Doppler Ultrasonography of the Fetal Tibial Artery in High-Risk Pregnancy and Its Value in Predicting and Monitoring Fetal Hypoxia in IUGR Fetuses
Abstract
:1. Introduction
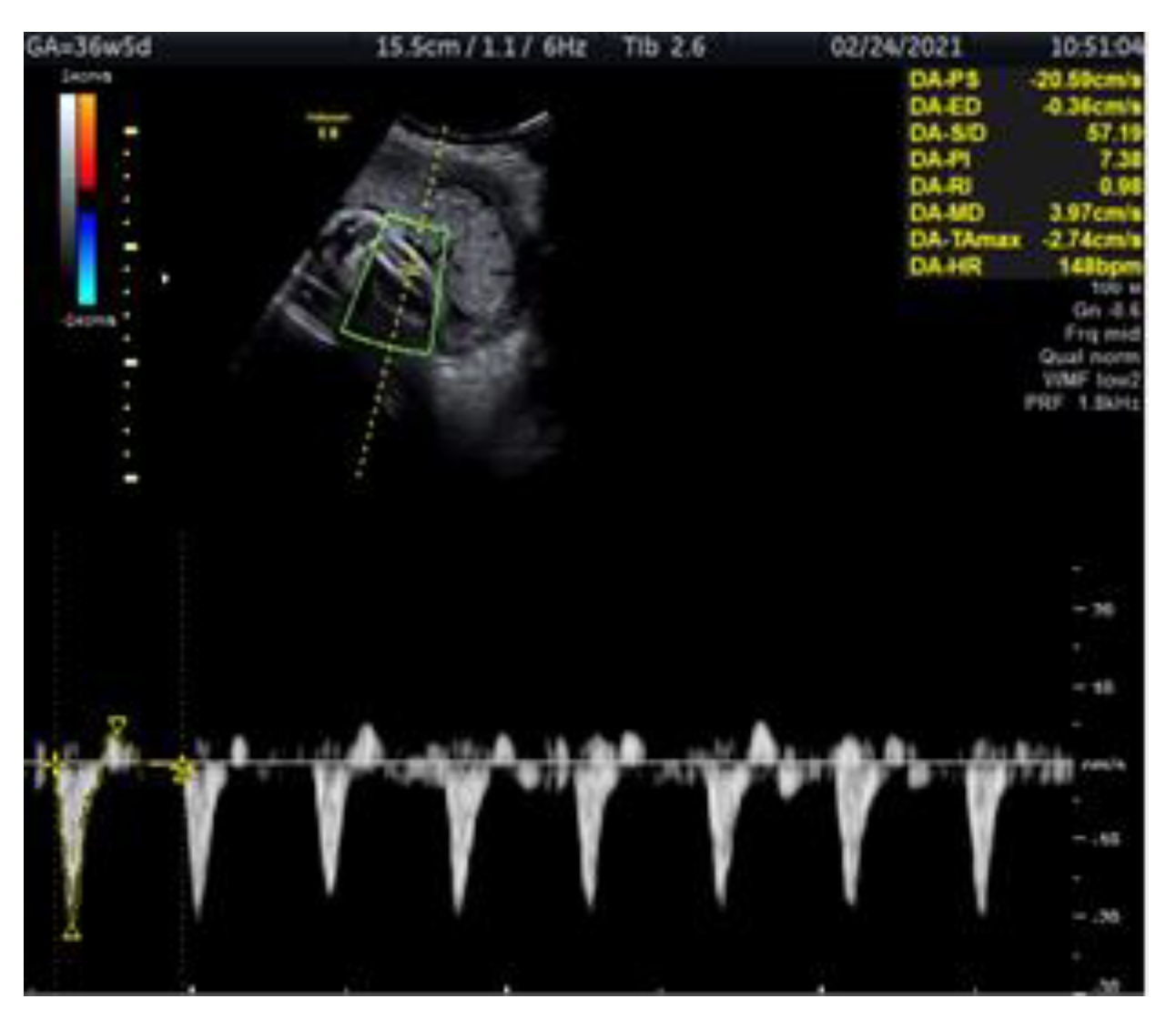
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jacqueline, E.A.; Bamfo, K.; Odibo, A.O. Diagnosis and Management of Fetal Growth Restriction. J. Pregnancy 2011, 2011, 640715. [Google Scholar] [CrossRef] [Green Version]
- Nardozza, L.M.M.; Caetano, A.C.R.; Zamarian, A.C.P.; Mazzola, J.B.; Silva, C.P.; Marçal, V.; Lobo, T.F.; Peixoto, A.B.; Júnior, E.A. Fetal growth restriction: Current knowledge. Arch. Gynecol. Obstet. 2017, 295, 1061–1077. [Google Scholar] [CrossRef] [PubMed]
- Nardozza, L.M.M.; Júnior, E.A.; Barbosa, M.M.; Caetano, A.C.R.; Lee, D.J.R.; Moron, A.F. Fetal growth restriction: Current knowledge to the general Obs/Gyn. Arch. Gynecol. Obstet. 2012, 286, 1–13. [Google Scholar] [CrossRef]
- Romo, A.; Carceller, R.; Tobajas, J. Intrauterine growth retardation (IUGR): Epidemiology and etiology. Pediatr. Endocrinol. Rev. 2009, 6 (Suppl. 3), 332–336. [Google Scholar] [PubMed]
- McCowan, L.M.; Figueras, F.; Anderson, N.H. Evidence-based national guidelines for the management of suspected fetal growth restriction: Comparison, consensus, and controversy. Am. J. Obstet. Gynecol. 2018, 218, S855–S868. [Google Scholar] [CrossRef] [Green Version]
- Tveit, J.V.H.; Saastad, E.; Stray-Pedersen, B.; Børdahl, P.E.; Flenady, V.; Fretts, R.; Frøen, J.F. Reduction of late stillbirth with the introduction of fetal movement information. BMC Pregnancy Childbirth 2009, 9, 32. [Google Scholar] [CrossRef]
- Schlembach, D. Fetal Growth Restriction—Diagnostic Work-up, Management and Delivery. Geburtshilfe Frauenheilkdactions 2020, 80, 1016–1025. [Google Scholar] [CrossRef]
- Passerini, K.; Kurmanavicius, J.; Burkhardt, T.; Balsyte, D. Influence of newborn head circumference and birth weight on the delivery mode of primipara: What is more important? J. Périnat. Med. 2020, 48, 681–686. [Google Scholar] [CrossRef]
- Žaliūnas, B.; Bartkevičienė, D.; Drąsutienė, G.; Utkus, A.; Kurmanavičius, J. Fetal biometry: Relevance in obstetrical practice. Medicina 2017, 53, 357–364. [Google Scholar] [CrossRef]
- Higgins, L.E.; Heazell, A.E.P.; Simcox, L.E.; Johnstone, E.D. Intra-placental arterial Doppler: A marker of fetoplacental vascularity in late-onset placental disease? Acta Obstet. Gynecol. Scand. 2020, 99, 865–874. [Google Scholar] [CrossRef]
- Lewkowitz, A.K.; Tuuli, M.G.; Cahill, A.G.; Macones, G.A.; Dicke, J.M. Perinatal outcomes after intrauterine growth restriction and umbilical artery Doppler pulsatility index of less than the fifth percentile. J. Matern. Neonatal Med. 2021, 34, 677–682. [Google Scholar] [CrossRef]
- Ferrazzi, E.; Bozzo, M.; Rigano, S.; Bellotti, M.; Morabito, A.; Pardi, G.; Battaglia, F.C.; Galan, H.L. Temporal sequence of abnormal Doppler changes in the peripheral and central circulatory systems of the severely growth-restricted fetus. Ultrasound Obstet. Gynecol. 2002, 19, 140–146. [Google Scholar] [CrossRef]
- Bilardo, C.M.; Baschat, A.A. The role of venous Doppler studies in the monitoring of growth-restricted fetuses. Int. Congr. Ser. 2005, 1279, 302–309. [Google Scholar] [CrossRef]
- Akolekar, R.; Ciobanu, A.; Zingler, E.; Syngelaki, A.; Nicolaides, K.H. Routine assessment of cerebroplacental ratio at 35–37 weeks’ gestation in the prediction of adverse perinatal outcome. Am. J. Obstet. Gynecol. 2019, 221, 65.e1–65.e18. [Google Scholar] [CrossRef] [PubMed]
- Figueras, F.; Gratacós, E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn. Ther. 2014, 36, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Dubiel, M.; Gudmundsson, S.; Gunnarsson, G.; Maršál, K. Middle cerebral artery velocimetry as a predictor of hypoxemia in fetuses with increased resistance to blood flow in the umbilical artery. Early Hum. Dev. 1997, 47, 177–184. [Google Scholar] [CrossRef]
- Konje, J.C.; Kaufmann, P.; Bell, S.C.; Taylor, D.J. A longitudinal study of quantitative uterine blood flow with the use of color power angiography in appropriate for gestational age pregnancies. Am. J. Obstet. Gynecol. 2001, 185, 608–613. [Google Scholar] [CrossRef]
- Maršál, K. Physiological adaptation of the growth-restricted fetus. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 49, 37–52. [Google Scholar] [CrossRef]
- Martins, J.G.; Biggio, J.R.; Abuhamad, A. Diagnosis and management of fetal growth restriction: (Replaces Clinical Guideline Number 3, April 2012). Am. J. Obstet. Gynecol. 2020, 223, B2–B17. [Google Scholar] [CrossRef]
- Lees, C.; Stampalija, T.; Baschat, A.A.; da Silva Costa, F.; Ferrazzi, E.; Figueras, F.; Hecher, K.; Kingdom, J.; Poon, L.C.; Salomon, L.J.; et al. ISUOG Practice Guidelines: Diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet. Gynecol. 2020, 56, 298–312. [Google Scholar] [CrossRef]
- Baschat, A.A. Fetal growth restriction—From observation to intervention. J. Périnat. Med. 2010, 38, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Frusca, T.; Todros, T.; Lees, C.; Bilardo, C.M.; Hecher, K.; Visser, G.H.A.; Papageorghiou, A.T.; Marlow, N.; Thilaganathan, B.; Van Wassenaer-Leemhuis, A.; et al. Outcome in early-onset fetal growth restriction is best combining computerized fetal heart rate analysis with ductus venosus Doppler: Insights from the Trial of Umbilical and Fetal Flow in Europe. Am. J. Obstet. Gynecol. 2018, 218, S783–S789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GRIT Study Group. A randomised trial of timed delivery for the compromised preterm fetus: Short term outcomes and Bayesian interpretation. BJOG Int. J. Obstet. Gynaecol. 2003, 110, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Unterscheider, J.; O’Donoghue, K.; Daly, S.; Geary, M.P.; Kennelly, M.M.; McAuliffe, F.M.; Hunter, A.; Morrison, J.J.; Burke, G.; Dicker, P.; et al. Fetal growth restriction and the risk of perinatal mortality-case studies from the multicentre PORTO study. BMC Pregnancy Childbirth Actions 2014, 14, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baschat, A.A. Neurodevelopment following fetal growth restriction and its relationship with antepartum parameters of placental dysfunction. Ultrasound Obstet. Gynecol. 2011, 37, 501–514. [Google Scholar] [CrossRef]
- Figueras, F.; Gratacos, E. Stage-based approach to the management of fetal growth restriction. Prenat. Diagn. 2014, 34, 655–659. [Google Scholar] [CrossRef]
- Baschat, A.A.; Hecher, K. Fetal growth restriction due to placental disease. Semin. Perinatol. 2004, 28, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Baschat, A.A. Planning management and delivery of the growth-restricted fetus. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 49, 53–65. [Google Scholar] [CrossRef]
- Harrington, K.; Thompson, M.O.; Carpenter, R.G.; Nguyen, M.; Campbell, S. Doppler fetal circulation in pregnancies complicated by pre-eclampsia or delivery of a small for gestational age baby: 2. Longitudinal analysis. Br. J. Obstet. Gynaecol. 1999, 106, 453–466. [Google Scholar] [CrossRef] [Green Version]
- Baschat, A.A. Arterial and venous Doppler in the diagnosis and management of early onset fetal growth restriction. Early Hum. Dev. 2005, 81, 877–887. [Google Scholar] [CrossRef]
- Edelstone, D.I. Regulation of blood flow through the ductus venosus. J. Dev. Physiol. 1980, 2, 219–238. [Google Scholar] [PubMed]
- Parer, J.T. The effect of atropine on heart rate and oxygen consumption of the hypoxic fetus. Am. J. Obstet. Gynecol. 1984, 148, 1118–1122. [Google Scholar] [CrossRef]
- Giussani, D.A.; Spencer, J.A.; Hanson, M.A. Fetal cardiovascular reflex responses to hypoxaemia. Fetal Matern. Med. Rev. 1994, 6, 17–37. [Google Scholar] [CrossRef]
- Kurmanavicius, J.; Florio, I.; Wisser, J.; Hebisch, G.; Zimmermann, R.; Müller, R.; Huch, R.; Huch, A. Reference resistance indices of the umbilical, fetal middle cerebral and uterine arteries at 24–42 weeks of gestation. Ultrasound Obstet. Gynecolactions 1997, 10, 112–120. [Google Scholar] [CrossRef]
- Wisser, J.; Kurmanavicius, J. Textbook of Perinatal Medicine, 2nd ed.; Kurjak, A., Ed.; The Parthenon Publishing Group Ltd.: London, UK, 1998; pp. 788–793. [Google Scholar]
- Wisser, J.; Kurmanavicius, J.; Muller, C.; Huch, A.; Huch, R. Pulsatility index in the fetal anterior tibial artery during the second half of normal pregnancy. Ultrasound Obstet. Gynecol. 1998, 11, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Mari, G. Arterial blood flow velocity waveforms of the pelvis and lower extremities in normal and growth-retarded fetuses. Am. J. Obstet. Gynecol. 1991, 165, 143–151. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norvilaitė, K.; Ramašauskaitė, D.; Bartkevičienė, D.; Žaliūnas, B.; Kurmanavičius, J. Doppler Ultrasonography of the Fetal Tibial Artery in High-Risk Pregnancy and Its Value in Predicting and Monitoring Fetal Hypoxia in IUGR Fetuses. Medicina 2021, 57, 1036. https://doi.org/10.3390/medicina57101036
Norvilaitė K, Ramašauskaitė D, Bartkevičienė D, Žaliūnas B, Kurmanavičius J. Doppler Ultrasonography of the Fetal Tibial Artery in High-Risk Pregnancy and Its Value in Predicting and Monitoring Fetal Hypoxia in IUGR Fetuses. Medicina. 2021; 57(10):1036. https://doi.org/10.3390/medicina57101036
Chicago/Turabian StyleNorvilaitė, Kristina, Diana Ramašauskaitė, Daiva Bartkevičienė, Bronius Žaliūnas, and Juozas Kurmanavičius. 2021. "Doppler Ultrasonography of the Fetal Tibial Artery in High-Risk Pregnancy and Its Value in Predicting and Monitoring Fetal Hypoxia in IUGR Fetuses" Medicina 57, no. 10: 1036. https://doi.org/10.3390/medicina57101036





