May Antitransglutaminase Levels Predict Severity of Duodenal Lesions in Adults with Celiac Disease?
Abstract
:1. Introduction
2. Materials and Methods
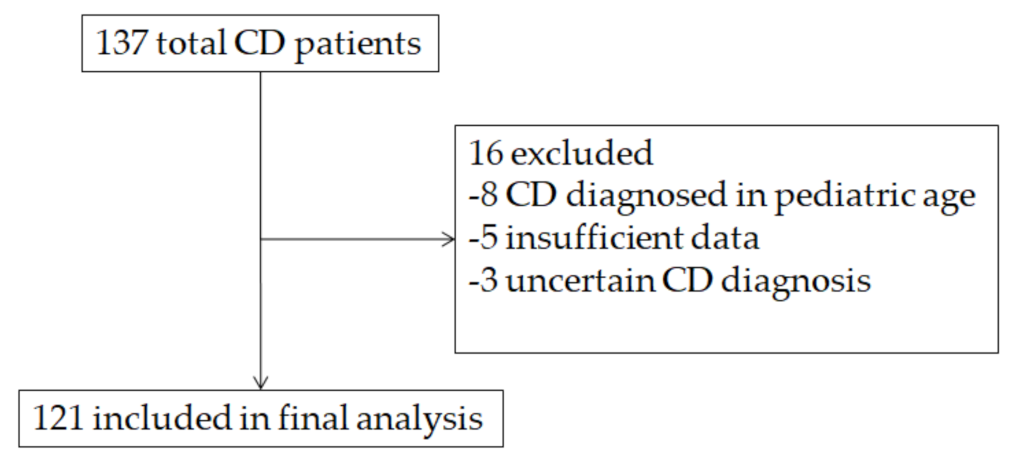
2.1. Patients
2.2. Serology and Other Laboratory Investigations
2.3. Endoscopic Procedures
2.4. Outcomes
2.5. Statistical Analysis
- (a)
- Previously reported sensitivity and specificity of TGA titer ≥10× ULN were 54% and 90%, respectively [16],
- (b)
- 1% estimated prevalence of CD, 90% precision, and 90% confidence interval, and
- (c)
- A p-value < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS 23 for Windows.
3. Results
3.1. Patients Characteristics
3.2. TGA Levels in Villous Atrophy Patients
3.3. Cutoffs for Serological Diagnosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: A comprehensive current review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husby, S.; Koletzko, S.; Korponay-Szabo, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar]
- Popp, A.; Kivelä, L.; Fuchs, V.; Kurppa, K. Diagnosing Celiac Disease: Towards Wide-Scale Screening and Serology-Based Criteria? Gastroenterol. Res. Pract. 2019, 2019, 2916024. [Google Scholar] [CrossRef]
- Collin, P.; Kaukinen, K.; Vogelsang, H.; Korponay-Szabó, I.; Sommer, R.; Schreier, E.; Volta, U.; Granito, A.; Veronesi, L.; Mascart, F.; et al. Antiendomysial and antihuman recombinant tissue transglutaminase antibodies in the diagnosis of coeliac disease: A biopsy-proven European multicentrestudy. Eur. J. Gastroenterol. Hepatol. 2005, 17, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Sugai, E.; Moreno, M.L.; Hwang, H.J.; Cabanne, A.; Crivelli, A.; Nachman, F.; Vazquez, H.; Niveloni, S.; Argonz, J.; Mazure, R.; et al. Celiac disease serology in patients with different pretest probabilities: Is biopsy avoidable? World J. Gastroenterol. 2010, 16, 3144–3152. [Google Scholar] [CrossRef]
- Salmi, T.T.; Collin, P.; Reunala, T.; Maki, M.; Kaukinen, K. Diagnostic methods beyond conventional histology in coeliac disease diagnosis. Dig. Liver Dis. 2010, 42, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Beltran, L.; Koenig, M.; Egner, W.; Howard, M.; Butt, A.; Austin, M.R.; Patel, D.; Sanderson, R.R.; Goubet, S.; Saleh, F.; et al. High-titre circulating tissue transglutaminase-2 antibodies predict small bowel villous atrophy, but decision cut-off limits must be locally validated. Clin. Exp. Immunol. 2014, 176, 190–198. [Google Scholar] [CrossRef]
- Fuchs, V.; Kurppa, K.; Huhtala, H.; Laurila, K.; Mäki, M.; Collin, P.; Salmi, T.; Luostarinen, L.; Saavalainen, P.; Kaukinen, K. Serology-based criteria for adult coeliac disease have excellent accuracy across the range of pre-test probabilities. Aliment. Pharmacol. Ther. 2019, 49, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Holmes, G.K.T.; Forsyth, J.M.; Knowles, S.; Seddon, H.; Hill, P.G.; Austin, A.S. Coeliac disease: Further evidence that biopsy is not always necessary for diagnosis. Eur. J. Gastroenterol. Hepatol. 2017, 29, 640–645. [Google Scholar]
- Zanini, B.; Magni, A.; Caselani, F.; Lanzarotto, F.; Carabellese, N.; Villanacci, V.; Ricci, C.; Lanzini, A. High tissue-transglutaminase antibody level predicts small intestinal villous atrophy in adult patients at high risk of celiac disease. Dig. Liver Dis. 2012, 44, 280–285. [Google Scholar] [CrossRef]
- Wakim-Fleming, J.; Pagadala, M.R.; Lemyre, M.S.; Lopez, R.; Kumaravel, A.; Carey, W.D.; Zein, N.N. Diagnosis of celiac disease in adults based on serology test results, without small-bowel biopsy. Clin. Gastroenterol. Hepatol. 2013, 11, 511–516. [Google Scholar]
- Oyaert, M.; Vermeersch, P.; De Hertogh, G.; Hiele, M.; Vandeputte, N.; Hoffman, I.; Bossuyt, X. Combining antibody tests and taking into account antibody levels improves serologic diagnosis of celiac disease. Clin. Chem. Lab. Med. 2015, 53, 1537–1546. [Google Scholar] [CrossRef]
- Efthymakis, K.; Serio, M.; Milano, A.; Laterza, F.; Bonitatibus, A.; Di Nicola, M.; Neri, M. Application of the Biopsy-Sparing ESPGHAN Guidelines for Celiac Disease Diagnosis in Adults: A Real-Life Study. Dig. Dis. Sci. 2017, 62, 2433–2439. [Google Scholar] [CrossRef] [PubMed]
- Latorre, M.; Lagana, S.M.; Freedberg, D.E.; Lewis, S.K.; Lebwohl, B.; Bhagat, G.; Green, P.H. Endoscopic biopsy technique in the diagnosis of celiac disease: One bite or two? Gastrointest. Endosc. 2015, 81, 1228–1233. [Google Scholar] [CrossRef]
- Oberhuber, G. Histopathology of celiac disease. Biomed. Pharmacother. 2000, 54, 368–372. [Google Scholar]
- Penny, H.A.; Raju, S.A.; Lau, M.S.; Marks, L.J.; Baggus, E.M.; Bai, J.C.; Bassotti, G.; Bontkes, H.J.; Carroccio, A.; Danciu, M.; et al. Accuracy of a no-biopsy approach for the diagnosis of coeliac disease across different adult cohorts. Gut 2021, 70, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Bai, J.C.; Biagi, F.; Card, T.R.; Ciacci, C.; Ciclitira, P.J.; Green, P.H.; Hadjivassiliou, M.; Holdoway, A.; van Heel, D.A.; et al. Diagnosis and management of adult coeliac disease: Guidelines from the British Society of Gastroenterology. Gut 2014, 63, 1210–1228. [Google Scholar] [PubMed]
- Hill, P.G.; Holmes, G.K. Coeliac disease: A biopsy is not always necessary for diagnosis. Aliment. Pharmacol. Ther. 2008, 27, 572–577. [Google Scholar] [CrossRef]
- Tortora, R.; Imperatore, N.; Capone, P.; De Palma, G.D.; De Stefano, G.; Gerbino, N.; Caporaso, N.; Rispo, A. The presence of anti-endomysial antibodies and the level of anti-tissue transglutaminases can be used to diagnose adult coeliac disease without duodenal biopsy. Aliment. Pharmacol. Ther. 2014, 40, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Alessio, M.G.; Tonutti, E.; Brusca, I.; Radice, A.; Licini, L.; Sonzogni, A.; Florena, A.; Schiaffino, E.; Marus, W.; Sulfaro, S.; et al. Correlation between IgA tissue transglutaminase antibody ratio and histological finding in celiac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 44–49. [Google Scholar] [PubMed] [Green Version]
- Di Tola, M.; Marino, M.; Goetze, S.; Casale, R.; Di Nardi, S.; Borghini, R.; Donato, G.; Tiberti, A.; Picarelli, A. Identification of a serum transglutaminase threshold value for the noninvasive diagnosis of symptomatic adult celiac disease patients: A retrospective study. J. Gastroenterol. 2016, 51, 1031–1039. [Google Scholar] [CrossRef]
- Singh, P.; Kurray, L.; Agnihotri, A.; Das, P.; Verma, A.K.; Sreenivas, V.; Dattagupta, S.; Makharia, G.K. Titers of anti-tissue transglutaminase antibody correlate well with severity of villous abnormalities in celiac disease. J. Clin. Gastroenterol. 2015, 49, 212–217. [Google Scholar] [PubMed] [Green Version]
- Losurdo, G.; Principi, M.; Iannone, A.; Amoruso, A.; Ierardi, E.; Di Leo, A.; Barone, M. Extra-intestinal manifestations of non-celiac gluten sensitivity: An expanding paradigm. World J. Gastroenterol. 2018, 24, 1521–1530. [Google Scholar] [CrossRef]
- Lebwohl, B.; Kapel, R.C.; Neugut, A.I.; Green, P.H.; Genta, R.M. Adherence to biopsy guidelines increases celiac disease diagnosis. Gastrointest. Endosc. 2011, 74, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Bañares, F.; Alsina, M.; Modolell, I.; Andújar, X.; Piqueras, M.; García-Puig, R.; Martín, B.; Rosinach, M.; Salas, A.; Viver, J.M.; et al. Are positive serum-IgA-tissue-transglutaminase antibodies enough to diagnose coeliac disease without a small bowel biopsy? Post-test probability of coeliac disease. J. Crohns. Colitis. 2012, 6, 861–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilus, T.; Kaukinen, K.; Virta, L.J.; Huhtala, H.; Mäki, M.; Kurppa, K.; Heikkinen, M.; Heikura, M.; Hirsi, E.; Jantunen, K.; et al. Refractory coeliac disease in a country with a high prevalence of clinically-diagnosed coeliac disease. Aliment. Pharmacol. Ther. 2014, 39, 418–425. [Google Scholar] [CrossRef] [Green Version]
- Ierardi, E.; Losurdo, G.; Piscitelli, D.; Giorgio, F.; Sorrentino, C.; Principi, M.; Montenegro, L.; Amoruso, A.; Di Leo, A. Seronegativeceliacdisease: Whereis the specificsetting? Gastroenterol. Hepatol. Bed Bench 2015, 8, 110–116. [Google Scholar] [PubMed]

| Variable. | n (%) or Mean ± SD |
|---|---|
| Male sex | 25 (20.7%) |
| Age (years) | 38.6 ± 12.3 (range 20–73) |
| Age at diagnosis (years) | 34.7 ± 12.3 (range 18–72) |
| First degree familiarity for CD | 26 (21.5%) |
| Diarrhea | 40 (33.1%) |
| Weight loss | 33 (27.3%) |
| Abdominal pain | 60 (49.6%) |
| Dyspepsia | 57 (47.1%) |
| Iron deficiency anemia | 54 (44.6%) |
| Autoimmune diseases | 40 (33.1%) |
| Dermatitis herpetiformis | 14 (11.6%) |
| DQ2/DQ8 homozygosis | 9 (7.4%) |
| Villous atrophy | 93 (76.9%) |
| TGA (IU/mL, n.v. 1–10) | 314.3 ± 1302.6 (range 1.8–13,500) |
| TGA normalized ×ULN | 30.5 ± 130.3 (range 0.11–1350) |
| EMA positivity | 100 (82.6%) |
| DGP positivity | 50 (41.3%) |
| Hemoglobin (g/dL, n.v. 12–15) | 12.9 ± 1.8 (range 7.1–16.9) |
| Blood iron (mcg/dL, n.v. 50–170) | 69.9 ± 42.9 (range 6.9–176) |
| Ferritin (mcg/dL, n.v. 8–252) | 37.4 ± 54.9 (range 1–297) |
| Transferrin (mg/dL, n.v. 200–360) | 271.1 ± 72.2 (range 190–465) |
| Albumin (g/dL, n.v. 3.4–5) | 4.1 ± 0.4 (range 2.93–4.83) |
| Vitamin D (ng/mL, n.v. > 20) | 22.8 ± 9.7 (range 4.2–55.7) |
| Vitamin B12(ng/mL, n.v. 150–650) | 389.5 ± 190.1 (range 44–859) |
| Folate (ng/mL, n.v. 3.9–26.8) | 4.7 ± 3.7 (range 1–20) |
| Variable | Atrophy (n = 93) | No Atrophy (n = 28) | p |
|---|---|---|---|
| Male sex | 20 (21.5%) | 5 (17.8%) | 0.79 |
| Age (years) | 38.7 ± 12.6 | 38.1 ± 11.4 | 0.79 |
| Age at diagnosis (years) | 35.0 ± 12.7 | 33.8 ± 11.1 | 0.63 |
| First degree familiarity for CD | 20 (21.5%) | 6 (21.4%) | 1.00 |
| Diarrhea | 29 (31.2%) | 11 (39.3%) | 0.45 |
| Weight loss | 21 (22.6%) | 12 (42.9%) | 0.06 |
| Abdominal pain | 43 (46.2%) | 17 (60.7%) | 0.22 |
| Dyspepsia | 44 (47.3%) | 13 (46.4%) | 0.66 |
| Iron deficiency anemia | 42 (45.1%) | 12 (42.9%) | 0.93 |
| Autoimmune diseases | 32 (34.4%) | 8 (28.6%) | 0.65 |
| Dermatitis herpetiformis | 10 (10.8%) | 4 (14.3%) | 0.74 |
| DQ2/DQ8 homozygosis | 6 (6.5%) | 3 (10.7%) | 0.43 |
| TGA (IU/mL) | 378.5 ± 1480.7 | 100.9 ± 95.0 | 0.08 |
| TGA normalized ×ULN | 37.2 ± 148.1 | 8.0 ± 7.1 | 0.06 |
| EMA positivity | 75 (80.6%) | 25 (89.3%) | 0.40 |
| DGP positivity | 37 (39.8%) | 13 (46.4%) | 0.67 |
| Hemoglobin (g/dL) | 12.9 ± 1.7 | 12.7 ± 2.1 | 0.68 |
| Blood iron (mcg/dL) | 72.1 ± 44.9 | 62.4 ± 35.4 | 0.31 |
| Ferritin (mcg/dL) | 36.5 ± 55.8 | 40.5 ± 52.6 | 0.8 |
| Transferrin (mg/dL) | 282.3 ± 76.2 | 226.3 ± 28.0 | 0.07 |
| Albumin (g/dL) | 4.0 ± 0.4 | 4.3 ± 0.3 | 0.06 |
| Vitamin D (ng/mL) | 23.7 ± 10.1 | 18.8 ± 6.9 | 0.08 |
| Vitamin B12 (ng/mL) | 398.6 ± 188.0 | 358.9 ± 201.0 | 0.52 |
| Folate (ng/mL) | 5.0 ± 3.9 | 3.8 ± 3.1 | 0.24 |
| TGA × ULN | |
|---|---|
| Cut off | 6.2 |
| Sensitivity | 57.14% |
| Specificity | 65.59% |
| Positive predictive value | 82.4% |
| Negative predictive value | 31.9% |
| AUC | 0.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Losurdo, G.; Di Leo, M.; Santamato, E.; Giangaspero, A.; Rendina, M.; Luigiano, C.; Ierardi, E.; Di Leo, A. May Antitransglutaminase Levels Predict Severity of Duodenal Lesions in Adults with Celiac Disease? Medicina 2021, 57, 1212. https://doi.org/10.3390/medicina57111212
Losurdo G, Di Leo M, Santamato E, Giangaspero A, Rendina M, Luigiano C, Ierardi E, Di Leo A. May Antitransglutaminase Levels Predict Severity of Duodenal Lesions in Adults with Celiac Disease? Medicina. 2021; 57(11):1212. https://doi.org/10.3390/medicina57111212
Chicago/Turabian StyleLosurdo, Giuseppe, Milena Di Leo, Edoardo Santamato, Antonio Giangaspero, Maria Rendina, Carmelo Luigiano, Enzo Ierardi, and Alfredo Di Leo. 2021. "May Antitransglutaminase Levels Predict Severity of Duodenal Lesions in Adults with Celiac Disease?" Medicina 57, no. 11: 1212. https://doi.org/10.3390/medicina57111212






