Effects of Mean Artery Pressure and Blood pH on Survival Rate of Patients with Acute Kidney Injury Combined with Acute Hypoxic Respiratory Failure: A Retrospective Study
Abstract
:1. Introduction
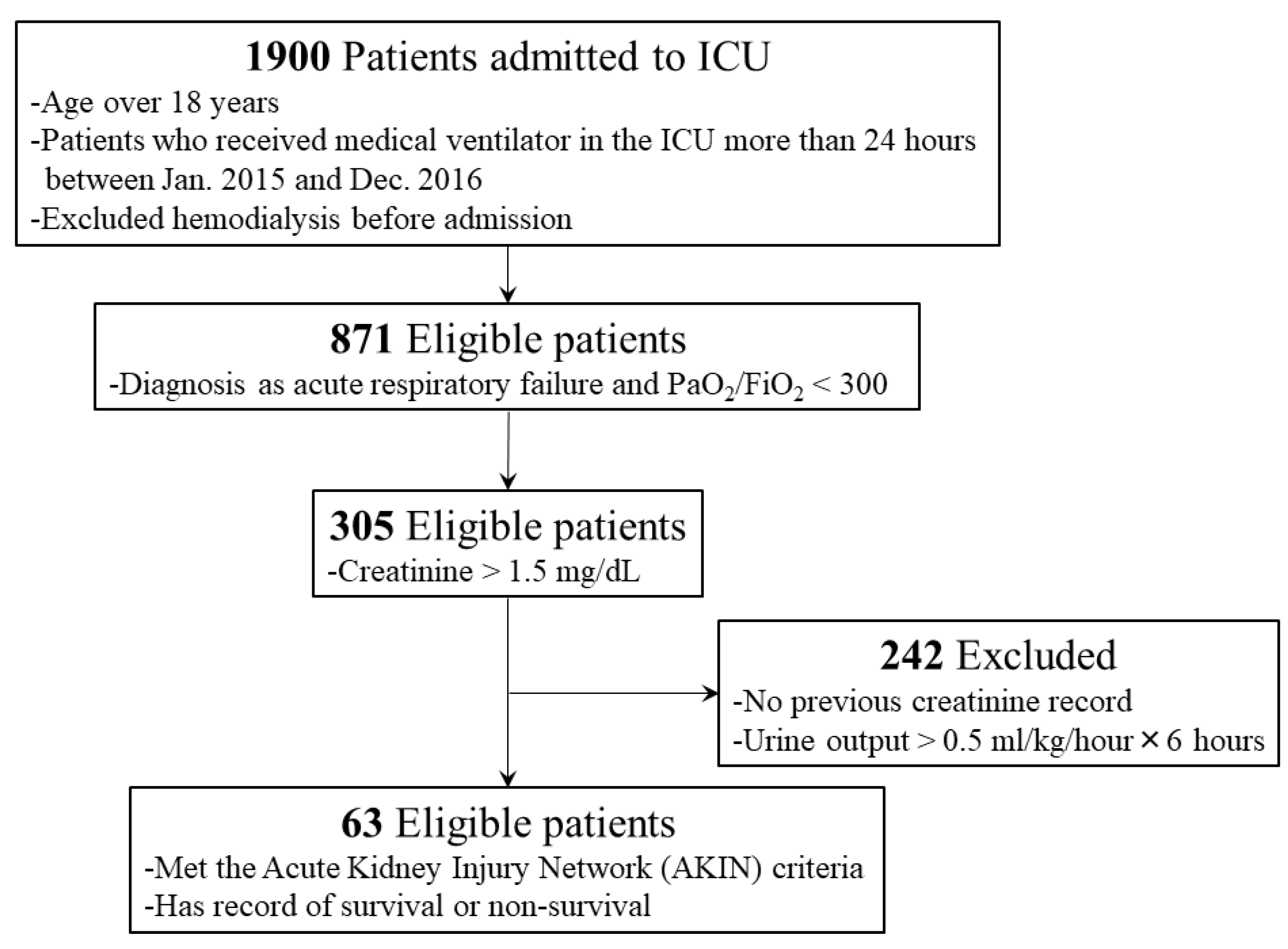
2. Materials and Methods
2.1. Study Populations
2.2. Data Collection
2.3. Acute Kidney Injury Characterization
2.4. Respiratory Failure Characterization
2.5. Outcome Measurement
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Basic Physiological and Hemodynamic Data
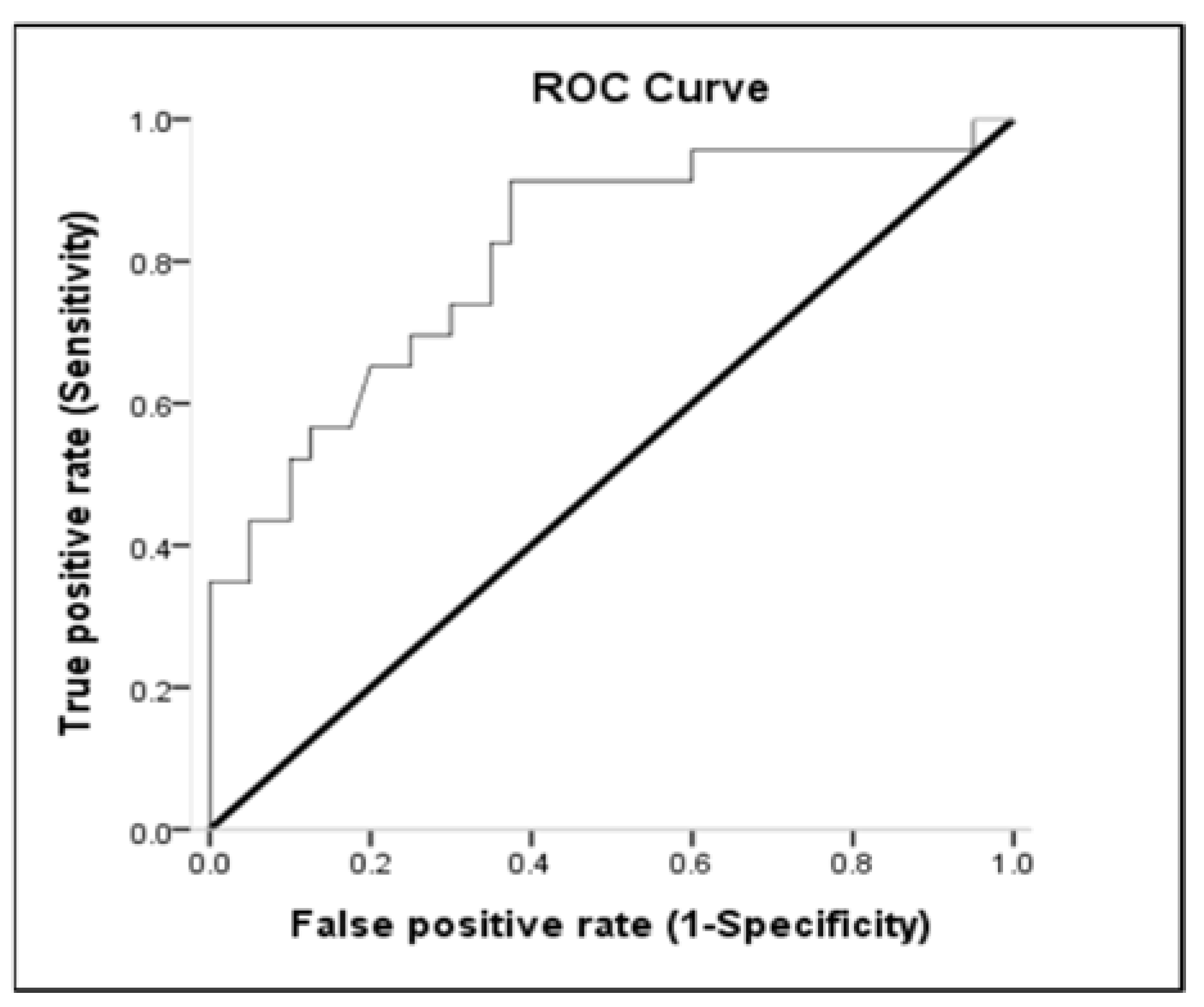
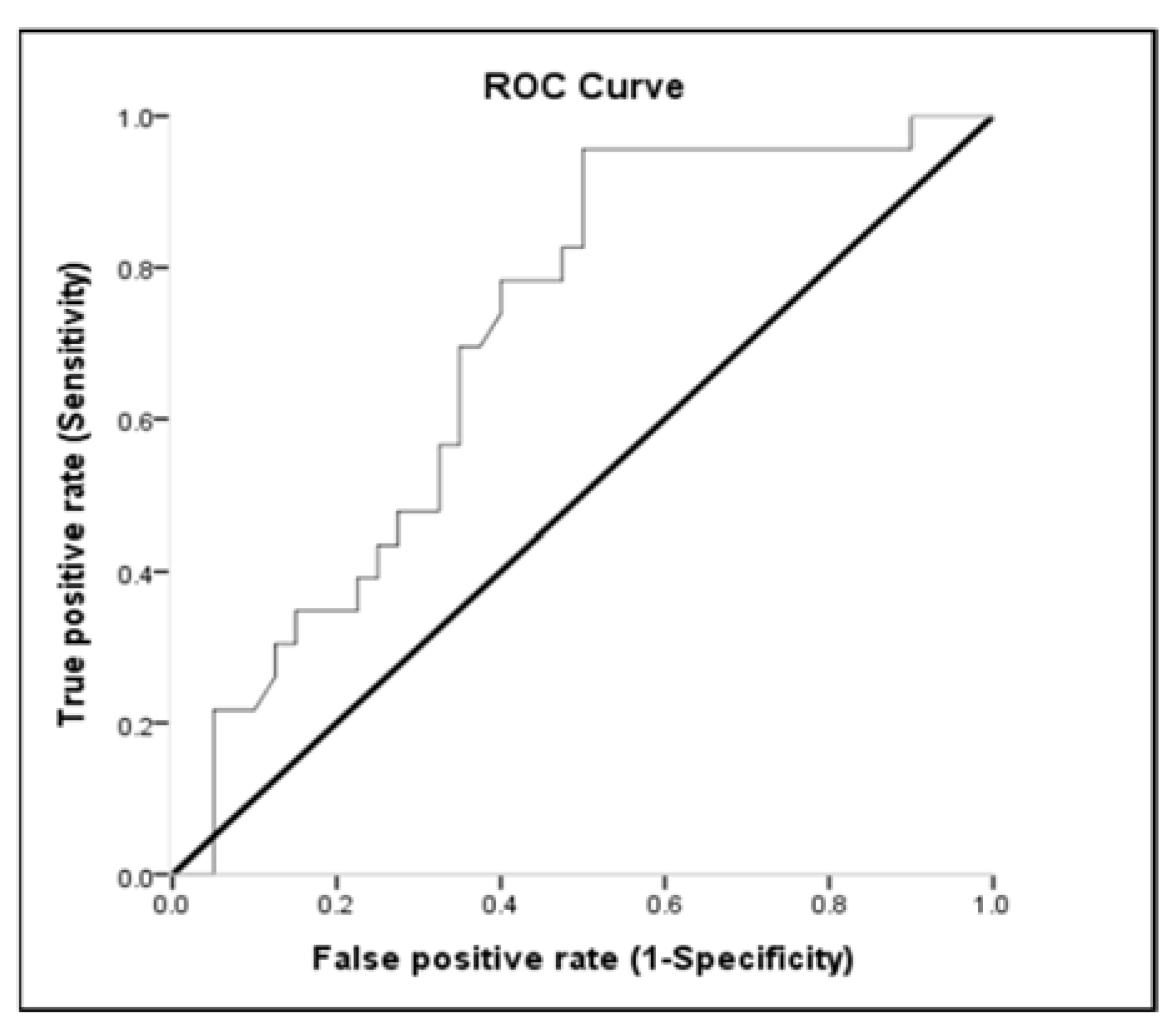
3.3. Receiver-Operating Characteristic (ROC) Curve
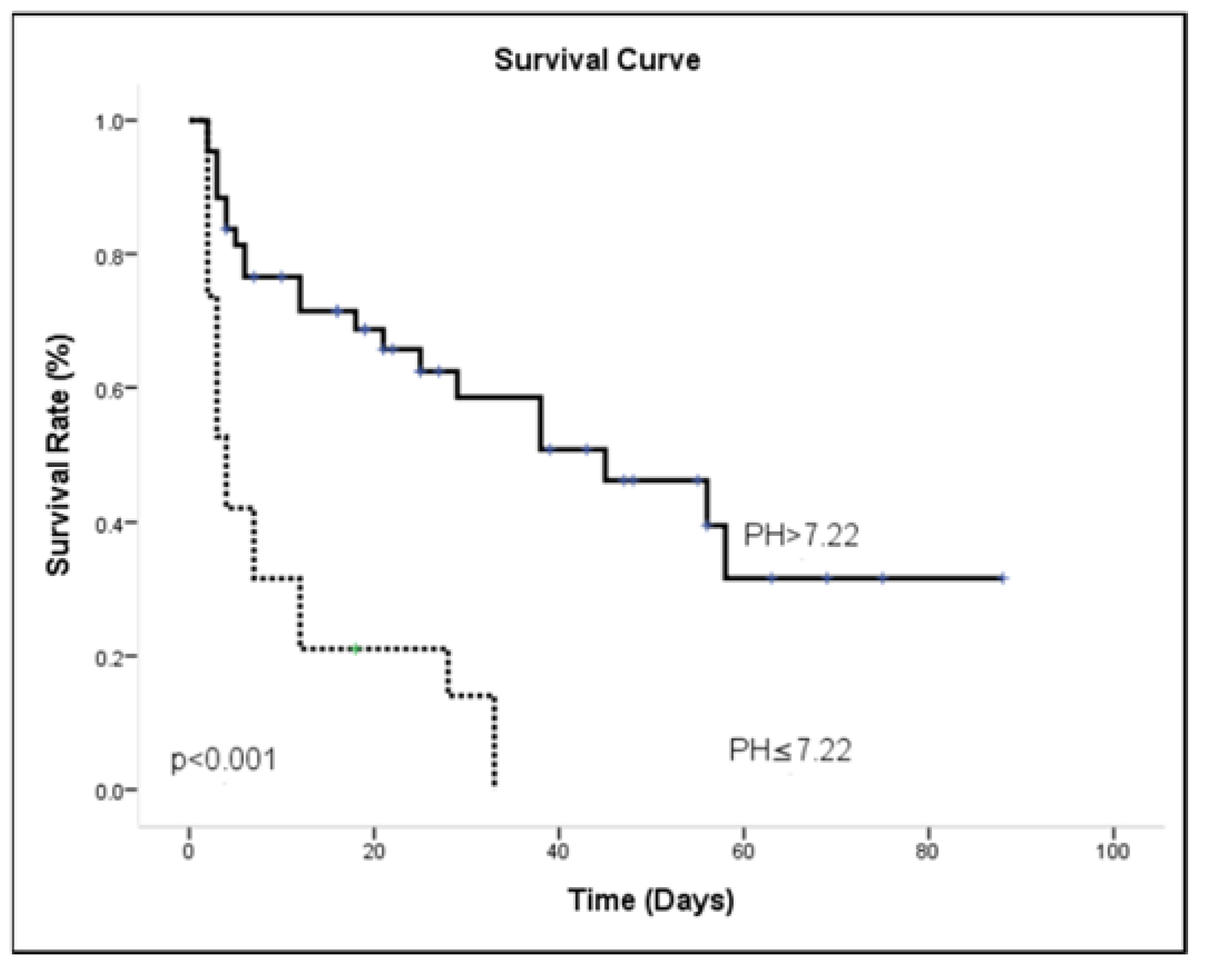
3.4. ICU Outcome Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakr, Y.; Lobo, S.M.; Moreno, R.P.; Gerlach, H.; Ranieri, V.M.; Michalopoulos, A.; Vincent, J.L.; The SOAP Investigators. Patterns and early evolution of organ failure in the intensive care unit and their relation to outcome. Crit. Care 2012, 16, R222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayr, V.D.; Dünser, M.W.; Greil, V.; Jochberger, S.; Luckner, G.; Ulmer, H.; Friesenecker, B.E.; Takala, J.; Hasibeder, W.R. Causes of death and determinants of outcome in critically ill patients. Crit. Care 2006, 10, R154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peres, L.A.; Wandeur, V.; Matsuo, T. Predictors of acute kidney injury and mortality in an Intensive Care Unit. J. Bras. Nefrol. 2015, 37, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dünser, M.W.; Takala, J.; Ulmer, H.; Mayr, V.D.; Luckner, G.; Jochberger, S.; Daudel, F.; Lepper, P.; Hasibeder, W.R.; Jakob, S.M. Arterial blood pressure during early sepsis and outcome. Intensiv. Care Med. 2009, 35, 1225–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.; et al. Surviving sepsis campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef]
- Lambden, S.; Laterre, P.F.; Levy, M.M.; Francois, B. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit. Care 2019, 23, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellum, J.A.; Kong, L.; Fink, M.P.; Weissfeld, L.A.; Yealy, D.M.; Pinsky, M.R.; Fine, J.; Krichevsky, A.; Delude, R.L.; Angus, D.C.; et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: Results of the genetic and inflammatory markers of sepsis (GenIMS) study. Arch. Intern. Med. 2007, 167, 1655–1663. [Google Scholar] [CrossRef] [Green Version]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef] [Green Version]
- Deruddre, S.; Cheisson, G.; Mazoit, J.X.; Vicaut, E.; Benhamou, D.; Duranteau, J. Renal arterial resistance in septic shock: Effects of increasing mean arterial pressure with norepinephrine on the renal resistive index assessed with Doppler ultrasonography. Intensiv. Care Med. 2007, 33, 1557–1562. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving sepsis campaign: International Guidelines for Management of Sepsis and Septic Shock 2016. Intensiv. Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Uchino, S.; Kellum, J.A.; Bellomo, R.; Doig, G.S.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA. 2005, 294, 813–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faubel, S. Pulmonary complications after acute kidney injury. Adv. Chronic Kidney Dis. 2008, 15, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, K.; Nathanson, B.H.; Munson, S.H.; Khangulov, V.; Stevens, M.; Badani, H.; Khanna, A.K.; Sessler, D.I. The relationship between ICU hypotension and in-hospital mortality and morbidity in septic patients. Intensiv. Care Med. 2018, 44, 857–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, M.M. Pathophysiology of hypertension in renal failure. Semin. Nephrol. 2002, 22, 17–26. [Google Scholar] [CrossRef]
- Bourgoin, A.; Leone, M.; Delmas, A.; Garnier, F.; Albanèse, J.; Martin, C. Increasing mean arterial pressure in patients with septic shock: Effects on oxygen variables and renal function. Crit. Care Med. 2005, 33, 780–786. [Google Scholar] [CrossRef]
- Acheampong, A.; Vincent, J.L. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit. Care 2015, 19, 51. [Google Scholar] [CrossRef] [Green Version]
- Badin, J.; Boulain, T.; Ehrmann, S.; Skarzynski, M.; Bretagnol, A.; Buret, J.; Benzekri-Lefevre, D.; Mercier, E.; Runge, I.; Garot, D.; et al. Relation between mean arterial pressure and renal function in the early phase of shock: A prospective, explorative cohort study. Crit. Care 2011, 15, R135. [Google Scholar] [CrossRef] [Green Version]
- Koomans, H.A.; Roos, J.C.; Boer, P.; Geyskes, G.G.; Mees, E.J. Salt sensitivity of blood pressure in chronic renal failure. Evidence for renal control of body fluid distribution in man. Hypertension 1982, 4, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.L.; Prowle, J.; Licari, E.; Uchino, S.; Bellomo, R. Changes in blood pressure before the development of nosocomial acute kidney injury. Nephrol. Dial. Transplan. 2009, 24, 504–511. [Google Scholar] [CrossRef] [Green Version]
- Macedo, E.; Malhotra, R.; Claure-Del Granado, R.; Fedullo, P.; Mehta, R.L. Defining urine output criterion for acute kidney injury in critically ill patients. Nephrol. Dial. Transplant. 2011, 26, 509–515. [Google Scholar] [CrossRef]
- Leone, M.; Asfar, P.; Radermacher, P.; Vincent, J.L.; Martin, C. Optimizing mean arterial pressure in septic shock: A critical reappraisal of the literature. Crit. Care 2015, 19, 101. [Google Scholar] [CrossRef] [Green Version]
- Strandgaard, S.; Olesen, J.; Skinhoj, E.; Lassen, N.A. Autoregulation of brain circulation in severe arterial hypertension. Br. Med. J. 1973, 1, 507–510. [Google Scholar] [CrossRef] [Green Version]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron. Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Azoulay, E.; Mokart, D.; Lambert, J.; Lemiale, V.; Rabbat, A.; Kouatchet, A.; Vincent, F.; Gruson, D.; Bruneel, F.; Epinette-Branche, G.; et al. Diagnostic strategy for hematology and oncology patients with acute respiratory failure: Randomized controlled trial. Am. J. Respir. Crit. Care Med. 2010, 182, 1038–1046. [Google Scholar] [CrossRef] [Green Version]
- Darmon, M.; Clec’h, C.; Adrie, C.; Argaud, L.; Allaouchiche, B.; Azoulay, E.; Bouadma, L.; Garrouste-Orgeas, M.; Haouache, H.; Schwebel, C.; et al. Acute respiratory distress syndrome and risk of AKI among critically ill patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1347–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.C.; Lin, S.F.; Liu, C.J.; Jiang, D.D.; Yang, P.C.; Chang, S.C. Risk factors for ICU mortality in critically ill patients. J. Formos. Med. Assoc. 2001, 100, 656–661. [Google Scholar] [PubMed]
- Sirio, C.A.; Tajimi, K.; Tase, C.; Knaus, W.A.; Wagner, D.P.; Hirasawa, H.; Sakanishi, N.; Katsuya, H.; Taenaka, N. An initial comparison of intensive care in Japan and the United States. Crit. Care Med. 1992, 20, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Bucuvic, E.M.; Ponce, D.; Balbi, A.L. Risk factors for mortality in acute kidney injury. Rev. Assoc. Med. Bras. (1992) 2011, 57, 158–163. [Google Scholar] [CrossRef]
- Santos, W.J.; Zanetta, D.M.; Pires, A.C.; Lobo, S.M.; Lima, E.Q.; Burdmann, E.A. Patients with ischaemic, mixed and nephrotoxic acute tubular necrosis in the intensive care unit--a homogeneous population? Crit. Care 2006, 10, R68. [Google Scholar] [CrossRef] [Green Version]
- Brivet, F.G.; Kleinknecht, D.J.; Loirat, P.; Landais, P.J. Acute renal failure in intensive care units-causes, outcome, and prognostic factors of hospital mortality; a prospective, multicenter study. French Study Group on Acute Renal Failure. Crit. Care Med. 1996, 24, 192–198. [Google Scholar] [CrossRef]
- Aldawood, A. Outcome and prognostic factors of critically ill patients with acute renal failure requiring continuous renal replacement therapy. Saudi J. Kidney Dis. Transpl. 2010, 21, 1106–1110. [Google Scholar]
- Azoulay, E.; Adrie, C.; De Lassence, A.; Pochard, F.; Moreau, D.; Thiery, G.; Cheval, C.; Moine, P.; Garrouste-Orgeas, M.; Alberti, C.; et al. Determinants of postintensive care unit mortality: A prospective multicenter study. Crit. Care Med. 2003, 31, 428–432. [Google Scholar] [CrossRef]
- Weisberg, L.S.; Allgren, R.L.; Genter, F.C.; Kurnik, B.R. Cause of acute tubular necrosis affects its prognosis. The Auriculin Anaritide Acute Renal Failure Study Group. Arch. Intern. Med. 1997, 157, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Liaño, F.; Gallego, A.; Pascual, J.; García-Martín, F.; Teruel, J.L.; Marcén, R.; Orofino, L.; Orte, L.; Rivera, M.; Gallego, N.; et al. Prognosis of acute tubular necrosis: An extended prospectively contrasted study. Nephron. 1993, 63, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Cheng, S.Y.; Chou, C.Y.; Liu, J.H.; Lin, H.H.; Tseng, Y.H.; Liu, Y.L.; Chen, W.; Huang, C.C. Association between mean arterial pressure and mortality in chronic hemodialysis patients. Kidney Blood Press. Res. 2009, 32, 99–105. [Google Scholar] [CrossRef]
- Zager, P.G.; Nikolic, J.; Brown, R.H.; Campbell, M.A.; Hunt, W.C.; Peterson, D.; Van Stone, J.; Levey, A.; Meyer, K.B.; Klag, M.J.; et al. “U” curve association of blood pressure and mortality in hemodialysis patients. Kidney Int. 1998, 54, 561–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, M.M. Hypertension in the hemodialysis population: A survey of 649 patients. Am. J. Kidney Dis. 1995, 26, 461–468. [Google Scholar] [CrossRef]
- Varpula, M.; Tallgren, M.; Saukkonen, K.; Voipio-Pulkki, L.M.; Pettilä, V. Hemodynamic variables related to outcome in septic shock. Intensiv. Care Med. 2005, 31, 1066–1071. [Google Scholar] [CrossRef]
- Asfar, P.; Meziani, F.; Hamel, J.F.; Grelon, F.; Megarbane, B.; Anguel, N.; Mira, J.P.; Dequin, P.F.; Gergaud, S.; Weiss, N.; et al. High versus low blood-pressure target in patients with septic shock. N. Eng. J. Med. 2014, 370, 1583–1593. [Google Scholar] [CrossRef] [Green Version]
- Moviat, M.; van Haren, F.; van der Hoeven, H. Conventional or physicochemical approach in intensive care unit patients with metabolic acidosis. Crit. Care 2003, 7, R41. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.W.; Hong, Y.S.; Park, D.W.; Choi, S.H.; Moon, S.W.; Park, J.S.; Kim, J.Y.; Baek, K.J. Lactic acidosis not hyperlactatemia as a predictor of in hospital mortality in septic emergency patients. Emerg. Med. J. 2008, 25, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Levraut, J.; Ichai, C.; Petit, I.; Ciebiera, J.P.; Perus, O.; Grimaud, D. Low exogenous lactate clearance as an early predictor of mortality in normolactatemic critically ill septic patients. Crit. Care Med. 2003, 31, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, X. Lactate clearance is a useful biomarker for the prediction of all-cause mortality in critically ill patients: A systematic review and meta-analysis*. Crit. Care Med. 2014, 42, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Essa, A.; Kousa, O.; Awad, D.; Stevenson, M.; DeVrieze, B.; Moore, D. The association of hypophosphatemia with resistant lactic acidosis in critical care illness. J. Investig. Med. High Impact Case Rep. 2020, 8, 2324709620934963. [Google Scholar] [CrossRef]
- Gil, A.; Carrizosa, F.; Herrero, A.; Martin, J.; González, J.; Jareño, A.; Rivero, J. Influence of mechanical ventilation on blood lactate in patients with acute respiratory failure. Intensiv. Care Med. 1998, 24, 924–930. [Google Scholar] [CrossRef]
- Gourhant, V.; Vuillot, O.; Claret, P.G.; Lefebvre, S.; Schaub, R.; Flacher, A.; Dumont, R.; Sebbane, M. Arterial pH selectively predicts critical care needs in emergency department obese patients with acute dyspnea: A prospective comparative study. Am. J. Emerg. Med. 2019, 37, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Devi, T.S.; Sharma, R.; Chhabra, P.; Gupta, R.; Rana, S.S.; Bhasin, D.K. Arterial pH, bicarbonate levels and base deficit at presentation as markers of predicting mortality in acute pancreatitis: A single-centre prospective study. Gastroenterol. Rep. (Oxf). 2014, 2, 226–231. [Google Scholar] [CrossRef]
- Mitchell, J.H.; Wildenthal, K.; Johnson, R.L., Jr. The effects of acid-base disturbances on cardiovascular and pulmonary function. Kidney Int. 1972, 1, 375–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zapol, W.M.; Snider, M.T. Pulmonary hypertension in severe acute respiratory failure. N. Engl. J. Med. 1977, 296, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Hesse, C.; Dehnert, C.; Siedler, H.; Kleinbongard, P.; Bardenheuer, H.J.; Kelm, M.; Bärtsch, P.; Haefeli, W.E. Hypoxia impairs systemic endothelial function in individuals prone to high-altitude pulmonary edema. Am. J. Respir. Crit. Care Med. 2005, 172, 763–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lien, J.; Chan, V. Risk factors influencing survival in acute renal failure treated by hemodialysis. Arch. Intern. Med. 1985, 145, 2067–2069. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Lin, C.Y.; Tian, Y.C.; Jenq, C.C.; Chang, M.Y.; Chen, Y.C.; Fang, J.T.; Yang, C.W. Acute kidney injury classification: Comparison of AKIN and RIFLE criteria. Shock 2010, 33, 247–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| All (n = 63) | Survival (n = 23) | Non-survival (n = 40) | p | |
|---|---|---|---|---|
| Age # ([min-max]) | 66.63 ± 16.40 [22.6–94.7] | 67.74 ± 14.64 [31.1–92.4] | 66 ± 17.48 [22.6–94.7] | 0.689 |
| Gender, male/female | 46/17 | 17/6 | 29/11 | 0.573 |
| SOFA score ([min-max]) | 11.06 ± 2.16 [7–17] | 11.38 ± 2.02 [7–17] | 10.52 ± 2.33 [8–15] | 0.133 |
| Diagnosis on admission * | ||||
| Respiratory diseases | 18 (28.5) | 3 (13) | 15 (37.5) | 0.021 |
| Neurological diseases | 6 (9.5) | 2 (8.7) | 4 (10) | |
| Nephrological disease | 3 (4.8) | 1 (4.3) | 2 (5) | |
| Infectious disease | 5 (7.9) | 0 (0) | 5 (12.5) | |
| Cardiologic diseases | 20 (31.7) | 13 (56.5) | 7 (17.5) | |
| Gastroenterological diseases | 10 (15.9) | 3 (13) | 7 (17.5) | |
| Hematologic diseases | 1 (1.6) | 1 (4.3) | 0 (0) | |
| AKIN stage | ||||
| Stage 1 | 25 (39.7) | 10 (43.5) | 15 (37.5) | 0.661 |
| Stage 2 | 15 (23.8) | 4 (17.4) | 11 (27.5) | |
| Stage 3 | 23 (36.5) | 9 (39.1) | 14 (35) | |
| Sepsis | 46 (73) | 14 (60.9) | 32 (80) | 0.141 |
| Lactic acidosis | 12 (19) | 4 (17) | 8 (20) | 0.800 |
| Metabolic acidosis | 47 (74.6) | 17 (73.9) | 30 (75) | 0.924 |
| Hemodialysis | 13 (20.6) | 5 (21.7) | 8 (20) | 0.653 |
| Hemofiltration | 12 (19) | 3 (13) | 9 (22.5) | |
| Length of hospital stay *([min-max]) | 22 ± 21.53 [1–88] | 14.15 ± 15.99 [4–88] | 35.3 ± 23.45 [1–58] | 0.001 |
| All (n = 63) | Survival (n = 23) | Non-survival (n = 40) | p | |
|---|---|---|---|---|
| Basic Data | ||||
| BUN (mg/dL) | 66.12 ± 22.17 [16.90–121.00] | 69.48 ± 22.13 [31.30–121.00] | 64.19 ± 22.24 [16.90–102.00] | 0.366 |
| Baseline creatinine (mg/dL) | 2.42 ± 2.16 [0.64–9.85] | 2.66 ± 2.08 [0.64–9.85] | 2.28 ± 2.22 [0.69–9.68] | 0.502 |
| Creatinine (mg/dL) | 4.73 ± 2.62 [1.34–11.38] | 5.30 ± 2.53 [1.34–11.10] | 4.40 ± 2.65 [1.52–11.38] | 0.193 |
| Lactate(mg/dL) * | 61.21 ± 42.54 [13.60–225] | 48.02 ± 32.09 [15.90–139.35] | 68.66 ± 46.15 [13.60–225] | 0.045 |
| Sodium (mg/dL) | 139.82 ± 6.36 [126.00–154.00] | 139.59 ± 5.96 [138.00–154.00] | 139.95 ± 6.66 [126.00–151.00] | 0.829 |
| Potassium (mg/dL) | 4.71 ± 1.02 [2.60–7.20] | 4.91 ± 0.87 [4.80–6.90] | 4.59 ± 1.08 [2.60–7.20] | 0.228 |
| Hb (g/dL) | 10.42 ± 2.49 [6.60–16.10] | 10.13 ± 2.12 [7.00–16.00] | 10.57 ± 2.68 [6.60–16.10] | 0.511 |
| WBC (109/L) * | 16.2 ± 7.84 [3.00–35.00] | 13.53 ± 6.61 [3.00–33.80] | 17.74 ± 8.15 [3.70–35.00] | 0.039 |
| Hemodynamic Data | ||||
| MAP (mmHg) * | 83.25 ± 20.93 [50.67–153.33] | 97.99 ± 23.55 [52.00–153.33] | 74.78 ± 13.48 [50.67–105.67] | 0.001 |
| Heart rate (beat/min) | 107.43 ± 17.87 [58.60–137.20] | 102.49 ± 17.67 [64.25–128.00] | 110.27 ± 17.58 [58.60–137.20] | 0.096 |
| Stroke volume (mL/beat) | 64.25 ± 10.81 [35.00–86.90] | 63.87 ± 11.66 [39.00–86.90] | 64.47 ± 10.44 [35.00–86.00] | 0.835 |
| Fluid balance (mL/day) | 894.41 ± 1362.87 [−1656.0–3978.3] | 581.79 ± 1323.08 [−1656.0–3752.0] | 1078.78 ± 1368.99 [−1100.0–3978.3] | 0.167 |
| Blood Gas Analysis | ||||
| pH * | 7.28 ± 0.11 [7.04–7.50] | 7.33 ± 0.08 [7.12–7.45] | 7.25 ± 0.11 [7.04–7.50] | 0.002 |
| PaCO2 (mmHg) | 40.82 ± 14.93 [21.20–84.03] | 39.43 ± 10.58 [24.33–63.20] | 41.64 ± 17.06 [21.20–84.03] | 0.531 |
| PEtCO2 (mmHg) | 31.92 ± 8.03 [18.50–50.00] | 32.32 ± 8.23 [18.50–49.50] | 31.67 ± 8.03 [21.00–50.00] | 0.774 |
| PaO2 (mmHg) | 116.92 ± 51.79 [33.80–233.20] | 110.53 ± 56.67 [33.80–233.20] | 120.6 ± 49.14 [34.95–221.37] | 0.462 |
| HCO3 (mmol/L) | 20.87 ± 7.35 [8.60–48.80] | 20.18 ± 4.3 [12.70–27.00] | 21.26 ± 8.66 [8.60–48.80] | 0.511 |
| BE (meq/L) | −4.79 ± 6.73 [−16.70–20.80] | −5.21 ± 4.75 [−12.67–3.20] | −4.55 ± 7.69 [−16.70–20.80] | 0.708 |
| PAaO2 (mmHg) | 419.75 ± 146.44 [102.90–625.79] | 462.42 ± 117.62 [127.40–625.79] | 398.41 ± 155.89 [102.90–619.00] | 0.111 |
| Pulmonary Characteristics # | ||||
| Respiratory rate (breaths/min) | 22.21 ± 3.96 [14.80–31.82] | 21.96 ± 4.24 [14.80–29.50] | 22.35 ± 3.84 [17.00–31.82] | 0.711 |
| Tidal volume (mL) | 517.11 ± 78.25 [378.40–701.00] | 521.79 ± 77.93 [400.14–659.25] | 514.43 ± 79.29 [378.40–701.00] | 0.722 |
| Minute volume (L/min) | 11.73 ± 3.43 [6.63–20.73] | 11.63 ± 3.77 [7.00–20.73] | 11.78 ± 3.28 [6.63–19.75] | 0.870 |
| Peak pressure (cmH2O) | 26.76 ± 3.68 [20.00–36.00] | 26.60 ± 3.87 [20.83–36.00] | 26.86 ± 3.61 [20.00–36.00] | 0.792 |
| PaO2/FIO2 ratio | 148.94 ± 68.98 [43.69–298.82] | 132.24 ± 68.7 [48.29–298.82] | 158.55 ± 68.13 [43.69–298.82] | 0.146 |
| Variables | Area under the Curve (%) | (95% Confidence Interval) | p |
|---|---|---|---|
| Lactate a | 0.654 | 0.513–0.795 | 0.047 |
| WBC a | 0.612 | 0.468–0.757 | 0.147 |
| MAP b | 0.810 | 0.694–0.926 | 0.000 |
| pH b | 0.693 | 0.560–0.826 | 0.013 |
| Factors | Sensitivity (%) | Specificity (%) | NPV (%) | PPV (%) |
|---|---|---|---|---|
| Lactate > 51.8 | 53.85 | 77.27 | 48.57 | 80.77 |
| MAP ≤ 77.16 | 62.50 | 91.30 | 58.33 | 92.59 |
| pH ≤ 7.22 | 47.50 | 95.65 | 51.16 | 95.00 |
| Variables | Multivariate Analysis | |||
|---|---|---|---|---|
| Coefficient, Mean (SE) | Odds Ratio | (95% Confidence Interval) | p | |
| pH ≤ 7.22 | 0.877 (0.389) | 2.403 | 1.122–5.147 | 0.024 |
| MAP ≤ 77.16 | 1.121 (0.410) | 3.068 | 1.374–6.853 | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, C.-H.; Lan, Y.-W.; Chen, Y.-C.; Cheng, T.-T.; Yu, S.-F.; Cidem, A.; Liu, Y.-H.; Kuo, C.-W.; Yen, C.-C.; Chen, W.; et al. Effects of Mean Artery Pressure and Blood pH on Survival Rate of Patients with Acute Kidney Injury Combined with Acute Hypoxic Respiratory Failure: A Retrospective Study. Medicina 2021, 57, 1243. https://doi.org/10.3390/medicina57111243
Ko C-H, Lan Y-W, Chen Y-C, Cheng T-T, Yu S-F, Cidem A, Liu Y-H, Kuo C-W, Yen C-C, Chen W, et al. Effects of Mean Artery Pressure and Blood pH on Survival Rate of Patients with Acute Kidney Injury Combined with Acute Hypoxic Respiratory Failure: A Retrospective Study. Medicina. 2021; 57(11):1243. https://doi.org/10.3390/medicina57111243
Chicago/Turabian StyleKo, Chi-Hua, Ying-Wei Lan, Ying-Chou Chen, Tien-Tsai Cheng, Shan-Fu Yu, Abdulkadir Cidem, Yu-Hsien Liu, Chia-Wen Kuo, Chih-Ching Yen, Wei Chen, and et al. 2021. "Effects of Mean Artery Pressure and Blood pH on Survival Rate of Patients with Acute Kidney Injury Combined with Acute Hypoxic Respiratory Failure: A Retrospective Study" Medicina 57, no. 11: 1243. https://doi.org/10.3390/medicina57111243
APA StyleKo, C.-H., Lan, Y.-W., Chen, Y.-C., Cheng, T.-T., Yu, S.-F., Cidem, A., Liu, Y.-H., Kuo, C.-W., Yen, C.-C., Chen, W., & Chen, C.-M. (2021). Effects of Mean Artery Pressure and Blood pH on Survival Rate of Patients with Acute Kidney Injury Combined with Acute Hypoxic Respiratory Failure: A Retrospective Study. Medicina, 57(11), 1243. https://doi.org/10.3390/medicina57111243









