Mitral Valve Surgery via Upper Ministernotomy: Single-Centre Experience in More than 400 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Clinical Data
2.2. Surgical Technique
2.3. Statistical Analysis
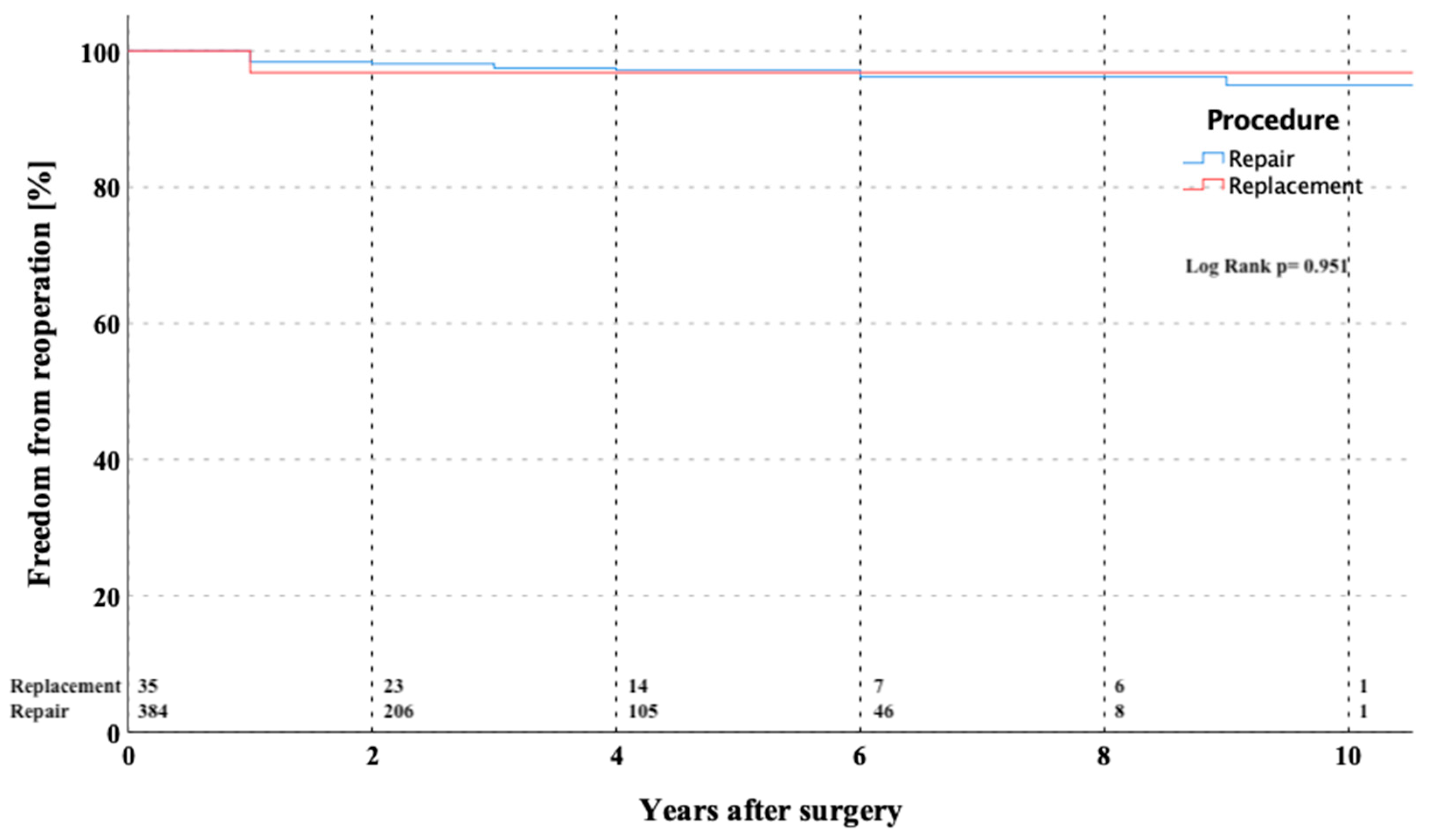
3. Results
3.1. Operative Data
3.2. Early Postoperative Outcomes
3.3. Survival and Late Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iribarne, A.; Karpenko, A.; Russo, M.J.; Cheema, F.; Umann, T.; Oz, M.C.; Smith, C.R.; Argenziano, M. Eight-year experience with minimally invasive cardiothoracic surgery. World J. Surg. 2010, 34, 611–615. [Google Scholar] [CrossRef] [Green Version]
- Totaro, P.; Carlini, S.; Pozzi, M.; Pagani, F.; Zattera, G.; D’Armini, A.M.; Vigano, M. Minimally invasive approach for complex cardiac surgery procedures. Ann. Thorac. Surg. 2009, 88, 462–466, discussion 7. [Google Scholar] [CrossRef] [PubMed]
- Kypson, A.P. Recent trends in minimally invasive cardiac surgery. Cardiology 2007, 107, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J. Minimally invasive cardiac surgery. Surg. Endosc. 2006, 20 (Suppl. S2), S488–S492. [Google Scholar] [CrossRef]
- Vanermen, H.; Farhat, F.; Wellens, F.; Geest, R.; Degrieck, I.; Praet, F.; Vermeulon, Y. Minimally invasive video-assisted mitral valve surgery: From port-access towards a totally endoscopic procedure. J. Card. Surg. 2000, 15, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Tatooles, A.J.; Pappas, P.S.; Gordon, P.J.; Slaughter, M.S. Minimally invasive mitral valve repair using the da vinci robotic system. Ann. Thorac. Surg. 2004, 77, 1978–1982, discussion 82–84. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Malhotra, R.; Mehta, Y.; Sharma, K.K.; Kasliwal, R.R.; Trehan, N. Minimally invasive mitral valve surgery through right anterolateral minithoracotomy. Ann. Thorac. Surg. 1999, 68, 1520–1524. [Google Scholar] [CrossRef]
- Karagoz, H.Y.; Bayazit, K.; Battaloglu, B.; Kurtoglu, M.; Özerdem, G.; Bakkaloglu, B.; Sönmez, B. Minimally invasive mitral valve surgery: The subxiphoid approach. Ann. Thorac. Surg. 1999, 67, 1328–1332, discussion 33. [Google Scholar] [CrossRef]
- Grossi, E.A.; Galloway, A.C.; LaPietra, A.; Ribakove, G.H.; Ursomanno, P.; Delianides, J.; Culliford, A.T.; Bizekis, C.; Esposito, R.A.; Baumann, F.; et al. Minimally invasive mitral valve surgery: A 6-year experience with 714 patients. Ann. Thorac. Surg. 2002, 74, 660–663, discussion 3–4. [Google Scholar] [CrossRef]
- Modi, P.; Hassan, A.; Chitwood, W.R.J. Minimally invasive mitral valve surgery: A systematic review and meta-analysis. Eur. J. Cardiothorac. Surg. 2008, 34, 943–952. [Google Scholar] [CrossRef] [Green Version]
- Cohn, L.H.; Adams, D.H.; Couper, G.S.; Bichell, D.; Rosborough, D.M.; Sears, S.P.; Aranki, S.F. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann. Surg. 1997, 226, 421–426, discussion 7–8. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Ochiai, R.; Takeda, J.; Shin, H.; Yozu, R. Comparison of early postoperative quality of life in minimally invasive versus conventional valve surgery. J. Anesth. 2003, 17, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Grossi, E.A.; Galoway, A.; Ribakove, G.H.; Zakow, P.K.; Derivaux, C.C.; Baumann, F.; Schwesinger, D.; Colvin, S.B. Impact of minimally invasive valvular heart surgery: A case-control study. Ann. Thorac. Surg. 2001, 71, 807–810. [Google Scholar] [CrossRef]
- de Vaumas, C.; Philip, I.; Daccache, G.; Depoix, J.-P.; Lecharny, J.-B.; Enguerand, D.; Desmonts, J.-M. Comparison of minithoracotomy and conventional sternotomy approaches for valve surgery. J. Cardiothorac. Vasc. Anesth. 2003, 17, 325–328. [Google Scholar] [CrossRef]
- Radwan, M.; Bon, D.; Dressen, L.; Walther, T.; Miscovic, A.; Moritz, A.; Papadopoulos, N. Propensity-matched comparison of two different access modes for minimally invasive mitral valve surgery. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Risteski, P.; Monsefi, N.; Miskovic, A.; Josic, T.; Bala, S.; Salem, R.; Zierer, A.; Moritz, A. Triple valve surgery through a less invasive approach: Early and mid-term results. Interact. Cardiovasc. Thorac. Surg. 2017, 24, 677–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risteski, P.; Ahmad, A.E.-S.; Monsefi, N.; Papadopoulos, N.; Radacki, I.; Herrmann, E.; Moritz, A.; Zierer, A. Minimally invasive aortic arch surgery: Early and late outcomes. Int. J. Surg. 2017, 45, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Akins, C.W.; Miller, D.C.; Turina, M.I.; Kouchoukos, N.T.; Blackstone, E.H.; Grunkemeier, G.L.; Takkenberg, J.; David, T.E.; Butchart, E.G.; Adams, D.H.; et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. J. Thorac. Cardiovasc. Surg. 2008, 135, 732–738. [Google Scholar] [CrossRef] [Green Version]
- Cheng, D.C.H.; Martin, J.; Lal, A.; Diegeler, A.; Folliguet, T.A.; Nifong, L.W.; Perier, P.; Raanani, E.; Smith, J.M.; Seeburger, J.; et al. Minimally invasive versus conventional open mitral valve surgery: A meta-analysis and systematic review. Innovations 2011, 6, 84–103. [Google Scholar] [CrossRef]
- Malaisrie, S.C.; Barnhart, G.R.; Farivar, R.S.; Mehall, J.; Hummel, B.; Rodriguez, E.; Anderson, M.; Lewis, C.; Hargrove, C.; Ailawadi, G.; et al. Current era minimally invasive aortic valve replacement: Techniques and practice. J. Thorac. Cardiovasc. Surg. 2014, 147, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, T.; Couper, G.S.; Borstlap, W.A.; Nauta, F.J.; Wollersheim, L.; McGurk, S.; Cohn, L.H. Minimal-access aortic valve replacement with concomitant aortic procedure: A 9-year experience. Innovations 2012, 7, 368–371. [Google Scholar] [CrossRef]
- Shrestha, M.; Krueger, H.; Umminger, J.; Koigeldiyev, N.; Beckmann, E.; Haverich, A.; Martens, A. Minimally invasive valve sparing aortic root replacement (david procedure) is safe. Ann. Cardiothorac. Surg. 2015, 4, 148–153. [Google Scholar] [PubMed]
- Franke, U.F.W.; Ursulescu, A.; Göbel, N.; Nagib, R.; Hansen, M.; Yadav, R.; Baumbach, H.; Albert, M. Results and quality of life after minimally invasive ross procedure. J. Heart Valve Dis. 2015, 24, 295–301. [Google Scholar] [PubMed]
- Seeburger, J.; Borger, M.; Falk, V.; Kuntze, T.; Czesla, M.; Walther, T.; Doll, N.; Mohr, F.W. Minimal invasive mitral valve repair for mitral regurgitation: Results of 1339 consecutive patients. Eur. J. Cardiothorac. Surg. 2008, 34, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Sündermann, S.H.; Sromicki, J.; Biefer, H.R.C.; Seifert, B.; Holubec, T.; Falk, V.; Jacobs, S. Mitral valve surgery: Right lateral minithoracotomy or sternotomy? A systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2014, 148, 1989–1995.e4. [Google Scholar] [CrossRef] [Green Version]
- Oezpeker, C.; Barbieri, F.; Hoefer, D.; Schneider, B.; Bonaros, N.; Grimm, M.; Mueller, L. Mitral valve surgery via partial upper sternotomy: Closing the gap between conventional sternotomy and right lateral minithoracotomy. Thorac. Cardiovasc. Surg. 2019, 67, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Little, S.; Flynn, M.; Pettersson, G.B.; Gillinov, A.M.; Blackstone, E.H. Revisiting the dome approach for partial sternotomy/minimally invasive mitral valve surgery. Ann. Thorac. Surg. 2009, 87, 694–697. [Google Scholar] [CrossRef]


| Variable | Median/Number/Mean | %/IQR/SD |
|---|---|---|
| Age [years] | 58.9 | 18.7 |
| Female | 133 | 31.7 |
| Hypertension | 326 | 53.3 |
| Diabetes | 34 | 8.1 |
| Peripheral vascular disease | 8 | 1.9 |
| Previous stroke | 27 | 6.4 |
| EuroSCORE II | 3.9 | ±3.6 |
| Sinus rhythm | 302 | 72.1 |
| Pulmonary hypertension | 147 | 35.1 |
| COPD | 38 | 9.1 |
| Etiology: | ||
| Myxomatous degenerative | 323 | 77.1 |
| Posterior leaflet prolapse | 257 | 79.6 |
| Bileaflet prolapse | 31 | 9.6 |
| Anterior leaflet prolapse | 26 | 8.0 |
| Commissural prolapse | 9 | 2.8 |
| Rheumatic | 22 | 5.3 |
| Infective | 32 | 7.6 |
| Isolated mitral ring dilatation | 30 | 7.2 |
| Other | 12 | 2.9 |
| Indication: | ||
| Elective | 340 | 80.7 |
| Urgent | 75 | 17.9 |
| Emergency | 4 | 1.0 |
| Variable | Number/Median | %/IQR |
|---|---|---|
| Mitral valve repair | 380 | 90.7 |
| Triangular or quadrangular resection | 276 | 65.8 |
| Artificial chord implantation | 139 | 33 |
| Ring annuloplasty | 380 | 100 |
| Cosgrove-Edwards ring (flexible) | 328 | 86.4 |
| Carpentier-Edwards physio ring (semirigid) | 47 | 12.3 |
| Profile 3D ring (rigid) | 5 | 1 |
| Mitral valve replacement | 39 | 9.3 |
| Mechanical prosthesis | 17 | 4.1 |
| Biological prothesis | 8 | 1.9 |
| Left atrial ablation | 61 | 14.5 |
| Amputation or occlusion of left atrial appendage | 261 | 62.2 |
| Aortic cross-clamp time [min] | 97.5 | 11.2 |
| Cardio-pulmonary bypass time [min] | 144.3 | 40.0 |
| Variable | Number (%)/Median (IQR) |
|---|---|
| Reoperation for bleeding | 31 (7.3%) |
| Blood loss during ICU stay [ml] | 450/363 |
| Myocardial infarction | 2 (0.4%) |
| Postoperative dialysis | 23 (5.4%) |
| Major neurological complications | 8 (2%) |
| Gastrointestinal bleeding/ischemia | 11 (2.6%) |
| Superficial sternal wound infection | 16 (3.8%) |
| Deep sternal wound infection | 4 (1%) |
| Postoperative pacemaker implantation | 24 (5.7%) |
| 30-day mortality | 13 (3.1%) |
| Variable | At Discharge (Mean ± SD) | Last Follow-Up (Mean ± SD) | p-Value |
|---|---|---|---|
| Mitral regurgitation [grade] | 0.21 ± 0.40 | 0.61 ± 0.66 | 0.01 |
| LVEDD [mm] | 51.84 ± 7.62 | 51.78 ± 8.79 | 0.92 |
| LVESD [mm] | 36.13 ± 8.15 | 35.39 ± 9.03 | 0.12 |
| MV Pmean [mmHg] | 4.02 ± 2.85 | 4.17 ± 2.89 | 0.51 |
| MVA [cm2] | 2.73 ± 1.05 | 2.44 ± 1.13 | 0.01 |
| Left atrial diameter [mm] | 45.44 ± 8.59 | 45.99 ± 8.60 | 0.68 |
| LVEF [%] | 55.4 ± 9.3 | 53.7 ± 8.8 | 0.23 |
| Patient | Age | Incidation to Operation | Operation | Time to Reoperation (Years) | Indication to Reoperation | Reoperationa |
|---|---|---|---|---|---|---|
| 1 | 46 | Myxomatous degenerative | MV repair | 0.01 | MV stenosis | MV replacement |
| 2 | 41 | Myxomatous degenerative | MV repair | 0.03 | MV stenosis | MV replacement |
| 3 | 46 | Myxomatous degenerative | MV replacement | 0.09 | MV regurgitation due to paravulvar leak | MV re-replacement |
| 4 | 71 | Myxomatous degenerative | MV repair | 0.2 | MV regurgitation due to endocarditis | MV replacement |
| 5 | 48 | Myxomatous degenerative | MV repair | 0.5 | MV regurgitation due to leaflet re-prolapse | MV re-repair |
| 6 | 44 | Rheumatic | MV replacement | 0.6 | MV regurgitation due to paravulvar leak | MV re-replacement |
| 7 | 65 | Myxomatous degenerative | MV repair | 0.8 | MV regurgitation due to leaflet re-prolapse | MV replacement |
| 8 | 68 | Myxomatous degenerative | MV repair | 1.3 | MV stenosis | MV replacement |
| 9 | 51 | Myxomatous degenerative | MV repair | 2 | MV regurgitation due to annulus re-dilatation | MV re-repair |
| 10 | 44 | Acute endocarditis | MV repair | 2.2 | MV regurgitation due to re-ndocarditis | MV replacement |
| 11 | 56 | Degenerative/calcification | MV repair | 3 | MV regurgitation due to annulus re-dilatation | MV re-repair |
| 12 | 49 | Myxomatous degenerative | MV repair | 6 | MV stenosis | MV replacement |
| 13 | 46 | Myxomatous degenerative | MV repair | 6 | MV regurgitation due to leaflet re-prolapse | MV re-repair |
| 14 | 51 | Degenerative/calcification | MV repair | 8 | MV regurgitation due to endocarditis | MV replacement |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radwan, M.; Salewski, C.; Hecker, F.; Miskovic, A.; Risteski, P.; Hlavicka, J.; Moritz, A.; Walther, T.; Holubec, T. Mitral Valve Surgery via Upper Ministernotomy: Single-Centre Experience in More than 400 Patients. Medicina 2021, 57, 1179. https://doi.org/10.3390/medicina57111179
Radwan M, Salewski C, Hecker F, Miskovic A, Risteski P, Hlavicka J, Moritz A, Walther T, Holubec T. Mitral Valve Surgery via Upper Ministernotomy: Single-Centre Experience in More than 400 Patients. Medicina. 2021; 57(11):1179. https://doi.org/10.3390/medicina57111179
Chicago/Turabian StyleRadwan, Medhat, Christoph Salewski, Florian Hecker, Aleksandra Miskovic, Petar Risteski, Jan Hlavicka, Anton Moritz, Thomas Walther, and Tomas Holubec. 2021. "Mitral Valve Surgery via Upper Ministernotomy: Single-Centre Experience in More than 400 Patients" Medicina 57, no. 11: 1179. https://doi.org/10.3390/medicina57111179
APA StyleRadwan, M., Salewski, C., Hecker, F., Miskovic, A., Risteski, P., Hlavicka, J., Moritz, A., Walther, T., & Holubec, T. (2021). Mitral Valve Surgery via Upper Ministernotomy: Single-Centre Experience in More than 400 Patients. Medicina, 57(11), 1179. https://doi.org/10.3390/medicina57111179






