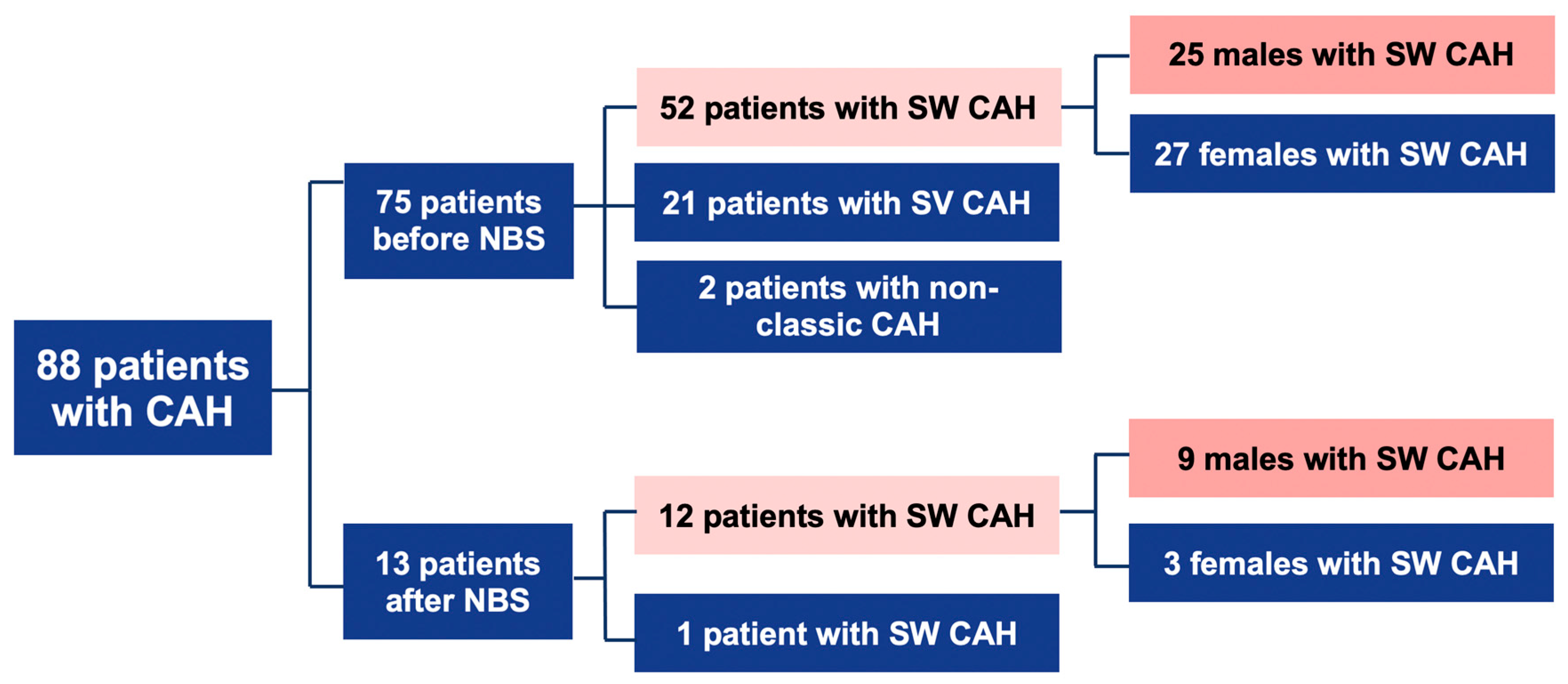
- Before NBS: 75 patients; 52 cases with SW form, 21-with SV, and 2-with NC form.
- (2)
- After NBS: 13 patients; 12 cases with SW, and 1 case with SV form (Figure 1).
2.5. Statistical Analyses
2.6. Bioethics
3. Results
3.1. Newborn Screening for CAH
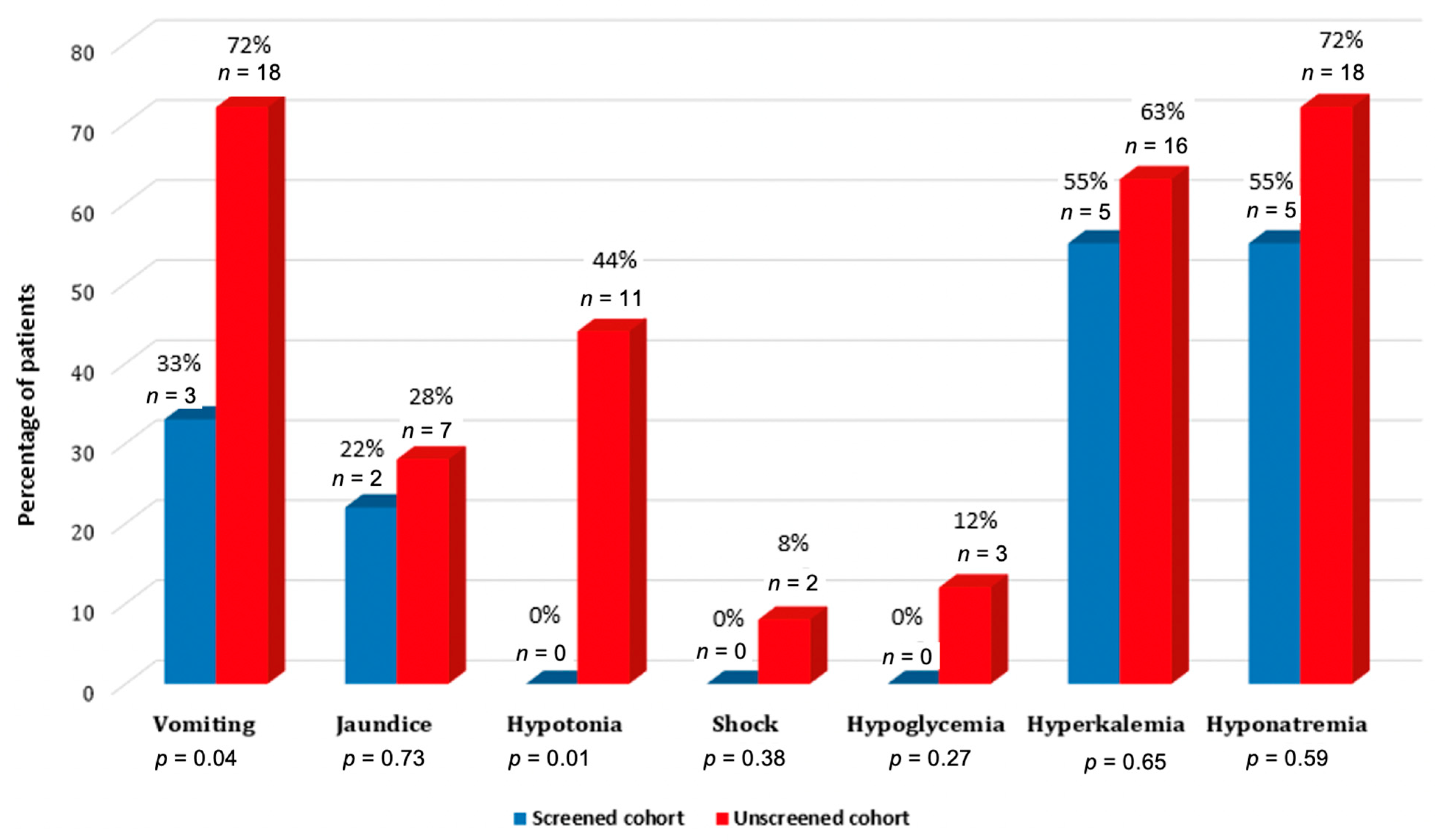
3.2. Clinical and Laboratory Assessment of Patients with SW CAH
3.3. Phenotype and Genotype Correlation in Patients of CAH Diagnosis in Lithuania Cohort
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Claahsen-van der Grinten, H.L.; Speiser, P.W.; Ahmed, S.F.; Arlt, W.; Auchus, R.J.; Falhammar, H.; Flück, C.E.; Guasti, L.; Huebner, A.; Kortmann, B.B.M.; et al. Congenital adrenal hyperplasia—Current insights in pathophysiology, diagnostics and management. Endocr. Rev. 2021, 7, 16. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–423. [Google Scholar] [CrossRef]
- Gong, L.; Gao, X.; Yang, N.; Zhao, J.; Yang, H.; Kong, Y. A pilot study on newborn screening for congenital adrenal hyperplasia in Beijing. J. Pediatric Endocrinol. Metab. 2019, 32, 253–258. [Google Scholar] [CrossRef]
- Witchel, S.F. Congenital Adrenal Hyperplasia. J. Pediatric Adolesc. Gynecol. 2017, 30, 520–534. [Google Scholar] [CrossRef] [PubMed]
- El-Maouche, D.; Arlt, W.; Merke, D.P. Congenital adrenal hyperplasia. Lancet 2017, 390, 2194–2210. [Google Scholar] [CrossRef]
- Therrell, B.L.; Padilla, C.D.; Loeber, J.G.; Kneisser, I.; Saadallah, A.; Borrajo, G.J.; Adams, J. Current status of newborn screening worldwide: 2015. Semin. Perinatol. 2015, 39, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Finkielstain, G.P.; Chen, W.; Mehta, S.P.; Fujimura, F.K.; Hanna, R.M.; Van Ryzin, C.; McDonnell, N.B.; Merke, D.P. Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 2011, 96, E161–E172. [Google Scholar] [CrossRef] [PubMed]
- Sherry, S.T.; Ward, M.-H.; Kholodov, M.; Baker, J.; Phan, L.; Smigielski, E.M.; Sirotkin, K. dbSNP: The NCBI database of genetic variation. Nucleic Acids Res. 2001, 29, 308–311. [Google Scholar] [CrossRef]
- Speiser, P.W.; Arlt, W.; Auchus, R.J.; Baskin, L.S.; Conway, G.S.; Merke, D.P.; Meyer-Bahlburg, H.F.L.; Miller, W.L.; Murad, M.H.; Oberfield, S.E.; et al. Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 4043–4088, Erratum in: J. Clin. Endocrinol. Metab. 2019, 104, 39–40. [Google Scholar] [CrossRef]
- Stenson, P.D.; Ball, E.V.; Mort, M.; Phillips, A.D.; Shiel, J.A.; Thomas, N.S.; Abeysinghe, S.; Krawczak, M.; Cooper, D.N. Human Gene Mutation Database (HGMD): 2003 update. Hum. Mutat. 2003, 21, 577–581. [Google Scholar] [CrossRef] [PubMed]
- den Dunnen, J.T.; Dalgleish, R.; Maglott, D.R.; Hart, R.K.; Greenblatt, M.S.; McGowan-Jordan, J.; Roux, A.F.; Smith, T.; Antonarakis, S.E.; Taschner, P.E.; et al. HGVS Recommendations for the Description of Sequence Variants: 2016 Update. Hum. Mutat. 2016, 37, 564–569. [Google Scholar] [CrossRef] [PubMed]
- van der Linde, A.A.A.; Schönbeck, Y.; van der Kamp, H.J.; van den Akker, E.L.T.; van Albada, M.E.; Boelen, A.; Finken, M.J.J.; Hannema, S.E.; Hoorweg-Nijman, G.; Odink, R.J.; et al. Evaluation of the Dutch neonatal screening for congenital adrenal hyperplasia. Arch. Dis. Child. 2019, 104, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Heather, N.L.; Seneviratne, S.N.; Webster, D.; Derraik, J.G.; Jefferies, C.; Carll, J.; Jiang, Y.; Cutfield, W.S.; Hofman, P.L. Newborn screening for congenital adrenal hyperplasia in New Zealand, 1994–2013. J. Clin. Endocrinol. Metab. 2015, 100, 1002–1008. [Google Scholar] [CrossRef]
- Tsuji, A.; Konishi, K.; Hasegawa, S.; Anazawa, A.; Onishi, T.; Ono, M.; Morio, T.; Kitagawa, T.; Kashimada, K. Newborn screening for congenital adrenal hyperplasia in Tokyo, Japan from 1989 to 2013: A retrospective population-based study. BMC Pediatrics 2015, 15, 209. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.; DeMartino, L.; McMahon, R.; Hamel, R.; Maloney, B.; Stansfield, D.-M.; McGrath, E.C.; Occhionero, A.; Gearhart, A.; Caggana, M.; et al. Newborn screening for congenital adrenal hyperplasia in New York State. Mol. Genet. Metab. Rep. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Fox, D.A.; Ronsley, R.; Khowaja, A.R.; Haim, A.; Vallance, H.; Sinclair, G.; Amed, S. Clinical Impact and Cost Efficacy of Newborn Screening for Congenital Adrenal Hyperplasia. J. Pediatrics 2020, 220, 101–108. [Google Scholar] [CrossRef]
- Tsuji-Hosokawa, A.; Kashimada, K. Thirty-Year Lessons from the Newborn Screening for Congenital Adrenal Hyperplasia (CAH) in Japan. Int. J. Neonatal Screen. 2021, 7, 36. [Google Scholar] [CrossRef]
- Sontag, M.K.; Miller, J.I.; McKasson, S.; Sheller, R.; Edelman, S.; Yusuf, C.; Singh, S.; Sarkar, D.; Bocchini, J.; Scott, J.; et al. Newborn screening timeliness quality improvement initiative: Impact of national recommendations and data repository. PLoS ONE 2020, 15, e0231050. [Google Scholar] [CrossRef]
- Gidlöf, S.; Wedell, A.; Guthenberg, C.; von Döbeln, U.; Nordenström, A. Nationwide neonatal screening for congenital adrenal hyperplasia in sweden: A 26-year longitudinal prospective population-based study. JAMA Pediatrics 2014, 168, 567–574. [Google Scholar] [CrossRef]
- Brosnan, C.A.; Brosnan, P.; Therrell, B.L.; Slater, C.H.; Swint, J.M.; Annegers, J.F.; Riley, W.J. A comparative cost analysis of newborn screening for classic congenital adrenal hyperplasia in Texas. Public Health Rep. 1998, 113, 170–178. [Google Scholar]
- de Miranda, M.C.; Haddad, L.B.P.; Trindade, E.; Cassenote, A.; Hayashi, G.Y.; Damiani, D.; Costa, F.C.; Madureira, G.; de Mendonca, B.B.; Bachega, T.A.S.S. The Cost-Effectiveness of Congenital Adrenal Hyperplasia Newborn Screening in Brazil: A Comparison between Screened and Unscreened Cohorts. Front. Pediatrics 2021, 9, 659492. [Google Scholar] [CrossRef]
- Donaldson, M.D.; Thomas, P.H.; Love, J.G.; Murray, G.D.; McNinch, A.W.; Savage, D.C. Presentation, acute illness, and learning difficulties in salt wasting 21-hydroxylase deficiency. Arch. Dis. Child. 1994, 70, 214–218. [Google Scholar] [CrossRef]
- Grosse, S.D. Cost effectiveness as a criterion for newborn screening policy decisions. In Ethics and Newborn Genetic Screening: New Technologies, New Challenges; Baily, M.A., Murray, T.H., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2009; pp. 58–88. [Google Scholar]
- Lousada, L.M.; Mendonca, B.B.; Bachega, T.A.S.S. Adrenal crisis and mortality rate in adrenal insufficiency and congenital adrenal hyperplasia. Arch. Endocrinol. Metab. 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, R.L.; Torpy, D.J.; Stratakis, C.A.; Falhammar, H. Adrenal Crises in Children: Perspectives and Research Directions. Horm. Res. Paediatr. 2018, 89, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Noel-Weiss, J.; Courant, G.; Woodend, A.K. Physiological weight loss in the breastfed neonate: A systematic review. Open Med. 2008, 2, e99–e110. [Google Scholar] [PubMed]
- Paul, I.M.; Schaefer, E.W.; Miller, J.R.; Kuzniewicz, M.W.; Li, S.X.; Walsh, E.M.; Walsh, E.M. Weight Change Nomograms for the First Month After Birth. Pediatrics 2016, 138, e20162625. [Google Scholar] [CrossRef] [PubMed]
- Balsamo, A.; Cacciari, E.; Piazzi, S.; Cassio, A.; Bozza, D.; Pirazzoli, P.; Zappulla, F. Congenital adrenal hyperplasia: Neonatal mass screening compared with clinical diagnosis only in the Emilia-Romagna region of Italy, 1980–1995. Pediatrics 1996, 98, 362–367. [Google Scholar] [PubMed]
- Ishii, T.; Adachi, M.; Takasawa, K.; Okada, S.; Kamasaki, H.; Kubota, T.; Kobayashi, H.; Sawada, H.; Nagasaki, K.; Numakura, C.; et al. Incidence and Characteristics of Adrenal Crisis in Children Younger than 7 Years with 21-Hydroxylase Deficiency: A Nationwide Survey in Japan. Horm. Res. Paediatr. 2018, 89, 166–171. [Google Scholar] [CrossRef]
- Pode-Shakked, N.; Blau, A.; Pode-Shakked, B.; Tiosano, D.; Weintrob, N.; Eyal, O.; Zung, A.; Levy-Khademi, F.; Tenenbaum-Rakover, Y.; Zangen, D.; et al. Combined Gestational Age- and Birth Weight-Adjusted Cutoffs for Newborn Screening of Congenital Adrenal Hyperplasia. J. Clin. Endocrinol. Metab. 2019, 104, 3172–3180. [Google Scholar] [CrossRef]
- Speiser, P.W.; Chawla, R.; Chen, M.; Diaz-Thomas, A.; Finlayson, C.; Rutter, M.M.; Sandberg, D.E.; Shimy, K.; Talib, R.; Cerise, J.; et al. Newborn Screening Protocols and Positive Predictive Value for Congenital Adrenal Hyperplasia Vary across the United States. Int. J. Neonatal Screen. 2020, 6, 37. [Google Scholar] [CrossRef]
- Nordenström, A.; Wedell, A.; Hagenfeldt, L.; Marcus, C.; Larsson, A. Neonatal screening for congenital adrenal hyperplasia: 17-hydroxyprogesterone levels and CYP21 genotypes in preterm infants. Pediatrics 2001, 108, E68. [Google Scholar] [CrossRef]
- Hingre, R.V.; Gross, S.J.; Hingre, K.S.; Mayes, D.M.; Richman, R.A. Adrenal steroidogenesis in very low birth weight preterm infants. J. Clin. Endocrinol. Metab. 1994, 78, 266–270. [Google Scholar] [CrossRef]
- Kopacek, C.; Prado, M.J.; da Silva, C.M.D.; de Castro, S.M.; Beltrão, L.A.; Vargas, P.R.; Grandi, T.; Rossetti, M.L.R.; Spritzer, P.M. Clinical and molecular profile of newborns with confirmed or suspicious congenital adrenal hyperplasia detected after a public screening program implementation. J. Pediatrics 2019, 95, 282–290. [Google Scholar] [CrossRef]
- Neocleous, V.; Shammas, C.; Phedonos, A.A.; Phylactou, L.A.; Skordis, N. Phenotypic variability of hyperandrogenemia in females heterozygous for CYP21A2 mutations. Indian J. Endocrinol. Metab. 2014, 18, 72–79. [Google Scholar] [CrossRef]
- Baumgartner-Parzer, S.M.; Nowotny, P.; Heinze, G.; Waldhäusl, W.; Vierhapper, H. Carrier frequency of congenital adrenal hyperplasia (21-hydroxylase deficiency) in a middle European population. J. Clin. Endocrinol. Metab. 2005, 90, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Dracopoulou-Vabouli, M.; Maniati-Christidi, M.; Dacou-Voutetakis, C. The spectrum of molecular defects of the CYP21 gene in the Hellenic population: Variable concordance between genotype and phenotype in the different forms of congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 2001, 86, 2845–2848. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoo, B.K.; Grosse, S.D. The cost effectiveness of screening newborns for congenital adrenal hyperplasia. Public Health Genom. 2009, 12, 67–72, Erratum in: Public Health Genom. 2018, 21, 100. [Google Scholar] [CrossRef] [PubMed]
- Lajic, S.; Karlsson, L.; Zetterström, R.H.; Falhammar, H.; Nordenström, A. The Success of a Screening Program Is Largely Dependent on Close Collaboration between the Laboratory and the Clinical Follow-Up of the Patients. Int. J. Neonatal Screen. 2020, 26, 68. [Google Scholar] [CrossRef] [PubMed]
- Grosse, S.D.; Van Vliet, G. Challenges in Assessing the Cost-Effectiveness of Newborn Screening: The Example of Congenital Adrenal Hyperplasia. Int. J. Neonatal Screen 2020, 6, 82. [Google Scholar] [CrossRef]
- Miranda, M.C.; Haddad, L.B.P.; Madureira, G.; De Mendonca, B.B.; Bachega, T.A.S.S. Adverse Outcomes and Economic Burden of Congenital Adrenal Hyperplasia Late Diagnosis in the Newborn Screening Absence. J. Endocr. Soc. 2019, 4, bvz013, Erratum in: J. Endocr. Soc. 2021, 5, 147. [Google Scholar] [CrossRef]
| Gestational Age (Weeks) | Cut-Off of 17OHP Serum Concentrations (ng/mL Serum *) | Reference Value to Recall for a Second Sample (ng/mL Serum *) | Reference Value to Urgent Medical Evaluation (ng/mL Serum *) |
|---|---|---|---|
| >38 | 28 | >28 <33.6 | ≥33.6 |
| 35–37 | 39 | >39 <46.8 | ≥46.8 |
| 32–34 | 53 | >53 <63.6 | ≥63.6 |
| 29–31 | 80 | >80 <96 | ≥96 |
| <29 | 198 | >198 <237.6 | ≥237.6 |
| Numbers of Newborns | |
|---|---|
| Screened newborns (2015–2020) | 158,486 |
| Positive tests (>cut off 17OHP): Recalled for the 2nd sample Notifications of positive case: Referrals for urgent medical evaluation after 1st DBS Referrals for medical evaluation after 2nd DBS | 320 204 118 116 2 |
| False-positive tests | 307 |
| True positive tests: From recalled for the 2nd sample From referred for urgent medical evaluation | 13 2 11 |
| False-negative tests * | 0 |
| Variables | Screened Cohort (n = 12) | Unscreened Cohort (n = 52) | Screened Males (n = 9) | Unscreened Males (n = 25) |
|---|---|---|---|---|
| Age at diagnosis in days, mean ± SD | 14.6 ± 9.6 | 16.5 ± 11.6 | 15.44 ± 7.79 | 19.13 ± 7.156 |
| p = 0.603 | p = 0.19 | |||
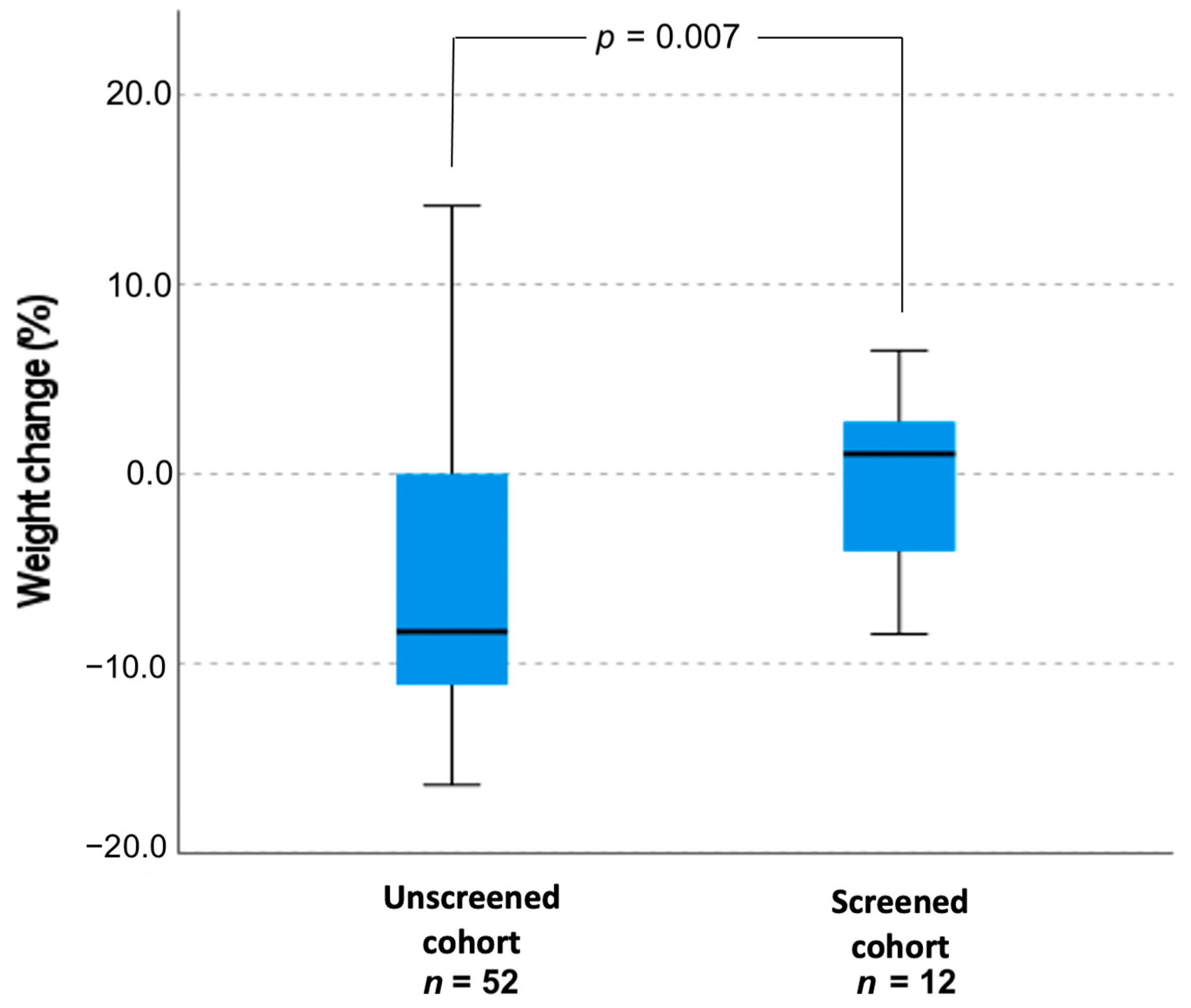
| Weight change (%), mean ± SD | −0.31 ± 4.9 | −6.2 ± 6.9 | 5.42 ± 17.72 | −7.81 ± 8.58 |
| p = 0.007 | p = 0.023 | |||
| Potassium mmol/L, mean ± SD | 6.7 ± 1.3 | 6.9 ± 1.6 | 6.89 ± 1.5 | 7.7 ± 1.5 |
| p = 0.79 | p = 0.33 | |||
| Sodium, mmol/L, mean ± SD | 127.5 ± 7.9 | 127.6 ± 11.6 | 126.31 ± 8.99 | 124.5 ± 9.7 |
| p = 0.67 | p = 0.64 | |||
| Sodium < 132 mmol/L, n (%) | 9 (75%) | 29 (56.4%) | 7 (78%) | 20 (80%) |
| p = 0.22 | p = 0.89 | |||
| Sodium < 130 mmol/L, n (%) | 5 (42%) | 25 (48%) | 5 (55%) | 18 (72%) |
| p = 0.67 | p = 0.59 | |||
| Pahtogenic Variant | Identification No. (dbSNP [10], HGMD [11]) | Expected Phenotype | Allele No. (Frequency) (n = 94) | |
|---|---|---|---|---|
| cDNA Level (NM_000500.9, NG_007941.3) | Predicted Protein Change (NP_000491.4) | |||
| c.1A > C | p.(Met1Leu) | SW | 1(0.01) | |
| c.293-13C > G | New splice acceptor site | rs6467 | SW | 16 (0.17) |
| c.[293-13C > G;1360C > T] | p.[(?);(Pro454Ser)] | - | SW | 3 (0.03) |
| c.[293-13C > G;332_339delGAGACTAC] | p.[(?);(Gly111Valfs) | SW | 1 (0.01) | |
| c.329T > A | p.(Leu110Ter) | - | SW | 2 (0.02) |
| c.332_339delGAGACTAC | p.(Gly111Valfs) | 1 (0.01) | ||
| c.518T > A | p.(Ile173Asn) | rs6475 | SV | 9 (0.10) |
| c.525C > A | p.(Tyr175Ter) | - | SW | 1 (0.01) |
| c.844G> | p.(Val282Leu) | rs6471 | NC | 4 (0.04) |
| c.916del | p.(Val306PhefsTer17) | - | - | 1 (0.01) |
| c.923dupT | p.(Leu308Phefs) | rs267606756 | SW | 1 (0.01) |
| c.[923dupT;955C > T] | p.[(Leu308Phefs); (p.Gln319Ter)] | - | SW | 1 (0.01) |
| c.955C > T | p.(Gln319Ter) | rs7755898 | SW | 3 (0.03) |
| c.1069C > | p.(Arg357Trp) | rs7769409 | SW | 4 (0.04) |
| c.1294G > A | p.(Glu432Lys) | rs1245238711 | NC | 1 (0.01) |
| c.1360C > T | p.(Pro454Ser) | rs6445 | NC | 2 (0.02) |
| ~30 kb deletion 1 | p.(0) | - | SW | 20 (0.21) |
| Deletion of exons 1–3 | p.(0) | - | SW | 7 (0.07) |
| Deletion of exons 1–7 | p.(0) | - | SW | 10 (0.11) |
| Unidentified pathogenic variant | - | 6 (0.06) | ||
| Genotype 1 | Age at Diagnosis | Clinical Manifes-Tation | Expected Phenotype | Gender |
|---|---|---|---|---|
| 1989–2014 | ||||
| c.[CYP21A1P(NR_040090.1)n.877+?_CYP21A2 (NM_000500.9)c.477+?del)];[CYP21A1P(NR_040090.1)n.877+?_CYP21A2(NM_000500.9)c.477+?del)], p.[(0)];[(0)] 2 | 1–5 days | SW | SW | 2 females |
| c.[CYP21A1P(NR_040090.1)n.877+?_CYP21A2(NM_000500.9)c.477+?del)];[(?-8)_(447+1_448-1)del], p.[(0)];[(0)] 3 | 6–20 days | SW | SW | 2 females, 3 males |
| c.[CYP21A1P(NR_040090.1)n.877+?_CYP21A2(NM_000500.9)c.477+?del)];[(?-8)_(939+1_940-1)del], p.[(0)];[(0)] 4 | 0 day | SW | SW | 1 female |
| c.[923dupT];[1451G>C], p.[(Leu308PhefsTer6(;)Arg484Pro)] | 17 days | SW | SV | 1 male |
| c.[CYP21A1P(NR_040090.1)n.877+?_CYP21A2(NM_000500.9)c.477+?del)];[(?-8)_(939+1_940-1)del], p.[(0)];[(0)] 4 | 13 days | SW | SW | 1 male |
| c.[CYP21A1P(NR_040090.1)n.877+?_CYP21A2(NM_000500.9)c.477+?del)];[(?-8)_(939+1_940-1)del], p.[(0)];[(0)] 4 | 34 days | SW | SW | 1 male |
| c.[CYP21A1P(NR_040090.1)n.877+?_CYP21A2(NM_000500.9)c.477+?del)];[293-13C>G], p.[(0)];[(?)] | 0–17 days | SW | SW | 1 female, 2 males |
| c.[(?-8)_(549+1_550-1)del];[293-13C>G], p.[(0)];[(?)] | 13 days | SW | SW | 1 male |
| c.[(?-8)_(939+1_940-1)del];[293-13C>G;518T>A;1360C>T], p.[(0)];[(?;Ile173Asn;Pro454Ser)] | 15 days | SW | SW | 1 male |
| c.[CYP21A1P(NR_040090.1)n.877+?_CYP21A2(NM_000500.9)c.477+?del)];[955C>T], p.[(0)];[(Gln319Ter)] | 42 days | SW | SW | 1 female |
| c.[CYP21A1P(NR_040090.1)n.877+?_CYP21A2(NM_000500.9)c.477+?del)];[1069C>T], p.[(0)];[(Arg357Trp)] | 30 days | SW | SW | 1 male |
| c.[(?-8)_(447+1_448-1)del];[293-13C>G], p.[(0)];[(?)] | 26 days | SW | SW | 1 male |
| c.[(?-8)_(447+1_448-1)del];[923dupT], p.[(0)];[(Leu308PhefsTer6)] | 27 days | SW | SW | 1 male |
| c.[(?-8)_(447+1_448-1)del];[923dupT; 955C>T], p.[(0)];[(Leu308PhefsTer6;Gln319Ter)] | 12 days | SW | SW | 1 female |
| c.[1A>C];[293-13C>G], p.[(Met1Leu)];[(?)] | 22 days | SW | SW | 1 male |
| c.[293-13C>G];[293-13C>G;1360C>T], p.[(?)];[(?;Pro454Ser)] | 1 day | SW | SW | 1 female |
| c.[293-13C>G];[c.332_339del], p.[(?)];[(Gly111ValfsTer21)] | 21 days | SW | SW | 1 male |
| c.293-13C>G(;)332_339del(;)518T>A, p.(?)(;)(Gly111ValfsTer21)(;)(Ile173Asn) | 6 days | SW | SW/SV | 1 male |
| c.293-13C>G(;)332_339del(;)1360C>T, p.(?)(;)(Gly111ValfsTer21)(;)(Pro454Ser) | 15 days | SW | SW/NC | 1 female |
| c.[293-13C>G];[518T>A], p.[(?)];[(Ile173Asn)] | 11 days–12.9 years | SV | SV | 3 females, 1 male |
| c.293-13C>G(;)955C>T(;)1069C>T, p.(?)(;)(Gln319Ter)(;)(Arg357Trp) | 30 days | SW | SW | 1 male |
| c.[293-13C>G];[1069C>T], p.[(?)];[(Arg357Trp) | 7 days | SW | SW | 1 female |
| c.844G>T(;)955C>T, p.(Val282Leu)(;)(Gln319Ter) | 6,2 years | SW | NC | 1 female |
| c.955C>T(;)1360C>T(;)1455del, p.(Gln319Ter)(;)(Pro454Ser)(;)(Met486TrpfsTer56) | 3 days | SW | SW/NC | 1 female |
| c.[518T>A];[518=], p.[(Ile173Asn)];[(Ile173=)] | 6.3 years | SW | SV? | 1 female |
| c.[(?-8)_(939+1_940-1)del];[518T>A], p.[(0)];[(Ile173Asn)] | 5 years | SV | SV | 1 female |
| c(?-8)_(939+1_940-1)del];[518T>A], p.[(0)];[(Ile173Asn)] | 9 years | SV | SV | 1 male |
| c.[(?-8)_(939+1_940-1)del];[844G>T], p.[(0)];[(Val282Leu)] | 7 days | SV | NC | 1 female |
| c.[(?-8)_(939+1_940-1)del]];[1294G>A], p.[(0)];[(Glu432Lys)] | 5 years | SV | NC | 1 female |
| c.[293-13C>G;1360C>T];[1360C>T], p.[(?;Pro454Ser)];[(Pro454Ser)] | 22.7 years | NC | NC | 1 female |
| c.293-13C>G(;)518T>A(;)1360C>T, p.(?)(;)(Ile173Asn)(;)(Pro454Ser) | 27 years | SV | SV/NC | 1 male |
| Heterozygous duplication of CYP21A2 gene. Homozygous or heterozygous genotype for c.955C>T, p.(Gln319Ter) variant | 5 years | SV | SW | 1 male |
| c.[CYP21A1P(NR_040090.1)n.877+?_CYP21A2(NM_000500.9)c.477+?del)];[844G>T] | 16 years | NC | NC | 1 female |
| c.[844G>T];[=], p.[(Val282Leu)];[(Val282=)] | 56 days | NC | - | 1 female |
| 2015–2020 | ||||
| c.[CYP21A1P(NR_040090.1)n.877+?_CYP21A2(NM_000500.9)c.477+?del)];[CYP21A1P(NR_040090.1)n.877+?_CYP21A2(NM_000500.9)c.477+?del)], p.[(0)];[(0)] | 8 days | SW | SW | 1 male |
| c.CYP21A1P(NR_040090.1)n.877+?_CYP21A2(NM_000500.9)c.477+?del)(;)(?-8)_(939+1_940-1)del], p.[(0)];[(0)] | 8 days | SW | SW | 1 male |
| c.CYP21A1P(NR_040090.1)n.877+?_CYP21A2(NM_000500.9)c.477+?del)(;)(c.293-13C>G), p.[(0)];[(?)] | 8 days | SW | SW | 1 female |
| c.(?-8)_(939+1_940-1)del(;)293-13C>G, p.[(0)];[(?)] | 10 days | SW | SW | 1 male |
| c.(?-8)_(939+1_940-1)del(;)329T>A, p.[(0)];[(Leu110Ter)] | 20 days | SW | SW | 1 male |
| c.293-13C>G(;)(293-13C>G) | 5 days | SW | SW | 1 female |
| c.[293-13C>G];[518T>A], p.[(?)];[(Ile173Asn)] | 32 days | SW | SV | 1 male |
| c.[293-13C>G];[1069C>T], p.[(?)];[(Arg357Trp) | 15 days | SW | SW | 1 male |
| c.329T>A(;)525C>A, p.(Leu110Ter)(;)(Tyr175Ter) | 14 days | SW | SW | 1 male |
| c.[518T>A];[916del], p.[(Ile173Asn)];[(Val306PhefsTer17)] | 43 days | SW | SV | 1 male |
| c.[518T>A];[916del], p.[(Ile173Asn)];[(Val306PhefsTer17)] | 15 days | SW | SV | 1 male |
| c.955C>T(;)1069C>T p.(Gln319Ter)(;)(Arg357Trp) | 2 days | SW | SW | 1 female |
| c.[518T>A];[518=], p.[(Ile173Asn)];[(Ile173=)] | 71 days | SV | 1 female | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navardauskaitė, R.; Banevičiūtė, K.; Songailienė, J.; Grigalionienė, K.; Čereškevičius, D.; Šukys, M.; Mockevicienė, G.; Smirnova, M.; Utkus, A.; Verkauskienė, R. Impact of Newborn Screening on Clinical Presentation of Congenital Adrenal Hyperplasia. Medicina 2021, 57, 1035. https://doi.org/10.3390/medicina57101035
Navardauskaitė R, Banevičiūtė K, Songailienė J, Grigalionienė K, Čereškevičius D, Šukys M, Mockevicienė G, Smirnova M, Utkus A, Verkauskienė R. Impact of Newborn Screening on Clinical Presentation of Congenital Adrenal Hyperplasia. Medicina. 2021; 57(10):1035. https://doi.org/10.3390/medicina57101035
Chicago/Turabian StyleNavardauskaitė, Rūta, Kornelija Banevičiūtė, Jurgita Songailienė, Kristina Grigalionienė, Darius Čereškevičius, Marius Šukys, Giedrė Mockevicienė, Marija Smirnova, Algirdas Utkus, and Rasa Verkauskienė. 2021. "Impact of Newborn Screening on Clinical Presentation of Congenital Adrenal Hyperplasia" Medicina 57, no. 10: 1035. https://doi.org/10.3390/medicina57101035
APA StyleNavardauskaitė, R., Banevičiūtė, K., Songailienė, J., Grigalionienė, K., Čereškevičius, D., Šukys, M., Mockevicienė, G., Smirnova, M., Utkus, A., & Verkauskienė, R. (2021). Impact of Newborn Screening on Clinical Presentation of Congenital Adrenal Hyperplasia. Medicina, 57(10), 1035. https://doi.org/10.3390/medicina57101035






