Perioperative Risk Stratification: A Need for an Improved Assessment in Surgery and Anesthesia—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
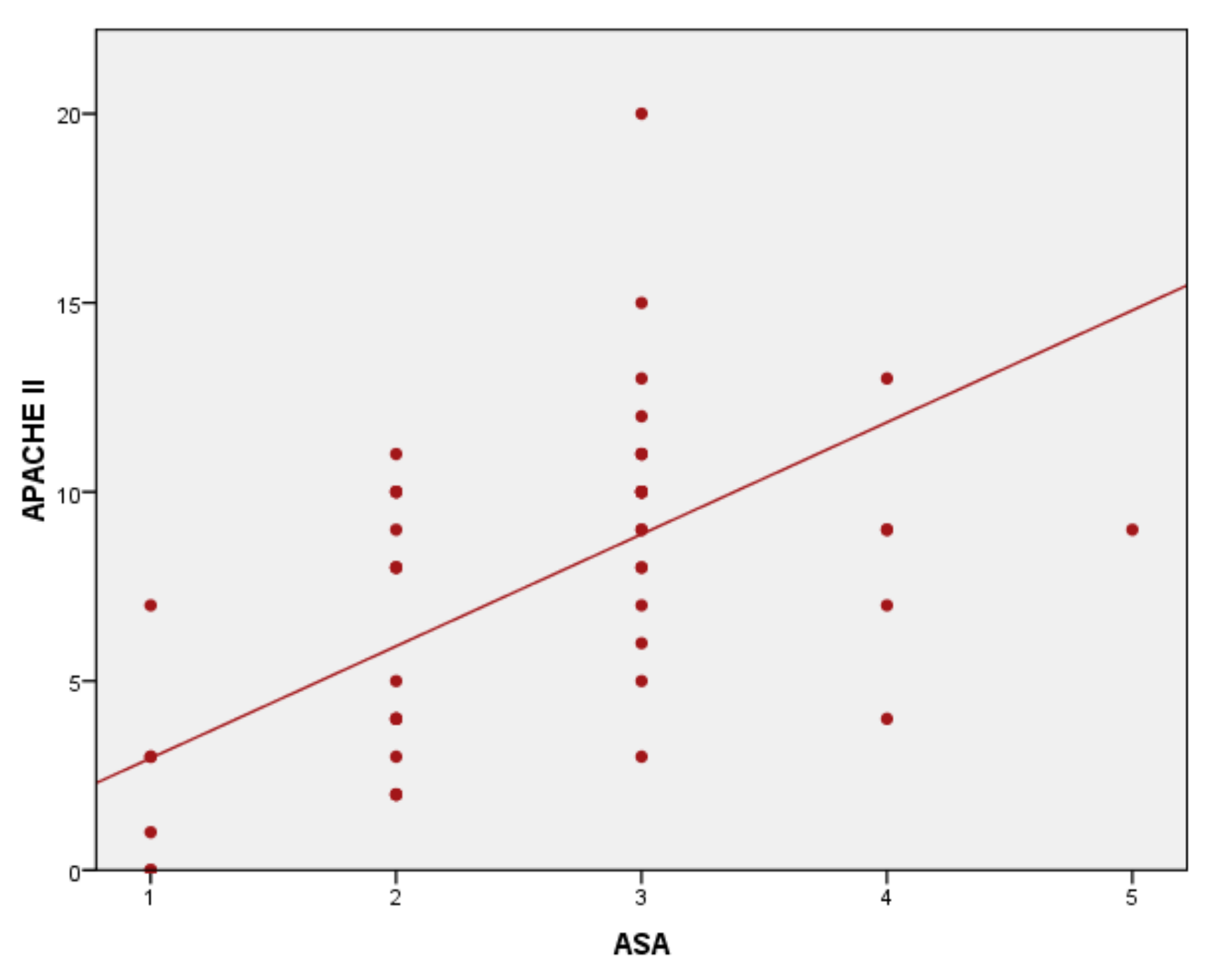
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stones, J.; Yates, D. Clinical risk assessment tools in anaesthesia. BJA Educ. 2018, 19, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Sibakoti, Y.C.; Devkota, H. Evaluation of P- POSSUM Scoring System in Patients Undergoing Emergency Laparotomy. Med J. Shree Birendra Hosp. 2017, 16, 28–36. [Google Scholar] [CrossRef]
- Snyders, P.C.S.; Swart, O.; Duvenage, R.C. Thirty-Day Readmission Rate: A Predictor of Initial Surgical Severity or Quality of Surgical Care? A Regional Hospital Analysis. S. Afr. Med. J. 2020, 110, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S. Scoring Systems for the Patients of Intensive Care Unit. Acute Crit. Care 2018, 33, 102–104. [Google Scholar] [CrossRef]
- Rapsang, A.G.; Shyam, D.C. Scoring systems in the intensive care unit: A compendium. Indian J. Crit. Care Med. 2014, 18, 220–228. [Google Scholar] [CrossRef]
- Bierle, D.M.; Raslau, D.; Regan, D.W.; Sundsted, K.K.; Mauck, K.F. Preoperative Evaluation Before Noncardiac Surgery. Mayo Clin. Proc. 2019, 95, 807–822. [Google Scholar] [CrossRef]
- Aminiahidashti, H.; Bozorgi, F.; Montazer, S.H.; Baboli, M.; Firouzian, A. Comparison of APACHE II and SAPS II Scoring Systems in Prediction of Critically Ill Patients’ Outcome. Emergency 2017, 5, e4. [Google Scholar]
- Flaatten, H.; Walther, S. Activity- or severity-based scoring in the ICU? Acta Anaesthesiol. Scand. 2016, 61, 2–4. [Google Scholar] [CrossRef]
- Balkan, B.; Essay, P.; Subbian, V. Evaluating ICU Clinical Severity Scoring Systems and Machine Learning Applications: APACHE IV/IVa Case Study. Annu Int Conf IEEE Eng Med Biol Soc 2018, 2018, 4073–4076. [Google Scholar] [CrossRef]
- Liu, V.X.; Lu, Y.; Carey, K.A.; Gilbert, E.R.; Afshar, M.; Akel, M.; Shah, N.S.; Dolan, J.; Winslow, C.; Kipnis, P.; et al. Comparison of Early Warning Scoring Systems for Hospitalized Patients With and Without Infection at Risk for In-Hospital Mortality and Transfer to the Intensive Care Unit. JAMA Netw. Open 2020, 3, e205191. [Google Scholar] [CrossRef]
- Oliver, C.M.; Walker, E.; Giannaris, S.; Grocott, M.P.W.; Moonesinghe, S.R. Risk Assessment Tools Validated for Patients Undergoing Emergency Laparotomy: A Systematic Review. Br. J. Anaesth. 2015, 115, 849–860. [Google Scholar] [CrossRef]
- Hackett, N.J.; De Oliveira, G.S.; Jain, U.K.; Kim, J.Y. ASA class is a reliable independent predictor of medical complications and mortality following surgery. Int. J. Surg. 2015, 18, 184–190. [Google Scholar] [CrossRef]
- Young, J.; Badgery-Parker, T.; Dobbins, T.; Jorgensen, M.; Gibbs, P.; Faragher, I.; Jones, I.; Currow, D. Comparison of ECOG/WHO Performance Status and ASA Score as a Measure of Functional Status. J. Pain Symptom Manag. 2014, 49, 258–264. [Google Scholar] [CrossRef]
- Curatolo, C.; Goldberg, A.; Maerz, D.; Lin, H.-M.; Shah, H.; Trinh, M. ASA Physical Status Assignment by Non-Anesthesia Providers: Do Surgeons Consistently Downgrade the ASA Score Preoperatively? J. Clin. Anesth. 2017, 38, 123–128. [Google Scholar] [CrossRef]
- Ngulube, A.; Muguti, G.I.; Muguti, E.G. Validation of POSSUM, P-POSSUM and the surgical risk scale in major general surgical operations in Harare: A prospective observational study. Ann. Med. Surg. 2019, 41, 33–39. [Google Scholar] [CrossRef]
- Thahir, A.; Pinto-Lopes, R.; Madenlidou, S.; Daby, L.; Halahakoon, V.C. Mortality risk scoring in emergency general surgery: Are we using the best tool? J. Perioper. Pract. 2020, 31, 153–158. [Google Scholar] [CrossRef]
- Liu, R.; Kidane, B. Esophagectomy Surgical Apgar Score: One Size Fits All? Semin. Thorac. Cardiovasc. Surg. 2019, 31, 581–582. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, Y.-C.; Yang, C.-H.; Su, N.-Y. Surgical Apgar score is strongly associated with postoperative ICU admission. Sci. Rep. 2021, 11, 115. [Google Scholar] [CrossRef]
- Pearson, A.C.S.; Subramanian, A.; Schroeder, D.R.; Findlay, J.Y. Adapting the Surgical Apgar Score for Perioperative Outcome Prediction in Liver Transplantation: A Retrospective Study. Transplant. Direct 2017, 3, e221. [Google Scholar] [CrossRef]
- Lee, H.; Lim, C.W.; Hong, H.P.; Ju, J.-W.; Jeon, Y.T.; Hwang, J.W.; Park, H.-P. Efficacy of the APACHE II Score at ICU Discharge in Predicting Post-ICU Mortality and ICU Readmission in Critically Ill Surgical Patients. Anaesth. Intensiv. Care 2015, 43, 175–186. [Google Scholar] [CrossRef]
- Basile-Filho, A.; Lago, A.F.; Menegueti, M.G.; Nicolini, E.A.; Rodrigues, L.A.d.B.; Nunes, R.S.; Auxiliadora-Martins, M.; Ferez, M.A. The Use of APACHE II, SOFA, SAPS 3, C-Reactive Protein/Albumin Ratio, and Lactate to Predict Mortality of Surgical Critically Ill Patients: A Retrospective Cohort Study. Medicine 2019, 98, e16204. [Google Scholar] [CrossRef]
- Helkin, A.; Jain, S.V.; Gruessner, A.; Fleming, M.; Kohman, L.; Costanza, M.; Cooney, R.N. Impact of ASA score misclassification on NSQIP predicted mortality: A retrospective analysis. Perioper. Med. 2017, 6, 23. [Google Scholar] [CrossRef]
- De Cassai, A.; Boscolo, A.; Tonetti, T.; Ban, I.; Ori, C. Assignment of ASA-Physical Status Relates to Anesthesiologists’ Experience: A Survey-Based National-Study. Korean J. Anesth. 2019, 72, 53–59. [Google Scholar] [CrossRef]
- Katz, A.D.; Mancini, N.; Karukonda, T.; Cote, M.; Moss, I.L. Comparative and Predictor Analysis of 30-day Readmission, Reoperation, and Morbidity in Patients Undergoing Multilevel ACDF Versus Single and Multilevel ACCF Using the ACS-NSQIP Dataset. Spine 2019, 44, E1379–E1387. [Google Scholar] [CrossRef]
- Al-Mazrou, A.M.; Haiqing, Z.; Guanying, Y.; Kiran, R.P. Sustained positive impact of ACS-NSQIP program on outcomes after colorectal surgery over the last decade. Am. J. Surg. 2019, 219. [Google Scholar] [CrossRef]
- Talmor, D.; Kelly, B. How to better identify patients at high risk of postoperative complications? Curr. Opin. Crit. Care 2017, 23, 417–423. [Google Scholar] [CrossRef]
- Doyle, D.J.; Goyal, A.; Bansal, P.; Garmon, E.H. American Society of Anesthesiologists Classification. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Hurwitz, E.E.; Simon, M.; Vinta, S.R.; Zehm, C.F.; Shabot, S.M.; Minhajuddin, A.; Abouleish, A.E. Adding Examples to the ASA-Physical Status Classification Improves Correct Assignment to Patients. Anesthesiology 2017, 126, 614–622. [Google Scholar] [CrossRef]
- Elias, A.C.G.P.; Matsuo, T.; Grion, C.M.C.; Cardoso, L.T.Q.; Verri, P.H. POSSUM escore como preditor de mortalidade em pacientes cirúrgicos. Rev. da Esc. de Enferm. da USP 2009, 43, 23–29. [Google Scholar] [CrossRef][Green Version]
- Hopkins, T.J.; Raghunathan, K.; Barbeito, A.; Cooter, M.; Stafford-Smith, M.; Schroeder, R.; Grichnik, K.; Gilbert, R.; Aronson, S. Associations between ASA Physical Status and Postoperative Mortality at 48 h: A Contemporary Dataset Analysis Compared to a Historical Cohort. Perioper Med. 2016, 5, 29. [Google Scholar] [CrossRef]
- Mayhew, D.; Mendonca, V.; Murthy, B.V.S. A review of ASA physical status—Historical perspectives and modern developments. Anaesthesia 2019, 74, 373–379. [Google Scholar] [CrossRef]
- Kinoshita, M.; Morioka, N.; Yabuuchi, M.; Ozaki, M. New surgical scoring system to predict postoperative mortality. J. Anesthesia 2016, 31, 198–205. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nag, D.S.; Dembla, A.; Mahanty, P.R.; Kant, S.; Chatterjee, A.; Samaddar, D.P.; Chugh, P. Comparative analysis of APACHE-II and P-POSSUM scoring systems in predicting postoperative mortality in patients undergoing emergency laparotomy. World J. Clin. Cases 2019, 7, 2227–2237. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Wang, S.; Xu, G.; Liu, J. Evaluation of the POSSUM, p-POSSUM, o-POSSUM, and APACHE II scoring systems in predicting postoperative mortality and morbidity in gastric cancer patients. Asian J. Surg. 2015, 40, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Kisa, N.G.; Kisa, E.; Cevik, B.E. Prediction of Mortality in Patients after Oncologic Gastrointestinal Surgery: Comparison of the ASA, APACHE II, and POSSUM Scoring Systems. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Jain, G.; Dosi, R.; Jain, N.; Pawar, K.S.; Sen, J. The predictive ability of SAPS II, APACHE II, SAPS III, and APACHE IV to assess outcome and duration of mechanical ventilation in respiratory intensive care unit. Lung India 2021, 38, 236–240. [Google Scholar] [CrossRef]
- Hosseini, M.; Ramazani, J. Evaluation of Acute Physiology and Chronic Health Evaluation II and sequential organ failure assessment scoring systems for prognostication of outcomes among Intensive Care Unit′s patients. Saudi J. Anaesth. 2016, 10, 168–173. [Google Scholar] [CrossRef]
- Busby, J.; Purdy, S.; Hollingworth, W. Calculating hospital length of stay using the Hospital Episode Statistics; a comparison of methodologies. BMC Health Serv. Res. 2017, 17, 347. [Google Scholar] [CrossRef]
- Buttigieg, S.C.; Abela, L.; Pace, A. Variables affecting hospital length of stay: A scoping review. J. Heal. Organ. Manag. 2018, 32, 463–493. [Google Scholar] [CrossRef]
- Gharacheh, L.; Torabipour, A.; Khiavi, F.F.; Malehi, A.S.; Haddadzadeh, M. Comparison of Statistical Models of Predict the Factors Affecting the Length of Stay (LOS) in the Intensive Care Unit (ICU) of a Teaching Hospital. Mater. Socio Med. 2017, 29, 88–91. [Google Scholar] [CrossRef]
- Baltazar, G.; Darnauer, T.; Akella, K.; Kanitsch, S.; Shafey, A.; Chendrasekhar, A. Surgical Apgar Score Predicts Postoperative Length of Stay Better Than American Society of Anesthesiologists Classification. Internet J. Surg. 2015, 32. [Google Scholar] [CrossRef]
- McDonald, M.R.; Sathiyakumar, V.; Apfeld, J.C.; Hooe, B.S.; Ehrenfeld, J.M.; Obremskey, W.T.; Sethi, M.K. Predictive factors of hospital length of stay in patients with operatively treated ankle fractures. J. Orthop. Traumatol. 2013, 15, 255–258. [Google Scholar] [CrossRef]
- McGuckin, D.G.; Mufti, S.; Turner, D.J.; Bond, C.; Moonesinghe, S.R. The association of peri-operative scores, including frailty, with outcomes after unscheduled surgery. Anaesthesia 2018, 73, 819–824. [Google Scholar] [CrossRef]


| Minimum | Maximum | Percentiles | IQR | |||
|---|---|---|---|---|---|---|
| 25th | 50th (Median) | 75th | ||||
| LOS | 0.20 | 30.00 | 5.00 | 9.00 | 11.00 | 6.00 |
| Predicted LOS | 0.00 | 30.00 | 2.00 | 4.00 | 6.00 | 4.00 |
| Minimum | Maximum | Percentiles | IQR | |||
|---|---|---|---|---|---|---|
| 25th | 50th (Median) | 75th | ||||
| P-POSSUM mortality rate | 0.20 | 22.70 | 0.40 | 1.35 | 4.87 | 4.48 |
| APACHE II mortality rate | 4.00 | 40.00 | 4.00 | 8.00 | 15.00 | 11.00 |
| SAS mortality rate | 0.00 | 14.00 | 1.00 | 1.00 | 1.02 | 0.02 |
| Spearman’s Rho | POSSUM | APACHE II | ASA | |
|---|---|---|---|---|
| Predicted LOS | Correlation Coefficient | 0.433 | 0.454 | 0.676 |
| Sig. (Two-tailed) | 0.002 | 0.001 | 0.000 | |
| Spearman’s Rho | LOS | Predicted LOS | ASA | |
|---|---|---|---|---|
| SAS | Correlation Coefficient | −0.326 | −0.486 | −0.446 |
| Sig. (2-tailed) | 0.021 | 0.000 | 0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigorescu, B.-L.; Săplăcan, I.; Petrișor, M.; Bordea, I.R.; Fodor, R.; Lazăr, A. Perioperative Risk Stratification: A Need for an Improved Assessment in Surgery and Anesthesia—A Pilot Study. Medicina 2021, 57, 1132. https://doi.org/10.3390/medicina57101132
Grigorescu B-L, Săplăcan I, Petrișor M, Bordea IR, Fodor R, Lazăr A. Perioperative Risk Stratification: A Need for an Improved Assessment in Surgery and Anesthesia—A Pilot Study. Medicina. 2021; 57(10):1132. https://doi.org/10.3390/medicina57101132
Chicago/Turabian StyleGrigorescu, Bianca-Liana, Irina Săplăcan, Marius Petrișor, Ioana Roxana Bordea, Raluca Fodor, and Alexandra Lazăr. 2021. "Perioperative Risk Stratification: A Need for an Improved Assessment in Surgery and Anesthesia—A Pilot Study" Medicina 57, no. 10: 1132. https://doi.org/10.3390/medicina57101132
APA StyleGrigorescu, B.-L., Săplăcan, I., Petrișor, M., Bordea, I. R., Fodor, R., & Lazăr, A. (2021). Perioperative Risk Stratification: A Need for an Improved Assessment in Surgery and Anesthesia—A Pilot Study. Medicina, 57(10), 1132. https://doi.org/10.3390/medicina57101132







