Current Challenges in Breast Implantation
Abstract
:1. Introduction
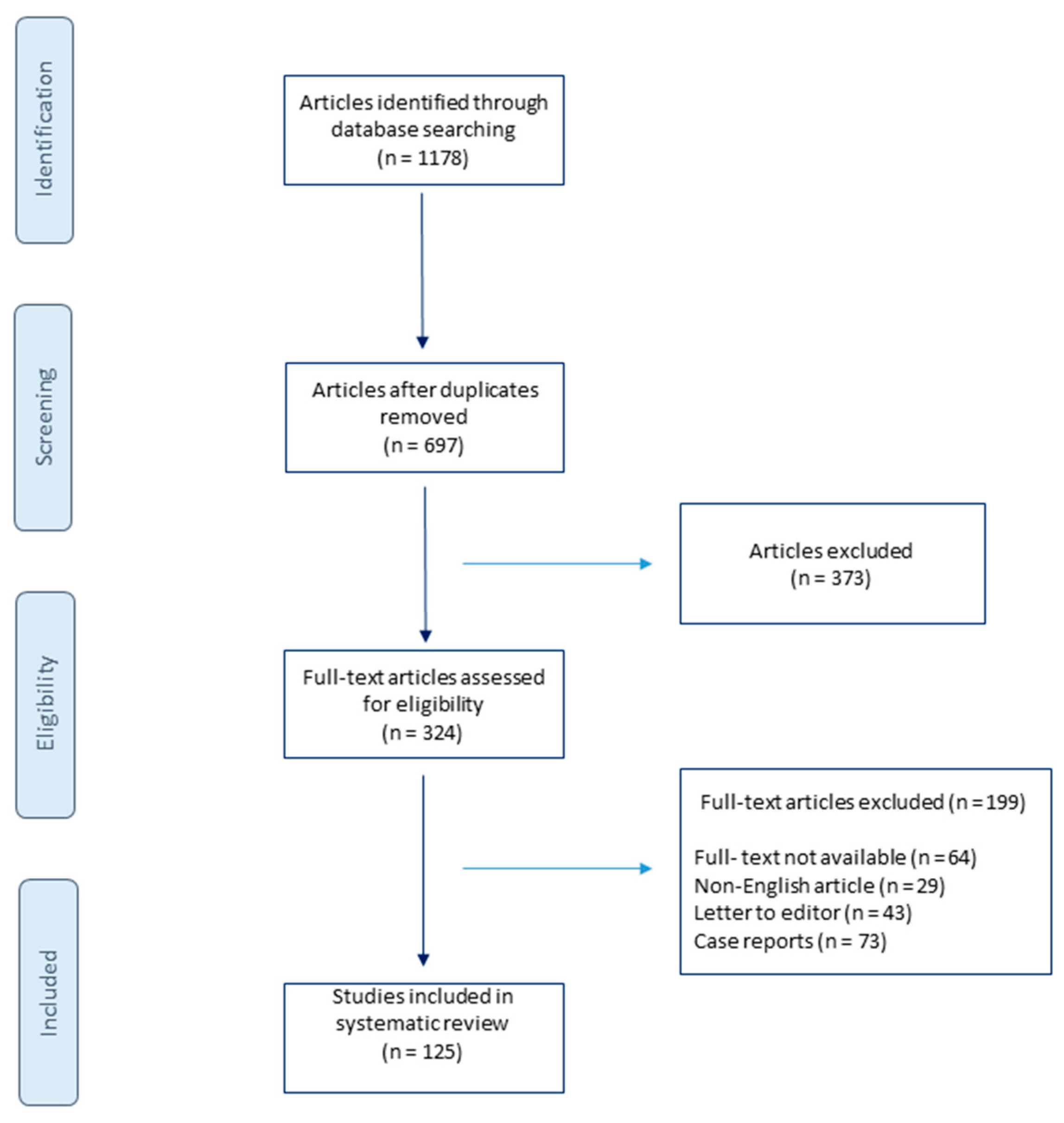
2. Materials and Methods
3. Step Back in Time
4. Silicone Breast Implants Crisis
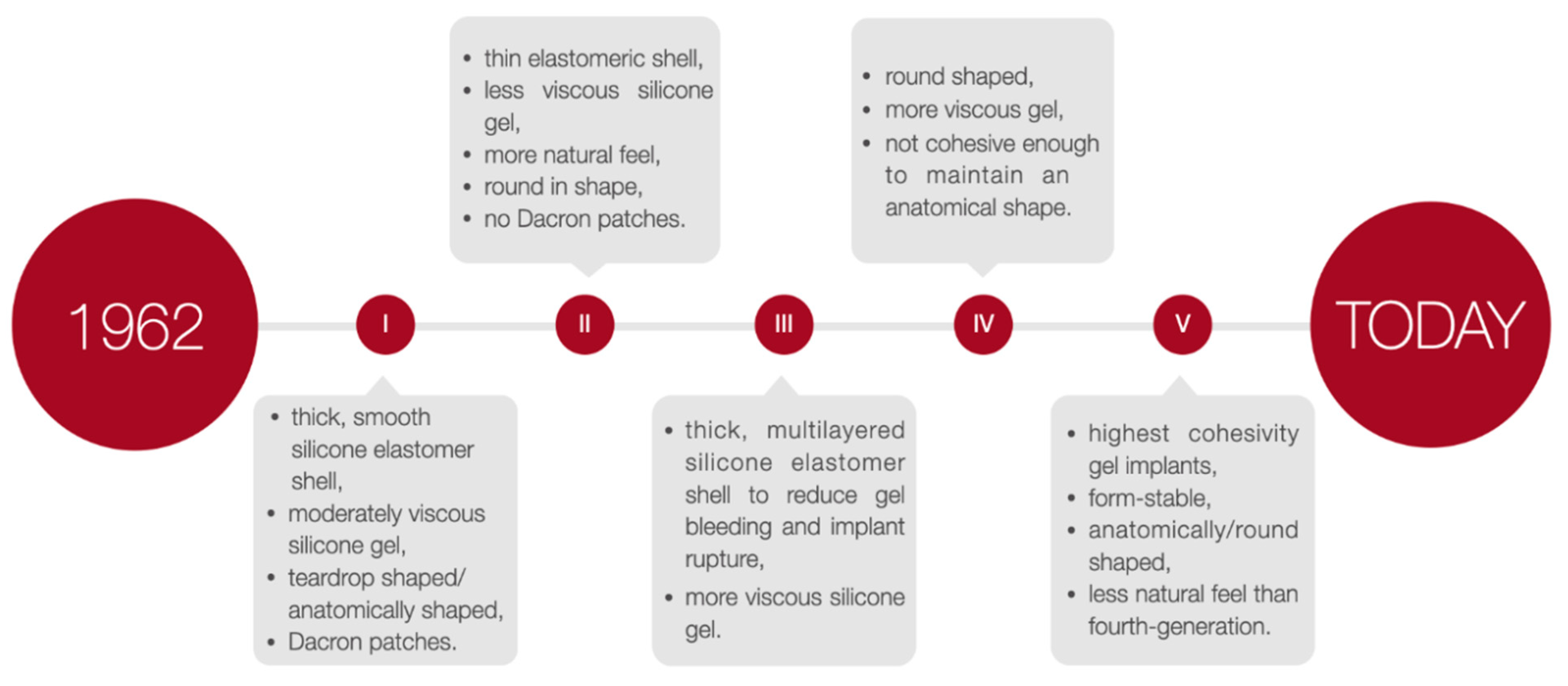
5. Characteristics of Breast Implants
6. Breast Augmentation Surgery—Preoperative Management
7. Incision Site and Implant Placement
8. Implant-Related Complications
9. Breast Implant-Associated Anaplastic Large Cell Lymphoma
10. Breast Implant Illness
11. Breast Implant Removal
12. Authors Perspective
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ISAPS. International Survey on Aesthetic/Cosmetic Procedures Performed in 2019. Available online: https://www.isaps.org/medical-professionals/isaps-global-statistics/ (accessed on 2 September 2021).
- National Plastic Surgery Statistics. Available online: https://www.plasticsurgery.org/news/plastic-surgery-statistics (accessed on 2 August 2021).
- Patel, B.C.; Wong, C.S.; Wright, T.; Schaffner, A.D. Breast Implants; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Song, W.J.; Kang, S.G.; Seo, B.F.; Choi, N.K.; Lee, J.H. A Systematic Review of the National Breast Implant Registry for Application in Korea: Can We Predict “Unpredictable” Complications? Medicina (Kaunas) 2020, 56, 370. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, B.G.; Hardt, N.S.; Abbitt, P.L.; Lanier, L.; Caffee, H.H. Breast implants, common complications, and concurrent breast disease. Radiographics 1993, 13, 95–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maxwell, G.P.; Gabriel, A. The evolution of breast implants. Plast. Reconstr. Surg. 2014, 134, 12S–17S. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, G.P.; Gabriel, A. Breast implant design. Gland Surg. 2017, 6, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Deva, A.K.; Cuss, A.; Magnusson, M.; Cooter, R. The “Game of Implants”: A Perspective on the Crisis-Prone History of Breast Implants. Aesthet. Surg. J. 2019, 39, S55–S65. [Google Scholar] [CrossRef] [Green Version]
- Jewell, M.L. Silicone gel breast implants at 50: The state of the science. Aesthet. Surg. J. 2012, 32, 1031–1034. [Google Scholar] [CrossRef] [Green Version]
- Calobrace, M.B.; Capizzi, P.J. The biology and evolution of cohesive gel and shaped implants. Plast. Reconstr. Surg. 2014, 134, 6S–11S. [Google Scholar] [CrossRef]
- Magnusson, M.R.; Cooter, R.D.; Rakhorst, H.; McGuire, P.A.; Adams, W.P., Jr.; Deva, A.K. Breast Implant Illness: A Way Forward. Plas.t Reconstr. Surg. 2019, 143, 74S–81S. [Google Scholar] [CrossRef]
- Calobrace, M.B.; Schwartz, M.R.; Zeidler, K.R.; Pittman, T.A.; Cohen, R.; Stevens, W.G. Long-Term Safety of Textured and Smooth Breast Implants. Aesthet. Surg. J. 2017, 38, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Cole, N.M. Consequences of the U.S. Food and Drug Administration-Directed Moratorium on Silicone Gel Breast Implants: 1992 to 2006. Plast. Reconstr. Surg. 2018, 141, 1137–1141. [Google Scholar] [CrossRef]
- McCarthy, C.M.; Cano, S.J.; Klassen, A.F.; Scott, A.; Van Laeken, N.; Lennox, P.A.; Cordeiro, P.G.; Pusic, A.L. The magnitude of effect of cosmetic breast augmentation on patient satisfaction and health-related quality of life. Plast. Reconstr. Surg. 2012, 130, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.fda.gov/media/80685/download (accessed on 20 August 2021).
- Shridharani, S.M.; Bellamy, J.L.; Mofid, M.M.; Singh, N.K. Breast augmentation. Eplasty 2013, 13, ic46. [Google Scholar] [PubMed]
- Rocco, N.; Rispoli, C.; Moja, L.; Amato, B.; Iannone, L.; Testa, S.; Spano, A.; Catanuto, G.; Accurso, A.; Nava, M.B. Different types of implants for reconstructive breast surgery. Cochrane Database Syst. Rev. 2016, 2016, CD010895. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.P., Jr.; Mallucci, P. Breast augmentation. Plast. Reconstr. Surg. 2012, 130, 597e–611e. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, D.A.; Spector, J.A. Breast augmentation. Plast. Reconstr. Surg. 2014, 133, 567e–583e. [Google Scholar] [CrossRef]
- Colwell, A.S.; Taylor, E.M. Recent Advances in Implant-Based Breast Reconstruction. Plast. Reconstr. Surg. 2020, 145, 421e–432e. [Google Scholar] [CrossRef]
- Tebbetts, J.B.; Adams, W.P. Five critical decisions in breast augmentation using five measurements in 5 minutes: The high five decision support process. Plast. Reconstr. Surg. 2006, 118, 35S–45S. [Google Scholar] [CrossRef]
- Frey, J.D.; Salibian, A.A.; Karp, N.S.; Choi, M. Implant-Based Breast Reconstruction: Hot Topics, Controversies, and New Directions. Plast. Reconstr. Surg. 2019, 143, 404e–416e. [Google Scholar] [CrossRef]
- Brown, M.H.; Somogyi, R.B.; Aggarwal, S. Secondary Breast Augmentation. Plast. Reconstr. Surg. 2016, 138, 119e–135e. [Google Scholar] [CrossRef]
- Schwartz, M.R. Evidence-Based Medicine: Breast Augmentation. Plast. Reconstr. Surg. 2017, 140, 109e–119e. [Google Scholar] [CrossRef]
- Goes, J.C.; Landecker, A. Optimizing outcomes in breast augmentation: Seven years of experience with the subfascial plane. Aesthet. Plast. Surg. 2003, 27, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Benito-Ruiz, J.; Redondo, A. Breast Augmentation Surgery: How Do We Do It? Results of a Joint Survey from European Association of Societies of Aesthetic Plastic Surgery. Aesthet. Plast. Surg. 2020, 44, 1957–1964. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Chahine, F. Comment on A Comprehensive Outcome Review of Subfascial Breast Augmentation over a 10-Year Period. Aesthet. Plast. Surg. 2021, 45, 1375–1377. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Gallardo, G.; Cuenca-Pardo, J.; Rodriguez-Olivares, E.; Iribarren-Moreno, R.; Contreras-Bulnes, L.; Vallarta-Rodriguez, A.; Kalixto-Sanchez, M.; Hernandez, C.; Ceja-Martinez, R.; Torres-Rivero, C. Breast Implant and Anaplastic Large Cell Lymphoma Meta-Analysis. J. Investig. Surg. 2017, 30, 56–65. [Google Scholar] [CrossRef]
- Holmich, L.R.; Vejborg, I.M.; Conrad, C.; Sletting, S.; Hoier-Madsen, M.; Fryzek, J.P.; McLaughlin, J.K.; Kjoller, K.; Wiik, A.; Friis, S. Untreated silicone breast implant rupture. Plast. Reconstr. Surg. 2004, 114, 204–214, discussion 215–206. [Google Scholar] [CrossRef]
- Hillard, C.; Fowler, J.D.; Barta, R.; Cunningham, B. Silicone breast implant rupture: A review. Gland Surg. 2017, 6, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Handel, N.; Garcia, M.E.; Wixtrom, R. Breast implant rupture: Causes, incidence, clinical impact, and management. Plast. Reconstr. Surg. 2013, 132, 1128–1137. [Google Scholar] [CrossRef]
- Necchi, S.; Molina, D.; Turri, S.; Rossetto, F.; Rietjens, M.; Pennati, G. Failure of silicone gel breast implants: Is the mechanical weakening due to shell swelling a significant cause of prostheses rupture? J. Mech. Behav. Biomed. Mater. 2011, 4, 2002–2008. [Google Scholar] [CrossRef]
- Pinchuk, V.; Tymofii, O.; Tkach, O.; Zamkovoy, V. Implant ruptures after augmentation mammoplasty. Aesthet. Plast. Surg. 2013, 37, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Rukanskiene, D.; Bytautaite, G.; Cesnauskaite, A.; Pilipaityte, L.; Astrauskas, T.; Jonaitiene, E. The Value of Ultrasound in the Evaluation of the Integrity of Silicone Breast Implants. Medicina (Kaunas) 2021, 57, 440. [Google Scholar] [CrossRef]
- Gorczyca, D.P.; Gorczyca, S.M.; Gorczyca, K.L. The diagnosis of silicone breast implant rupture. Plast. Reconstr. Surg. 2007, 120, 49S–61S. [Google Scholar] [CrossRef]
- Lin, M.-F.; Lai, L.-H.; Hsiao, W.-T.; Yao, M.M.-S.; Chan, W.-P. Developing a Specific MRI Technology to Identify Complications Caused by Breast Implants. Appl. Sci. 2021, 11, 3434. [Google Scholar] [CrossRef]
- Diehm, Y.F.; Jost, Y.; Kotsougiani-Fischer, D.; Haug, V.; Splinter, M.; Haring, P.; Berger, M.R.; Debus, J.; Kneser, U.; Fischer, S. The Treatment of Capsular Contracture Around Breast Implants Induced by Fractionated Irradiation: The Collagenase of the Bacterium Clostridium Histolyticum as a Novel Therapeutic Approach. Aesthet. Plast. Surg. 2021, 45, 1273–1281. [Google Scholar] [CrossRef]
- Gundeslioglu, O.; Altundag, O.; Altundag, K. Nanobacteria and breast implant capsule contracture and calcification: A hypothesis. Aesthet. Plast. Surg. 2005, 29, 582. [Google Scholar] [CrossRef]
- Bachour, Y. Capsular Contracture in Breast Implant Surgery: Where Are We Now and Where Are We Going? Aesthet. Plast. Surg. 2021, 45, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Bachour, Y.; Bargon, C.A.; de Blok, C.J.M.; Ket, J.C.F.; Ritt, M.; Niessen, F.B. Risk factors for developing capsular contracture in women after breast implant surgery: A systematic review of the literature. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, e29–e48. [Google Scholar] [CrossRef] [PubMed]
- Araco, A.; Caruso, R.; Araco, F.; Overton, J.; Gravante, G. Capsular contractures: A systematic review. Plast. Reconstr. Surg. 2009, 124, 1808–1819. [Google Scholar] [CrossRef]
- Prantl, L.; Schreml, S.; Fichtner-Feigl, S.; Poppl, N.; Eisenmann-Klein, M.; Schwarze, H.; Fuchtmeier, B. Clinical and morphological conditions in capsular contracture formed around silicone breast implants. Plast. Reconstr. Surg. 2007, 120, 275–284. [Google Scholar] [CrossRef]
- Prantl, L. Serologic and Histologic Findings in Capsule Contracture Patients with Silicone Gel Implants. In Breast Augmentation: Principles and Practice; Shiffman, M.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 649–654. [Google Scholar] [CrossRef]
- de Bakker, E.; Rots, M.; Buncamper, M.E.; Niessen, F.B.; Smit, J.M.; Winters, H.A.H.; Ozer, M.; de Vet, H.C.W.; Mullender, M.G. The Baker Classification for Capsular Contracture in Breast Implant Surgery Is Unreliable as a Diagnostic Tool. Plast. Reconstr. Surg. 2020, 146, 956–962. [Google Scholar] [CrossRef]
- Groth, A.K.; Graf, R. Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) and the Textured Breast Implant Crisis. Aesthet. Plast. Surg. 2020, 44, 1–12. [Google Scholar] [CrossRef]
- Jones, J.L.; Hanby, A.M.; Wells, C.; Calaminici, M.; Johnson, L.; Turton, P.; Deb, R.; Provenzano, E.; Shaaban, A.; Ellis, I.O.; et al. Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL): An overview of presentation and pathogenesis and guidelines for pathological diagnosis and management. Histopathology 2019, 75, 787–796. [Google Scholar] [CrossRef]
- Rondon-Lagos, M.; Rangel, N.; Camargo-Villalba, G.; Forero-Castro, M. Biological and genetic landscape of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). Eur. J. Surg. Oncol. 2021, 47, 942–951. [Google Scholar] [CrossRef]
- Keech, J.A., Jr.; Creech, B.J. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast. Reconstr. Surg. 1997, 100, 554–555. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [Green Version]
- di Pompeo, S.F.; Sorotos, M.; Clemens, M.W.; Firmani, G.; European Association of Plastic Surgeons (EURAPS) Committee on Device Safety and Development. Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL): Review of Epidemiology and Prevalence Assessment in Europe. Aesthet. Surg. J. 2021, 41, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Nava, M.B.; Adams, W.P., Jr.; Botti, G.; Campanale, A.; Catanuto, G.; Clemens, M.W.; Del Vecchio, D.A.; De Vita, R.; Di Napoli, A.; Hall-Findlay, E.; et al. MBN 2016 Aesthetic Breast Meeting BIA-ALCL Consensus Conference Report. Plast. Reconstr. Surg. 2018, 141, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, R. The State of the Art about Etiopathogenetic Models on Breast Implant Associated-Anaplastic Large Cell Lymphoma (BIA-ALCL): A Narrative Review. J. Clin. Med. 2021, 10, 2082. [Google Scholar] [CrossRef] [PubMed]
- Clemens, M.W.; Brody, G.S.; Mahabir, R.C.; Miranda, R.N. How to Diagnose and Treat Breast Implant-Associated Anaplastic Large Cell Lymphoma. Plast. Reconstr. Surg. 2018, 141, 586e–599e. [Google Scholar] [CrossRef]
- Turton, P.; El-Sharkawi, D.; Lyburn, I.; Sharma, B.; Mahalingam, P.; Turner, S.D.; MacNeill, F.; Johnson, L.; Hamilton, S.; Burton, C.; et al. UK Guidelines on the Diagnosis and Treatment of Breast Implant-Associated Anaplastic Large Cell Lymphoma on behalf of the Medicines and Healthcare products Regulatory Agency Plastic, Reconstructive and Aesthetic Surgery Expert Advisory Group. Br. J. Haematol. 2021, 192, 444–458. [Google Scholar] [CrossRef] [PubMed]
- McGuire, P.A.; Haws, M.J.; Nahai, F. Breast Implant Illness: How Can We Help? Aesthet. Surg. J. 2019, 39, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Deva, A.K.; Turner, S.D.; Kadin, M.E.; Magnusson, M.R.; Prince, H.M.; Miranda, R.N.; Inghirami, G.G.; Adams, W.P., Jr. Etiology of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL): Current Directions in Research. Cancers 2020, 12, 3861. [Google Scholar] [CrossRef]
- Mempin, M.; Hu, H.; Chowdhury, D.; Deva, A.; Vickery, K. The A, B and C’s of Silicone Breast Implants: Anaplastic Large Cell Lymphoma, Biofilm and Capsular Contracture. Materials 2018, 11, 2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oishi, N.; Miranda, R.N.; Feldman, A.L. Genetics of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). Aesthet. Surg. J. 2019, 39, S14–S20. [Google Scholar] [PubMed]
- Clemens, M.W.; Jacobsen, E.D.; Horwitz, S.M. 2019 NCCN Consensus Guidelines on the Diagnosis and Treatment of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). Aesthet. Surg. J. 2019, 39, S3–S13. [Google Scholar] [CrossRef] [Green Version]
- Duvic, M.; Tetzlaff, M.T.; Gangar, P.; Clos, A.L.; Sui, D.; Talpur, R. Results of a Phase II Trial of Brentuximab Vedotin for CD30+ Cutaneous T-Cell Lymphoma and Lymphomatoid Papulosis. J. Clin. Oncol. 2015, 33, 3759–3765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prince, H.M.; Kim, Y.H.; Horwitz, S.M.; Dummer, R.; Scarisbrick, J.; Quaglino, P.; Zinzani, P.L.; Wolter, P.; Sanches, J.A.; Ortiz-Romero, P.L.; et al. Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): An international, open-label, randomised, phase 3, multicentre trial. Lancet 2017, 390, 555–566. [Google Scholar] [CrossRef]
- Sanchez Rubio, N.; Lannegrand Menendez, B.; Duque Munoz, M.; Montes Fernandez, M.; Ciudad Fernandez, M.J. Uncommon complications of breast prostheses. Radiologia (Engl. Ed.) 2020, 62, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Newby, J.M.; Tang, S.; Faasse, K.; Sharrock, M.J.; Adams, W.P. Understanding Breast Implant Illness. Aesthet. Surg. J. 2020. [Google Scholar] [CrossRef]
- Atiyeh, B.; Emsieh, S. Breast Implant Illness (BII): Real Syndrome or a Social Media Phenomenon? A Narrative Review of the Literature. Aesthet. Plast. Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wee, C.E.; Younis, J.; Isbester, K.; Smith, A.; Wangler, B.; Sarode, A.L.; Patil, N.; Grunzweig, K.; Boas, S.; Harvey, D.J.; et al. Understanding Breast Implant Illness, Before and After Explantation: A Patient-Reported Outcomes Study. Ann. Plast. Surg. 2020, 85, S82–S86. [Google Scholar] [CrossRef]
- Tang, S.Y.Q.; Israel, J.S.; Afifi, A.M. Breast Implant Illness: Symptoms, Patient Concerns, and the Power of Social Media. Plast. Reconstr. Surg. 2017, 140, 765e–766e. [Google Scholar] [CrossRef]
- Lee, M.; Ponraja, G.; McLeod, K.; Chong, S. Breast Implant Illness: A Biofilm Hypothesis. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2755. [Google Scholar] [CrossRef]
- Keane, G.; Chi, D.; Ha, A.Y.; Myckatyn, T.M. En Bloc Capsulectomy for Breast Implant Illness: A Social Media Phenomenon? Aesthet. Surg. J. 2021, 41, 448–459. [Google Scholar] [CrossRef]
- Rohrich, R.J.; Kaplan, J.; Dayan, E. Silicone Implant Illness: Science versus Myth? Plast. Reconstr. Surg. 2019, 144, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Swanson, E. Evaluating the Necessity of Capsulectomy in Cases of Textured Breast Implant Replacement. Ann. Plast. Surg. 2020, 85, 691–698. [Google Scholar] [CrossRef]
- Swanson, E. The Case for Breast Implant Removal or Replacement Without Capsulectomy. Aesthet. Plast. Surg. 2021, 45, 1338–1341. [Google Scholar] [CrossRef]
- Gurunluoglu, R.; Sacak, B.; Arton, J. Outcomes analysis of patients undergoing autoaugmentation after breast implant removal. Plast. Reconstr. Surg. 2013, 132, 304–315. [Google Scholar] [CrossRef]
- Kaplan, J.; Rohrich, R. Breast implant illness: A topic in review. Gland Surg. 2021, 10, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Gerzenshtein, J. The Dishonesty of Referring to Total Intact Capsulectomy as “En Bloc” Resection or Capsulectomy. Plast. Reconstr. Surg. 2020, 145, 227e–228e. [Google Scholar] [CrossRef] [PubMed]
- Tevis, S.E.; Hunt, K.K.; Clemens, M.W. Stepwise En Bloc Resection of Breast Implant-Associated Anaplastic Large Cell Lymphoma with Oncologic Considerations. Aesthet. Surg. J. Open Forum. 2019, 1, ojz005. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Grade | Baker | Wilflingseder |
|---|---|---|
| I | Implant shell not palpable and not visible | Thin and uncontracted capsule |
| II | Implant shell slightly firm, but not visible | “Constrictive fibrosis”, no giant cells |
| III | Implant shell clearly firm and implant visible | “Constrictive fibrosis”, giant cells present |
| IV | Implant shell very firm, implant dislocation and deformation | Inflammatory cells, foreign body granulomas, neovascularization, possible neuromas |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelc, Z.; Skórzewska, M.; Kurylcio, A.; Olko, P.; Dryka, J.; Machowiec, P.; Maksymowicz, M.; Rawicz-Pruszyński, K.; Polkowski, W. Current Challenges in Breast Implantation. Medicina 2021, 57, 1214. https://doi.org/10.3390/medicina57111214
Pelc Z, Skórzewska M, Kurylcio A, Olko P, Dryka J, Machowiec P, Maksymowicz M, Rawicz-Pruszyński K, Polkowski W. Current Challenges in Breast Implantation. Medicina. 2021; 57(11):1214. https://doi.org/10.3390/medicina57111214
Chicago/Turabian StylePelc, Zuzanna, Magdalena Skórzewska, Andrzej Kurylcio, Paweł Olko, Joanna Dryka, Piotr Machowiec, Marcela Maksymowicz, Karol Rawicz-Pruszyński, and Wojciech Polkowski. 2021. "Current Challenges in Breast Implantation" Medicina 57, no. 11: 1214. https://doi.org/10.3390/medicina57111214
APA StylePelc, Z., Skórzewska, M., Kurylcio, A., Olko, P., Dryka, J., Machowiec, P., Maksymowicz, M., Rawicz-Pruszyński, K., & Polkowski, W. (2021). Current Challenges in Breast Implantation. Medicina, 57(11), 1214. https://doi.org/10.3390/medicina57111214







