Predictors of Atrial Fibrillation Recurrences after a First Radiofrequency Catheter Ablation Intervention for Paroxysmal Atrial Fibrillation—Experience of a Low Volume Ablation Centre
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
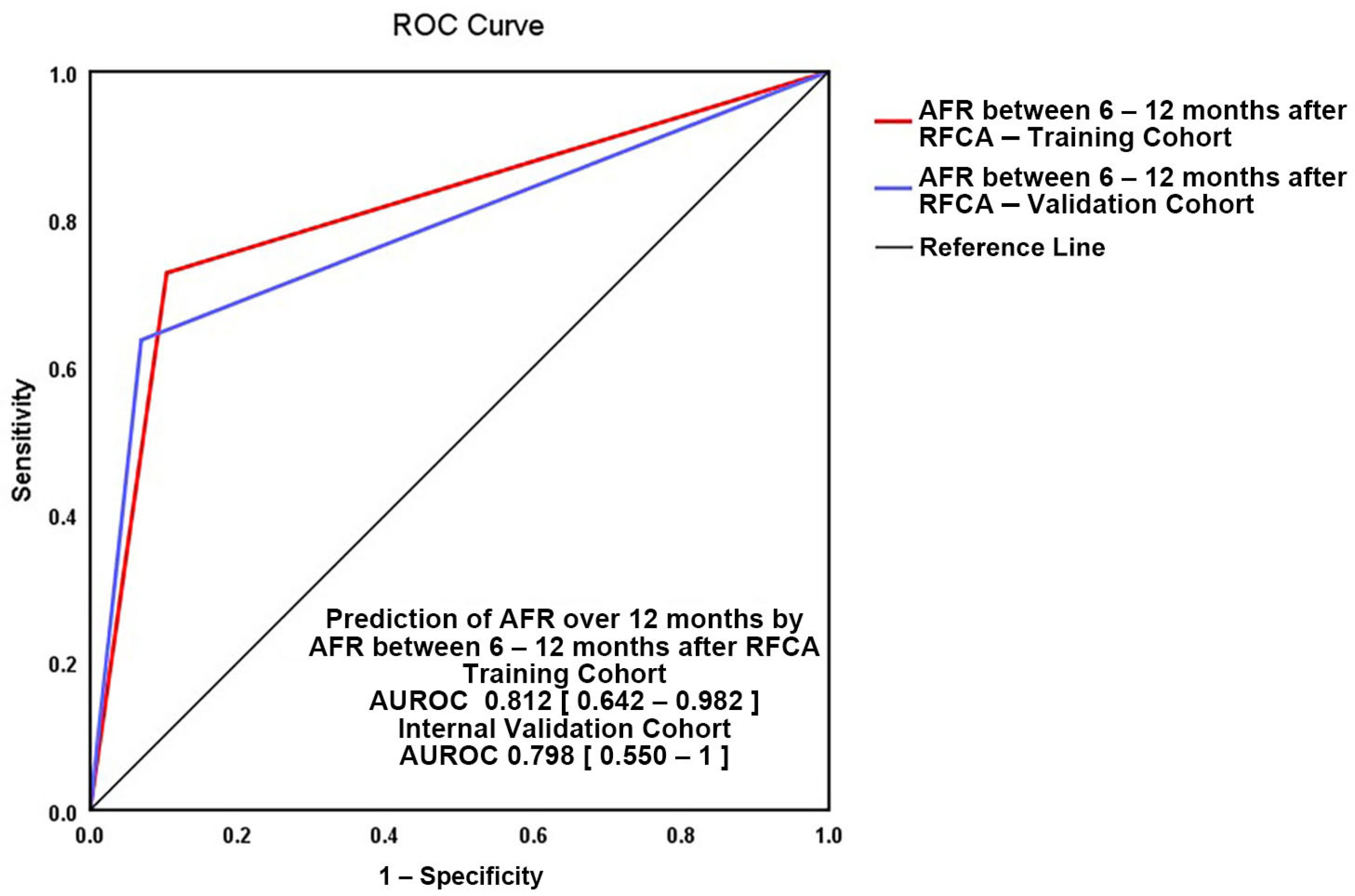
3.2. Independent Predictors for AFR between 6 and 12 Months
3.3. Independent Predictors for AFR after 12 Months
4. Discussion
4.1. Rationale for the Study
4.2. Added Value to Current Literature
4.3. Comparison with Published Data
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Outcome | Independent Characteristics | r Correlation | p-Value |
|---|---|---|---|
| Presence of AFR (0–3 months) | Complete PVI | −0.401 | 0.010 |
| AF history from first diagnosis | 0.033 | 0.840 | |
| Number of AFR 0–3 months | 0.939 | 0.001 | |
| AFR 3–12 months | 0.608 | 0.001 | |
| Number of AFR 3–12 months | 0.601 | 0.001 | |
| AFR after 12 months | 0.159 | 0.350 | |
| SVPB/24 h at 1 month ECG monitoring | 0.301 | 0.059 | |
| SVPB/24 h at 3 months ECG monitoring | 0.451 | 0.003 | |
| Presence of AFR (3–12 months) | Complete PVI | −0.387 | 0.014 |
| Number of AFR 3–12 months | 0.969 | 0.001 | |
| AF on ECG monitoring | 0.542 | 0.001 | |
| AFR after 12 months | 0.523 | 0.001 | |
| SVPB/24 h at 1 month | 0.221 | 0.170 | |
| SVPB/24 h at 3 months | 0.615 | 0.001 | |
| SVPB/24 h at 6 months | 0.494 | 0.001 | |
| SVPB/24 h at 12 months | 0.646 | 0.001 | |
| Presence of AFR after 12 months | Complete PVI | −0.116 | 0.528 |
| AFR 3–12 months | 0.523 | 0.001 | |
| AFR 3–6 months | 0.262 | 0.123 | |
| AFR 6–12 months | 0.585 | 0.001 | |
| Number of AFR 3–6 months | 0.224 | 0.189 | |
| Number of AFR 6–12 months | 0.568 | 0.001 | |
| SVPB/24 h at 1 month | 0.376 | 0.024 | |
| SVPB/24 h at 3 months | 0.370 | 0.026 | |
| SVPB/24 h at 6 months | 0.388 | 0.021 | |
| SVPB /24 h at 12 months | 0.609 | 0.001 | |
| AF on 48 h ECG monitor (0–3 months) | AF on self-monitoring 0–3 months | 0.329 | 0.038 |
| AF on 48 h ECG monitor (3–12 months) | AF on self-monitoring 3–12 months | 0.542 | 0.001 |
| Variables | Nagelkerke R2 | Hosmer–Lemeshow Test | p-Value Regression Coefficient | AUROC | p-Value AUROC | |
|---|---|---|---|---|---|---|
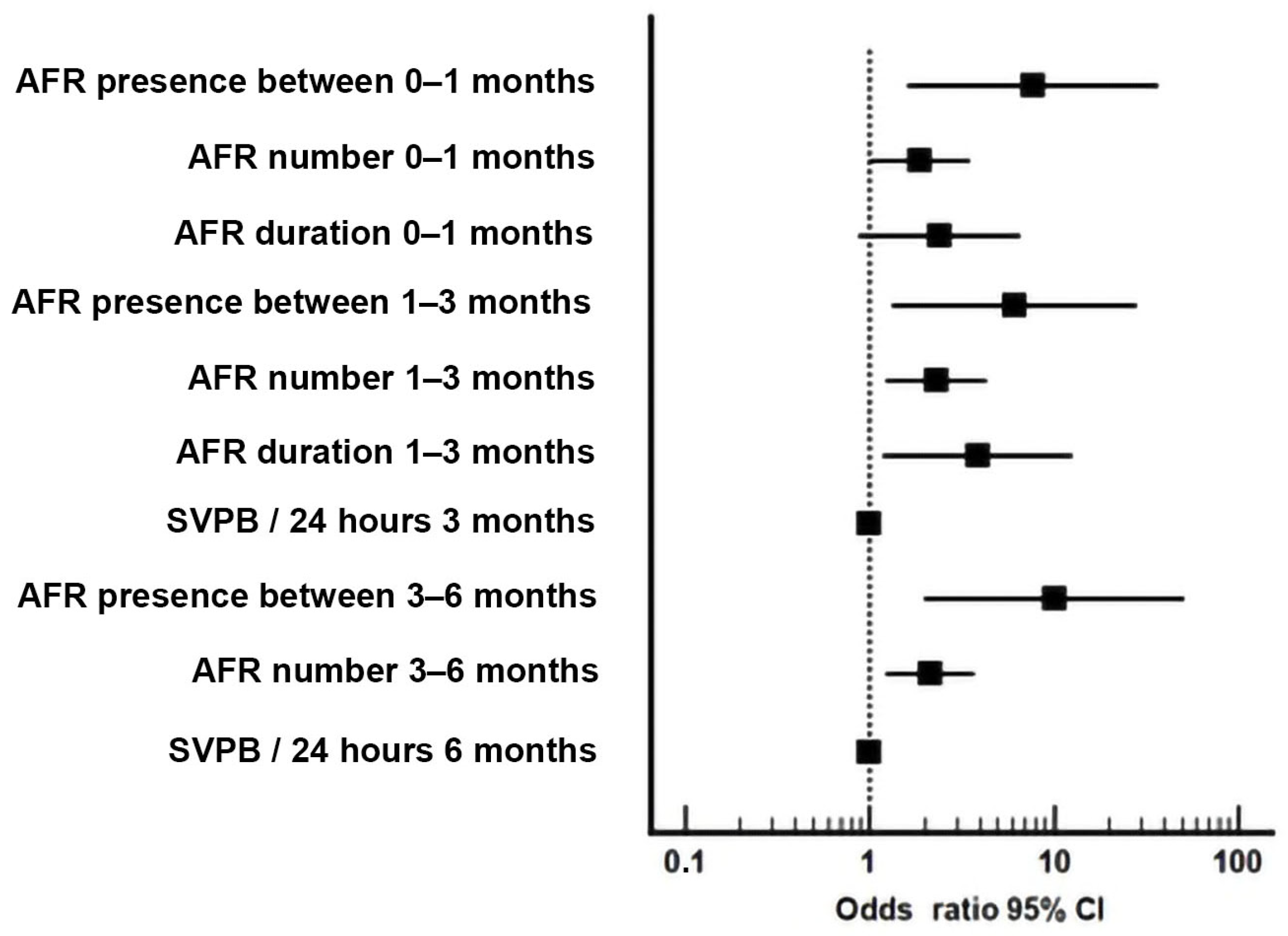
| 1 | AFR presence 0–1 month | 0.244 | - | 0.011 | 0.726 | 0.031 |
| 2 | AFR number 0–1 month | 0.165 | 0.135 | 0.044 | 0.719 | 0.036 |
| 3 | AFR duration 0–1 month | 0.114 | 0.063 | 0.091 | 0.672 | 0.101 |
| 4 | AFR 1–3 months | 0.202 | - | 0.020 | 0.707 | 0.048 |
| 5 | AFR number 1–3 months | 0.298 | 0.564 | 0.009 | 0.746 | 0.019 |
| 6 | AFR duration 1–3 months | 0.202 | 0.598 | 0.025 | 0.715 | 0.039 |
| 7 | SVPB/24 h at 3 months ECG monitor | 0.270 | 0.407 | 0.059 | 0.769 | 0.012 |
| 8 | AFR 3–6 months | 0.291 | - | 0.005 | 0.744 | 0.020 |
| 9 | AFR number 3–6 months | 0.315 | 0.747 | 0.007 | 0.759 | 0.013 |
| 10 | SVPB/24 h at 6 months ECG monitor | 0.480 | 0.381 | 0.096 | 0.776 | 0.009 |
| Model Number | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Parameters | RN01, RD01, RN13, RN36, SVPB/24 h6 m | RN01, RD01, RN13, SVPB/24 h6 m | RD01, RN13, SVPB/24 h 6 m |
| Nagelkerke R2 | 0.735 | 0.728 | 0.707 |
| Hosmer–Lemeshow Test | 0.126 | 0.129 | 0.131 |
| AIC | 30.05 | 28.45 | 27.55 |
| BIC | 39.71 | 36.50 | 34.00 |
| Multivariate Model | Variable 1 Arctan (Duration of AFR during the First Month) | Variable 2 Arctan (Number of AFR 1–3 m) | Variable 3 Loge (SVPB/24 h at 6 m Holter ECG) |
|---|---|---|---|
| VIF | 1.029 | 1.291 | 1.264 |
| Coefficient | 2.255 | 2.081 | 0.752 |
| Coefficient–Standard Error | 1.159 | 1.042 | 0.342 |
| Coefficient–Significance | p = 0.05 | p = 0.04 | p = 0.02 |
| Intercept | 6.05 | ||
| Intercept–Standard Error | 2.1 | ||
| Intercept–Significance | p = 0.004 | ||
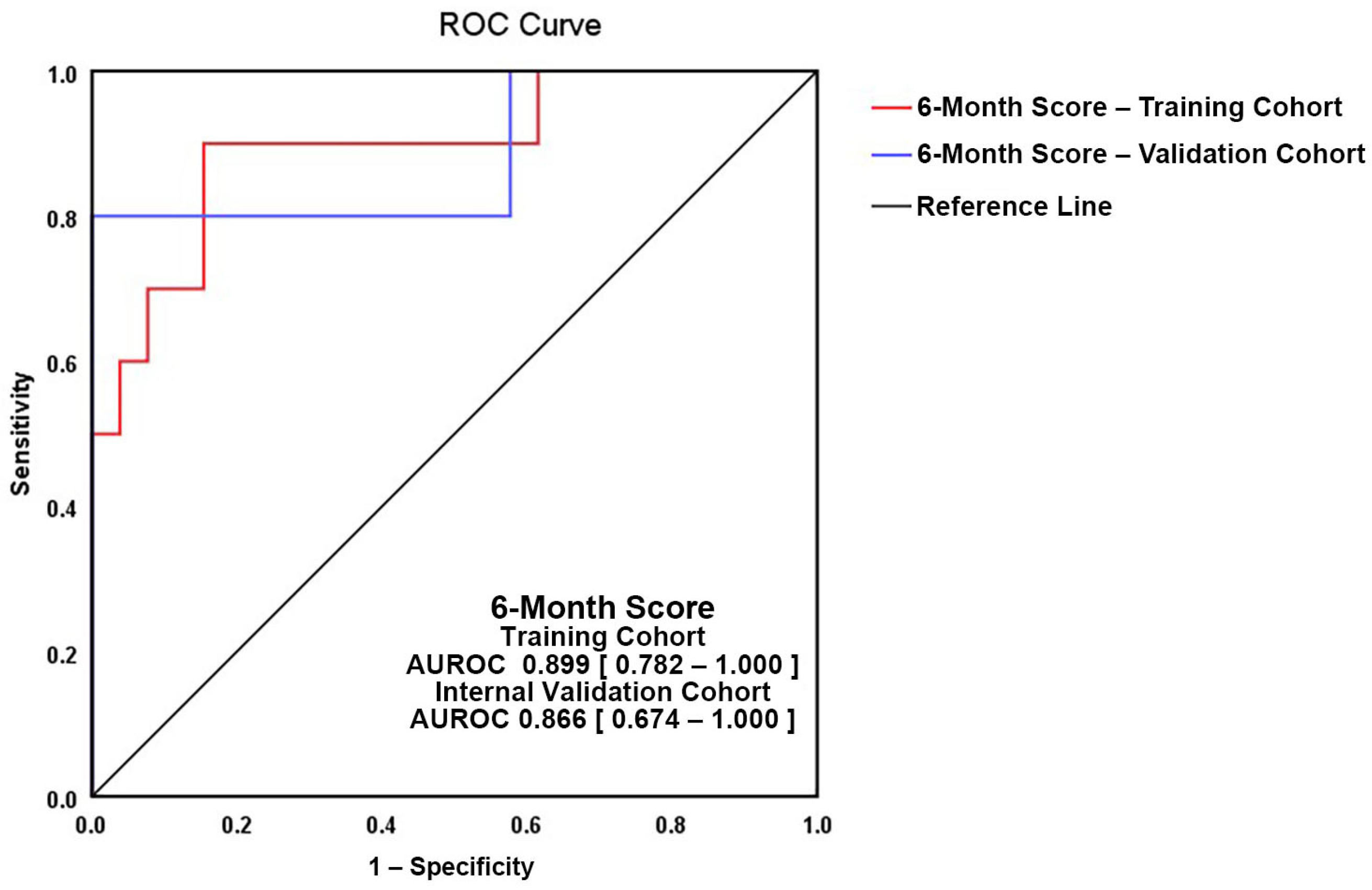
| AUROC | 0.899 (0.782–1.0) | ||
| Nagelkerke Pseudo-R2 | 0.591 | ||
| Hosmer–Lemeshow p-value | 0.452 | ||
| AIC | 33.135 | ||
| BIC | 39.579 | ||
| Variables | Nagelkerke R2 | Hosmer–Lemeshow Test | p-Value Regression Coefficient | AUROC | p-Value AUROC | |
|---|---|---|---|---|---|---|
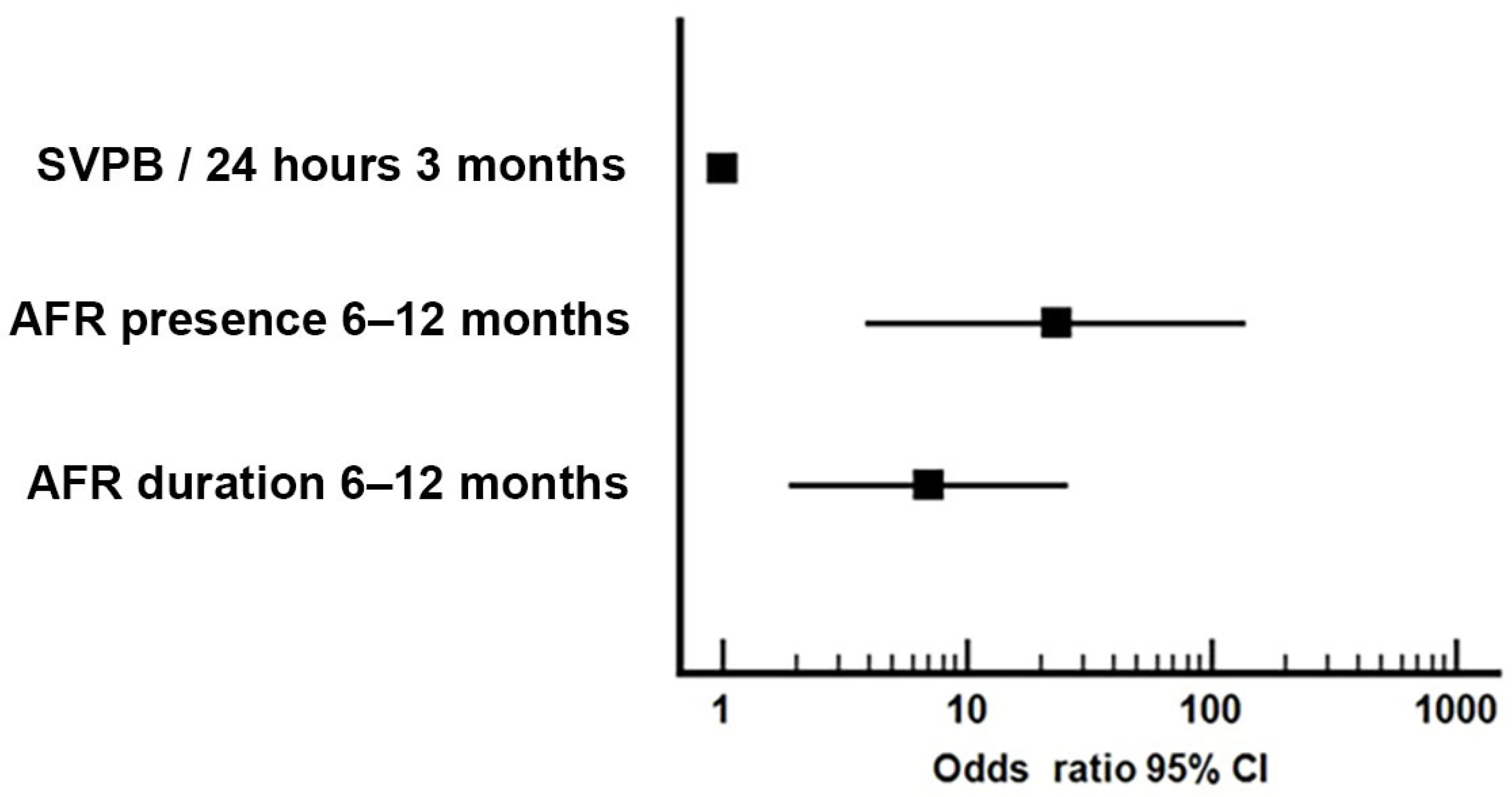
| 1 | AFR presence 6–12 months | 0.401 | - | 0.002 | 0.792 | 0.007 |
| 2 | AFR duration 6–12 months | 0.410 | 0.446 | 0.005 | 0.804 | 0.005 |
| 3 | SVPB/24 hours at 3 months ECG monitor | 0.215 | 0.440 | 0.078 | 0.704 | 0.066 |
| Model Number | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
|---|---|---|---|---|---|
| Parameters | AFR 6–12 m | RD 6–12 m | AFR 6–12 m, SVPB/24 h3 m | RD 6–12 m, SVPB/24 h3 m | AFR 6–12 m, RD 6–12 m |
| Nagelkerke R2 | 0.449 | 0.428 | 0.455 | 0.421 | 0.452 |
| Hosmer–Lemeshow Test | - | 0.271 | 0.630 | 0.609 | 0.882 |
| AIC | 9.56 | 12.05 | 36.75 | 37.12 | 13.11 |
| BIC | 12.94 | 15.32 | 41.58 | 41.78 | 18.02 |
References
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, F.; Antz, M.; Ernst, S.; Hachiya, H.; Mavrakis, H.; Deger, F.T.; Schaumann, A.; Chun, J.; Falk, P.; Hennig, D.; et al. Recovered Pulmonary Vein Conduction as a Dominant Factor for Recurrent Atrial Tachyarrhythmias After Complete Circular Isolation of the Pulmonary Veins: Lessons From Double Lasso Technique. Circulation 2005, 111, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, F.; Ernst, S.; Vogtmann, T.; Goya, M.; Volkmer, M.; Schaumann, A.; Bänsch, D.; Antz, M.; Kuck, K.-H. Characterization of Reentrant Circuits in Left Atrial Macroreentrant Tachycardia: Critical Isthmus Block Can Prevent Atrial Tachycardia Recurrence. Circulation 2002, 105, 1934–1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayan, S.M.; Krummen, D.E.; Shivkumar, K.; Clopton, P.; Rappel, W.-J.; Miller, J.M. Treatment of Atrial Fibrillation by the Ablation of Localized Sources. J. Am. Coll. Cardiol. 2012, 60, 628–636. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Mounsey, J.P. Atrial Substrate Modification for Atrial Fibrillation: Striving to Get Smarter. Circ. Arrhythm. Electrophysiol. 2017, 10, e005840. [Google Scholar] [CrossRef]
- Wilber, D.J.; Pappone, C.; Neuzil, P.; De Paola, A.; Marchlinski, F.; Natale, A.; Macle, L.; Daoud, E.G.; Calkins, H.; Hall, B.; et al. Comparison of Antiarrhythmic Drug Therapy and Radiofrequency Catheter Ablation in Patients With Paroxysmal Atrial Fibrillation: A Randomized Controlled Trial. JAMA 2010, 303, 333. [Google Scholar] [CrossRef]
- Ma, Y.; Bai, F.; Qin, F.; Li, Y.; Tu, T.; Sun, C.; Zhou, S.; Liu, Q. Catheter Ablation for Treatment of Patients with Atrial Fibrillation and Heart Failure: A Meta-Analysis of Randomized Controlled Trials. BMC Cardiovasc. Disord. 2018, 18, 165. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, A.N.; Shipp, N.J.; Brooks, A.G.; Kuklik, P.; Lau, D.H.; Lim, H.S.; Sullivan, T.; Roberts-Thomson, K.C.; Sanders, P. Long-term Outcomes of Catheter Ablation of Atrial Fibrillation: A Systematic Review and Meta-analysis. JAHA 2013, 2, e004549. [Google Scholar] [CrossRef] [Green Version]
- Hachem, A.H.; Marine, J.E.; Tahboub, H.A.; Kamdar, S.; Kanjwal, S.; Soni, R.; Kanjwal, K. Radiofrequency Ablation versus Cryoablation in the Treatment of Paroxysmal Atrial Fibrillation: A Meta-Analysis. Cardiol. Res. Pract. 2018, 2018, 6276241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, L.-F.; Jaïs, P.; Sanders, P.; Garrigue, S.; Hocini, M.; Sacher, F.; Takahashi, Y.; Rotter, M.; Pasquié, J.-L.; Scavée, C.; et al. Catheter Ablation for Atrial Fibrillation in Congestive Heart Failure. N. Engl. J. Med. 2004, 351, 2373–2383. [Google Scholar] [CrossRef] [PubMed]
- Gentlesk, P.J.; Sauer, W.H.; Gerstenfeld, E.P.; Lin, D.; Dixit, S.; Pa-C, E.Z.; Callans, D.; Marchlinski, F.E. Reversal of Left Ventricular Dysfunction Following Ablation of Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2007, 18, 9–14. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, B.; Arora, S.; Kumar, V.; Abdelrahman, M.; Lahewala, S.; Dave, M.; Shah, M.; Tan, B.; Savani, S.; Badheka, A.; et al. Temporal Trends of In-Hospital Complications Associated with Catheter Ablation of Atrial Fibrillation in the United States: An Update from Nationwide Inpatient Sample Database (2011–2014). J. Cardiovasc. Electrophysiol. 2018, 29, 715–724. [Google Scholar] [CrossRef]
- Andrade, J.G.; Khairy, P.; Verma, A.; Guerra, P.G.; Dubuc, M.; Rivard, L.; Deyell, M.W.; Mondesert, B.; Thibault, B.; Talajic, M.; et al. Early Recurrence of Atrial Tachyarrhythmias Following Radiofrequency Catheter Ablation of Atrial Fibrillation: EARLY RECURRENCE POST AF ABLATION. Pacing Clin. Electrophysiol. 2012, 35, 106–116. [Google Scholar] [CrossRef]
- Cappato, R.; Calkins, H.; Chen, S.-A.; Davies, W.; Iesaka, Y.; Kalman, J.; Kim, Y.-H.; Klein, G.; Natale, A.; Packer, D.; et al. Updated Worldwide Survey on the Methods, Efficacy, and Safety of Catheter Ablation for Human Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2010, 3, 32–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherian, T.S.; Callans, D.J. Recurrent Atrial Fibrillation After Radiofrequency Ablation. Card. Electrophysiol. Clin. 2020, 12, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Oral, H.; Knight, B.P.; Özaydın, M.; Tada, H.; Chugh, A.; Hassan, S.; Scharf, C.; Lai, S.W.K.; Greenstein, R.; Pelosi, F.; et al. Clinical Significance of Early Recurrences of Atrial Fibrillation after Pulmonary Vein Isolation. J. Am. Coll. Cardiol. 2002, 40, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Allam, L.E.; El Moteleb, A.M.A.; Ghanem, M.T. Predictors of Short and Long Term Recurrences of Paroxysmal AF after Radiofrequency Ablation. Is Blanking Period Really Benign? J. Atr. Fibrillation 2018, 11, 2012. [Google Scholar] [CrossRef]
- Pokushalov, E.; Romanov, A.; Corbucci, G.; Bairamova, S.; Losik, D.; Turov, A.; Shirokova, N.; Karaskov, A.; Mittal, S.; Steinberg, J.S. Does Atrial Fibrillation Burden Measured by Continuous Monitoring during the Blanking Period Predict the Response to Ablation at 12-Month Follow-Up? Heart Rhythm. 2012, 9, 1375–1379. [Google Scholar] [CrossRef]
- Gang, U.J.O.; Nalliah, C.J.; Lim, T.W.; Thiagalingam, A.; Kovoor, P.; Ross, D.L.; Thomas, S.P. Atrial Ectopy Predicts Late Recurrence of Atrial Fibrillation After Pulmonary Vein Isolation. Circ. Arrhythm. Electrophysiol. 2015, 8, 569–574. [Google Scholar] [CrossRef] [Green Version]
- Alhede, C.; Johannessen, A.; Dixen, U.; Jensen, J.S.; Raatikainen, P.; Hindricks, G.; Walfridsson, H.; Kongstad, O.; Pehrson, S.; Englund, A.; et al. Higher Burden of Supraventricular Ectopic Complexes Early after Catheter Ablation for Atrial Fibrillation Is Associated with Increased Risk of Recurrent Atrial Fibrillation. EP Eur. 2018, 20, 50–57. [Google Scholar] [CrossRef]
- Dretzke, J.; Chuchu, N.; Agarwal, R.; Herd, C.; Chua, W.; Fabritz, L.; Bayliss, S.; Kotecha, D.; Deeks, J.J.; Kirchhof, P.; et al. Predicting Recurrent Atrial Fibrillation after Catheter Ablation: A Systematic Review of Prognostic Models. Europace 2020, 22, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Im, S.I.; Park, D.H.; Kim, B.J.; Cho, K.I.; Kim, H.S.; Heo, J.H. Clinical and Electrocardiographic Characteristics for Prediction of New-Onset Atrial Fibrillation in Asymptomatic Patients with Atrial Premature Complexes. IJC Heart Vasc. 2018, 19, 70–74. [Google Scholar] [CrossRef]
- Chong, B.-H.; Pong, V.; Lam, K.-F.; Liu, S.; Zuo, M.-L.; Lau, Y.-F.; Lau, C.-P.; Tse, H.-F.; Siu, C.-W. Frequent Premature Atrial Complexes Predict New Occurrence of Atrial Fibrillation and Adverse Cardiovascular Events. Europace 2012, 14, 942–947. [Google Scholar] [CrossRef]
- Dewland, T.A.; Vittinghoff, E.; Mandyam, M.C.; Heckbert, S.R.; Siscovick, D.S.; Stein, P.K.; Psaty, B.M.; Sotoodehnia, N.; Gottdiener, J.S.; Marcus, G.M. Atrial Ectopy as a Predictor of Incident Atrial Fibrillation: A Cohort Study. Ann. Intern. Med. 2013, 159, 721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acharya, T.; Tringali, S.; Bhullar, M.; Nalbandyan, M.; Ilineni, V.K.; Carbajal, E.; Deedwania, P. Frequent Atrial Premature Complexes and Their Association With Risk of Atrial Fibrillation. Am. J. Cardiol. 2015, 116, 1852–1857. [Google Scholar] [CrossRef]
- Persson, A.P.; Fedorowski, A.; Hedblad, B.; Persson, M.; Juul-Möller, S.; Engström, G.; Johnson, L.S.B. Heart Rate and Premature Atrial Contractions at 24hECG Independently Predict Atrial Fibrillation in a Population-Based Study. Heart 2020, 106, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical Power Analyses Using G*Power 3.1: Tests for Correlation and Regression Analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Sambataro, G.; Giuffrè, M.; Sambataro, D.; Palermo, A.; Vignigni, G.; Cesareo, R.; Crimi, N.; Torrisi, S.E.; Vancheri, C.; Malatino, L.; et al. The Model for Early COvid-19 Recognition (MECOR) Score: A Proof-of-Concept for a Simple and Low-Cost Tool to Recognize a Possible Viral Etiology in Community-Acquired Pneumonia Patients during COVID-19 Outbreak. Diagnostics 2020, 10, 619. [Google Scholar] [CrossRef]
- Mesquita, J.; Ferreira, A.M.; Cavaco, D.; Moscoso Costa, F.; Carmo, P.; Marques, H.; Morgado, F.; Mendes, M.; Adragão, P. Development and Validation of a Risk Score for Predicting Atrial Fibrillation Recurrence after a First Catheter Ablation Procedure—ATLAS Score. EP Eur. 2018, 20, f428–f435. [Google Scholar] [CrossRef]
- Kornej, J.; Hindricks, G.; Shoemaker, M.B.; Husser, D.; Arya, A.; Sommer, P.; Rolf, S.; Saavedra, P.; Kanagasundram, A.; Patrick Whalen, S.; et al. The APPLE Score: A Novel and Simple Score for the Prediction of Rhythm Outcomes after Catheter Ablation of Atrial Fibrillation. Clin. Res. Cardiol. 2015, 104, 871–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkle, R.A.; Jarman, J.W.E.; Mead, R.H.; Engel, G.; Kong, M.H.; Fleming, W.; Patrawala, R.A. Predicting Atrial Fibrillation Ablation Outcome: The CAAP-AF Score. Heart Rhythm. 2016, 13, 2119–2125. [Google Scholar] [CrossRef] [Green Version]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.-H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2017, 14, e275–e444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamane, T.; Date, T.; Kanzaki, Y.; Inada, K.; Matsuo, S.; Shibayama, K.; Miyanaga, S.; Miyazaki, H.; Sugimoto, K.; Mochizuki, S. Behavior of Atrial Ectopic Beats before and after Pulmonary Vein Isolation in Patients with Atrial Fibrillation: A Reduction in the Number and Arrhythmogenicity of Ectopic Firings. Heart Rhythm. 2006, 3, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Gaztañaga, L.; Frankel, D.S.; Kohari, M.; Kondapalli, L.; Zado, E.S.; Marchlinski, F.E. Time to Recurrence of Atrial Fibrillation Influences Outcome Following Catheter Ablation. Heart Rhythm. 2013, 10, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Mulder, A.A.W.; Wijffels, M.C.E.F.; Wever, E.F.D.; Boersma, L.V.A. Early Recurrence of Atrial Fibrillation as a Predictor for 1-Year Efficacy after Successful Phased RF Pulmonary Vein Isolation: Evaluation of Complaints and Multiple Holter Recordings. Int. J. Cardiol. 2013, 165, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Willems, S.; Khairy, P.; Andrade, J.G.; Hoffmann, B.A.; Levesque, S.; Verma, A.; Weerasooriya, R.; Novak, P.; Arentz, T.; Deisenhofer, I.; et al. Redefining the Blanking Period After Catheter Ablation for Paroxysmal Atrial Fibrillation: Insights From the ADVICE (Adenosine Following Pulmonary Vein Isolation to Target Dormant Conduction Elimination) Trial. Circ. Arrhythm. Electrophysiol. 2016, 9, e003909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mujović, N.; Marinković, M.; Lenarczyk, R.; Tilz, R.; Potpara, T.S. Catheter Ablation of Atrial Fibrillation: An Overview for Clinicians. Adv. Ther. 2017, 34, 1897–1917. [Google Scholar] [CrossRef] [Green Version]
- Potpara, T.S.; Mujovic, N.; Sivasambu, B.; Shantsila, A.; Marinkovic, M.; Calkins, H.; Spragg, D.; Lip, G.Y.H. Validation of the MB-LATER Score for Prediction of Late Recurrence after Catheter-Ablation of Atrial Fibrillation. Int. J. Cardiol. 2019, 276, 130–135. [Google Scholar] [CrossRef]
- Canpolat, U.; Aytemir, K.; Yorgun, H.; Şahiner, L.; Kaya, E.B.; Oto, A. A Proposal for a New Scoring System in the Prediction of Catheter Ablation Outcomes: Promising Results from the Turkish Cryoablation Registry. Int. J. Cardiol. 2013, 169, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Bavishi, A.A.; Kaplan, R.M.; Peigh, G.; Diaz, C.L.; Baman, J.R.; Trivedi, A.; Wasserlauf, J.; Shen, M.J.; Sattayaprasert, P.; Chicos, A.B.; et al. Patient Characteristics as Predictors of Recurrence of Atrial Fibrillation Following Cryoballoon Ablation. Pacing Clin. Electrophysiol. 2019, 42, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Hatle, L.; Angelsen, B.; Tromsdal, A. Noninvasive Assessment of Atrioventricular Pressure Half-Time by Doppler Ultrasound. Circulation 1979, 60, 1096–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristics | Results |
|---|---|
| Age at ablation time, years, mean ± SD (95% CI) | 56.43 ± 9.66 (53.33–59.52) |
| Time from AF diagnosis, years, mean ± SD (95% CI) | 3.45 ± 2.34 (2.70–4.20) |
| Treatment, n (%) | |
| Propafenone | 16 (40%) |
| Amiodarone | 13 (32%) |
| Flecainide | 4 (10%) |
| Betablockers | 29 (72%) |
| Statins | 16 (40%) |
| ACEI | 25 (62%) |
| Associated conditions, n (%) | |
| Arterial hypertension | 27 (67%) |
| Dyslipidemia | 19 (47%) |
| Type 2 diabetes mellitus | 7 (17%) |
| BMI (kg/m2), mean ± SD (95%CI) | 29.83 ± 4.13 (28.51–31.15) |
| Procedure characteristics, n (%) | |
| Complete pulmonary vein isolation | 32 (80%) |
| Cavo-tricuspid ablation | 3 (7%) |
| Echocardiographic parameters, mean ± SD (95% CI) | |
| LVEF, % | 56.00 ± 5.71 (54.17–57.83) |
| LV mass, g/m2 | 87.20 ± 13.24 (82.96–91.44) |
| LA anteroposterior diameter, mm | 39.82 ± 4.44 (38.40–41.24) |
| LA volume, mL/m2 | 38.14 ± 8.78 (35.33–40.95) |
| RA diameter, mm | 37.33 ± 5.19 (35.66–38.99) |
| Characteristics | 0–3 Months | 3–6 Months | 6–12 Months | After 12 Months |
|---|---|---|---|---|
| Patients with AFR, n (%) | 19/40 (47) | 11/40 (27) | 11/40 (27) | 11/40 (27) |
| AFR/patient, n (%) | ||||
| 1 episode | 5/19 (26) | 3/11 (27) | 3/11 (27) | 3/11 (27) |
| 2 episodes | 6/19 (32) | 2/11 (18) | 3/11 (27) | 0 (0) |
| 3 episodes | 3/19 (16) | 0 (0) | 0 (0) | 1/11 (9) |
| More than 4 episodes | 5/19 (26) | 6/11 (55) | 5/11 (45) | 7/11 (64) |
| AFR duration/patient * | ||||
| <12 h | 12/19 (63) | 10/11 (91) | 6/11 (54) | 2/11 (18) |
| 12–24 h | 5/19 (26) | 1/11 (9) | 4/11 (36) | 5/11 (45) |
| >24 h | 1/19 (5) | 0 (0) | 1/11 (9) | 4/11 (36) |
| Symptomatic AFR ** | 17/19 (89) | 10/11 (91) | 10/11 (91) | 11/11 (100) |
| AFR diagnosed by self-monitoring, n (%) | 17/19 (89) | 11/11 (100) | 10/11 (91) | 11/11 (100) |
| AFR diagnosed by continuous ECG monitoring, n (%) | 8/19 (42) | 4/11 (36) | 5/11 (45) | 8/11 (73) |
| Characteristics | No AFR | AFR | p-Value |
|---|---|---|---|
| Patients, n (%) | 29/40 (72.5) | 11/40 (27.5) | 0.006 |
| Age, years, mean ± SD (95% CI) | 57.48 ± 9.95 (53.70–61.27) | 53.64 ± 8.65 (48.82–59.45) | 0.266 |
| Gender M, n (%) | 19/29 (66) | 10/11 (91) | 0.1 |
| AF history, years, mean ± SD (95% CI) | 3 (4.3) | 3 (3) | 0.929 |
| Body mass index, kg/m2, mean ± SD (95% CI) | 29.32 ± 4.33 (27.38–30.88) | 31.88 ± 3.99 (29.02–34.73) | 0.138 |
| PVI, n (%) | |||
| Complete | 25/29 (86.2) | 7/11 (63.6) | 0.11 |
| Incomplete | 4/29 (13.8) | 4/11 (36.4) | 0.11 |
| Associated conditions, n (%) | |||
| Arterial hypertension | 19/29 (65) | 8/11 (72) | 0.66 |
| Dyslipidemia | 13/29 (44) | 6/11 (54) | 0.58 |
| Diabetes | 5/29 (17) | 2/11 (18) | 0.94 |
| AFR presence, n (%) | |||
| 0–1 month | 5/29 (17) | 7/11 (64) | 0.004 |
| 1–3 months | 6/29 (21) | 7/11 (64) | 0.01 |
| 3–6 months | 4/29 (14) | 7/11 (64) | 0.002 |
| SVPB/24 h at ECG monitors, median (IQR) | |||
| at 1 month | 57.5 (215) | 450 (1201) | 0.079 |
| at 3 months | 29.5 (110) | 124 (961.98) | 0.005 |
| at 6 months | 36.23 (99.16) | 830 (2424.34) | 0.009 |
| Treatment, n (%) | |||
| Class I AAD | 1/29 (3) | 6/11 (54) | 0.001 |
| Class III AAD | 0 | 2/11 (18) | 0.018 |
| Betablockers | 22/29 (76) | 10/11 (91) | 0.28 |
| Characteristics | No AFR | AFR | p-Value |
|---|---|---|---|
| Patients, n (%) | 29 (72.5) | 11 (27.5) | 0.006 |
| Age, years, mean ± SD (95% CI) | 57.46 ±9.3 (53.70–61.22) | 53.50 ±8.86 (47.16–59.84) | 0.257 |
| Gender M, n (%) | 21/29 (72) | 8/1 (73) | 0.98 |
| AF history, years, mean ± SD (95% CI) | 3.4 ± 2.34 (2.45–4.35) | 3.65 ± 2.76 (1.66–5.63) | 0.915 |
| Body mass index, kg/m2, mean ± SD (95% CI) | 29.98 ± 4.24 (28.26–31.69) | 31.22 ± 4.30 (28.15–34.30) | 0.621 |
| PVI, n (%) | |||
| Incomplete | 5/29 (17.2) | 3/11 (27.3) | 0.47 |
| Complete | 24/29 (82.8) | 8/11 (72.7) | 0.47 |
| Associated conditions, n (%) | |||
| Arterial hypertension | 18/29 (62) | 9/11 (82) | 0.28 |
| Dyslipidemia | 13/29 (45) | 6/11 (54) | 0.58 |
| Diabetes mellitus | 4/29 (14) | 3/11 (27) | 0.31 |
| AFR presence, n (%) | |||
| 0–1 month | 7/29 (24) | 5/11 (45) | 0.18 |
| 1–3 months | 8/29 (28) | 5/11 (45) | 0.28 |
| 3–6 months | 6/29 (21) | 5/11 (45) | 0.11 |
| 6–12 months | 3/29 (10) | 8/11 (73) | 0.001 |
| SVPB/24 h at ECG monitors, median (IQR) | |||
| at 1 month | 57.5 (215) | 450 (1201) | 0.079 |
| at 3 months | 40 (108.5) | 132 (1148) | 0.068 |
| at 6 months | 38 (105.75) | 277 (2275) | 0.023 |
| at 12 months | 31.5 (73.78) | 1290 (2288.56) | 0.001 |
| Treatment, n (%) | |||
| Class I AAD | 3/29 (10.3) | 2/11 (18.2) | 0.5 |
| Class III AAD | 0 | 1/11 (9.1) | 0.062 |
| Betablockers | 23/29 (79.3) | 9/11 (81.8) | 0.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matei, L.-L.; Siliște, C.; Stoica, S.; Bejan, G.-C.; Ghilencea, L.-N.; Vinereanu, D. Predictors of Atrial Fibrillation Recurrences after a First Radiofrequency Catheter Ablation Intervention for Paroxysmal Atrial Fibrillation—Experience of a Low Volume Ablation Centre. Medicina 2021, 57, 1139. https://doi.org/10.3390/medicina57111139
Matei L-L, Siliște C, Stoica S, Bejan G-C, Ghilencea L-N, Vinereanu D. Predictors of Atrial Fibrillation Recurrences after a First Radiofrequency Catheter Ablation Intervention for Paroxysmal Atrial Fibrillation—Experience of a Low Volume Ablation Centre. Medicina. 2021; 57(11):1139. https://doi.org/10.3390/medicina57111139
Chicago/Turabian StyleMatei, Lavinia-Lucia, Călin Siliște, Sebastian Stoica, Gabriel-Cristian Bejan, Liviu-Nicolae Ghilencea, and Dragoș Vinereanu. 2021. "Predictors of Atrial Fibrillation Recurrences after a First Radiofrequency Catheter Ablation Intervention for Paroxysmal Atrial Fibrillation—Experience of a Low Volume Ablation Centre" Medicina 57, no. 11: 1139. https://doi.org/10.3390/medicina57111139
APA StyleMatei, L.-L., Siliște, C., Stoica, S., Bejan, G.-C., Ghilencea, L.-N., & Vinereanu, D. (2021). Predictors of Atrial Fibrillation Recurrences after a First Radiofrequency Catheter Ablation Intervention for Paroxysmal Atrial Fibrillation—Experience of a Low Volume Ablation Centre. Medicina, 57(11), 1139. https://doi.org/10.3390/medicina57111139








