Correlations between PNPLA3 Gene Polymorphisms and NAFLD in Type 2 Diabetic Patients
Abstract
:1. Introduction
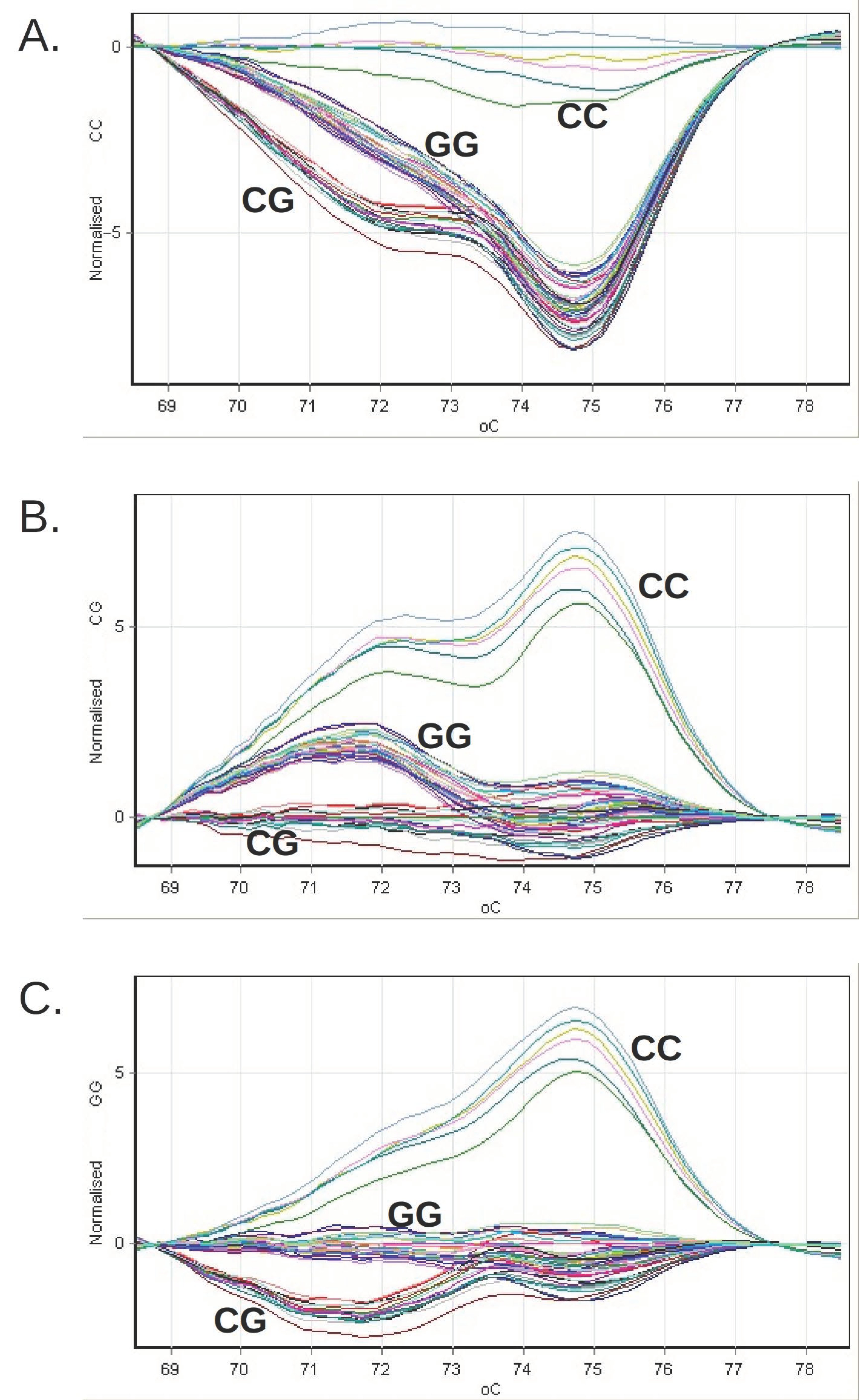
2. Materials and Methods
- Waist circumference (WC)—normal values for WC were <80 cm in women and <94 cm in men; values exceeding these limits led to the diagnosis of abdominal obesity;
- Blood pressure—previous diagnosis of hypertension or values higher than 130 mmHg systolic blood pressure or higher than 85 mmHg diastolic blood pressure at the time of the clinical examination;
- Hypertriglyceridemia (triglycerides >150 mg/dL);
- Low values of high-density lipoprotein cholesterol (HDLc) (<40 mg/dL in men, <50 mg/dL in women);
- Hyperglycemia or type 2 diabetes mellitus (all the subjects met this criteria).
- Three genotyping standards (CC, CG, GG), consisting of synthetic DNA molecules with a sequence that includes the genomic 46 bp region amplified with the help of the pair of primers used in the reaction;
- Three genotyping controls (rs738409 CC, CG, GG), consisting of genomic DNA sampled with a known PNPLA3 genotype; a negative amplification control, in which no DNA was introduced.
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matteoni, C.A.; Younossi, Z.M.; Gramlich, T.; Boparai, N.; Liu, Y.C.; McCullough, A.J. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology 1999, 116, 1413–1419. [Google Scholar] [CrossRef]
- Rinella, M.; Charlton, M. The globalization of nonalcoholic fatty liver disease: Prevalence and impact on world health. Hepatology 2016, 64, 19–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Sima, A.; Timar, R.; Vlad, A.; Timar, B.; Rosu, M.; Dan, I.; Sirli, R.; Popescu, A.; Sporea, I. Nonalcoholic fatty liver disease: A frequent condition in type 2 diabetic patients. Wien Klin. Wochenschr. 2014, 126, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Targher, G. Type 2 diabetes mellitus and risk of hepatocellular carcinoma: Spotlight on nonalcoholic fatty liver disease. Ann. Transl. Med. 2017, 5, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basaranoglu, M.; Canbakan, B.; Yildiz, K.; Ceylan, B.; Baysa, B.; Uysal, O.; Senturk, H. Nonalcoholic fatty liver may increase the risk of operation in patients with fatty liver and the frequency of cancer in their first-degree relatives. Wien Klin. Wochenschr. 2016, 128, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Cassader, M.; Pagano, G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann. Med. 2011, 43, 617–649. [Google Scholar] [CrossRef]
- Romeo, S.; Sanyal, A.; Valenti, L. Leveraging Human Genetics to Identify Potential New Treatments for Fatty Liver Disease. Cell Metab. 2020, 7, 35–45. [Google Scholar] [CrossRef]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef] [Green Version]
- Kienesberger, P.C.; Oberer, M.; Lass, A.; Zechner, R. Mammalian patatin domain containing proteins: A family with diverse lipolytic activities involved in multiple biological functions. J. Lipid. Res. 2009, 50, S63–S68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotronen, A.; Johansson, L.E.; Johansson, L.M.; Roos, C.; Westerbacka, J.; Hamsten, A.; Bergholm, R.; Arkkila, P.; Arola, J.; Kiviluoto, T.; et al. A common variant in PNPLA3, which encodes adiponutrin, is associated with liver fat content in humans. Diabetologia 2009, 52, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.M.; Mancuso, D.J.; Yan, W.; Sims, H.F.; Gibson, B.; Gross, R.W. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglyceroltransacylase activities. J. Biol. Chem. 2004, 279, 48968–48975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sookoian, S.; Pirola, C.J. PNPLA3, the triacylglycerol synthesis/hydrolysis/storage dilemma, and nonalcoholic fatty liver disease. World J. Gastroenterol. 2012, 18, 6018–6026. [Google Scholar] [CrossRef]
- Lake, A.C.; Sun, Y.; Li, J.L.; Kim, J.E.; Johnson, J.W.; Li, D.; Revett, T.; Shih, H.H.; Liu, W.; Paulsen, J.E.; et al. Expression, regulation, and triglyceride hydrolase activity of Adiponutrin family members. J. Lipid Res. 2005, 46, 2477–2487. [Google Scholar] [CrossRef] [Green Version]
- Chamoun, Z.; Vacca, F.; Parton, R.G.; Gruenberg, J. PNPLA3/adiponutrin functions in lipid droplet formation. Biol. Cell. 2013, 105, 219–233. [Google Scholar] [CrossRef]
- Ruhanen, H.; Perttila, J.; Holtta-Vuori, M.; Zhou, Y.; Yki-Jarvinen, H.; Ikonen, E.; Käkelä, R.; Olkkonen, V.M. PNPLA3 mediates hepatocyte triacylglycerol remodeling. J. Lipid Res. 2014, 55, 739–746. [Google Scholar] [CrossRef] [Green Version]
- He, S.; McPhaul, C.; Li, J.Z.; Garuti, R.; Kinch, L.; Grishin, N.V.; Cohen, J.C.; Hobbs, H.H. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J. Biol. Chem. 2010, 285, 6706–6715. [Google Scholar] [CrossRef] [Green Version]
- Anstee, Q.M.; Day, C.P. The genetics of NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 645–655. [Google Scholar] [CrossRef]
- Lazo, M.; Hernaez, R.; Eberhardt, M.S.; Bonekamp, S.; Kamel, I.; Guallar, E.; Koteish, A.; Brancati, F.L.; Clark, J.M. Prevalence of nonalcoholic fatty liver disease in the United States: The Third National Health and Nutrition Examination Survey, 1988-1994. Am. J. Epidemiol. 2013, 178, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Hernaez, R.; McLean, J.; Lazo, M.; Brancati, F.L.; Hirschhorn, J.N.; Borecki, I.B.; Harris, T.B.; Nguyen, T.; Kamel, I.R.; Bonekamp, S.; et al. Association between variants in or near PNPLA3, GCKR, and PPP1R3B with ultrasound-defined steatosis based on data from the third National Health and Nutrition Examination Survey. Clin. Gastroenterol. Hepatol. 2013, 11, 1183–1190. [Google Scholar] [CrossRef] [Green Version]
- Stender, S.; Kozlitina, J.; Nordestgaard, B.G.; Tybjaerg-Hansen, A.; Hobbs, H.H.; Cohen, J.C. Adiposity amplifies the genetic risk of fatty liver disease conferred by multiple loci. Nat. Genet. 2017, 49, 842–847. [Google Scholar] [CrossRef]
- Sookoian, S.; Castano, G.; Burgueno, A.L.; Gianotti, T.F.; Rosselli, M.S.; Pirola, C.J. A diagnostic model to differentiate simple steatosis from nonalcoholic steatohepatitis based on the likelihood ratio form of Bayes theorem. Clin. Biochem. 2009, 42, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Valenti, L.; Marchesini, G.; Di Marco, V.; Licata, A.; Camma, C.; Barcellona, M.R.; Cabibi, D.; Donati, B.; Fracanzani, A.; et al. PNPLA3 GG genotype and carotid atherosclerosis in patients with non-alcoholic fatty liver disease. PLoS ONE 2013, 8, e74089. [Google Scholar]
- Yki-Jarvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Tanoglu, A.; Beyazit, Y. Liver fatty acid-binding protein may be a useful marker for non-alcoholic fatty liver disease but obesity is a major concern. Wien Klin. Wochenschr. 2016, 128, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefan, N.; Häring, H.U.; Cusi, K. Non-alcoholic fatty liver disease: Causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019, 7, 313–324. [Google Scholar] [CrossRef]
- Stefan, N.; Fritsche, A.; Schick, F.; Häring, H.U. Phenotypes of prediabetes and stratification of cardiometabolic risk. Lancet Diabetes Endocrinol. 2016, 4, 789–798. [Google Scholar] [CrossRef]
- Sliz, E.; Sebert, S.; Wurtz, P.; Kangas, A.J.; Soininen, P.; Lehtimaki, T.; Kähönen, M.; Viikari, J.; Männikkö, M.; Ala-Korpela, M.; et al. NAFLD risk alleles in PNPLA3, TM6SF2, GCKR and LYPLAL1 show divergent metabolic effects. Hum. Mol. Genet. 2018, 27, 2214–2223. [Google Scholar] [CrossRef] [Green Version]
- Franko, A.; Merkel, D.; Kovarova, M.; Hoene, M.; Jaghutriz, B.A.; Heni, M.; Königsrainer, A.; Papan, C.; Lehr, S.; Häring, H.U.; et al. Dissociation of Fatty Liver and Insulin Resistance in I148M PNPLA3 Carriers: Differences in Diacylglycerol (DAG) FA18:1 Lipid Species as a Possible Explanation. Nutrients 2018, 10, 1314. [Google Scholar] [CrossRef] [Green Version]
- Kantartzis, K.; Peter, A.; Machicao, F.; Machann, J.; Wagner, S.; Königsrainer, I.; Königsrainer, A.; Schick, F.; Fritsche, A.; Häring, H.U.; et al. Dissociation between fatty liver and insulin resistance in humans carrying a variant of the patatin-like phospholipase 3 gene. Diabetes 2009, 58, 2616–2623. [Google Scholar] [CrossRef] [Green Version]
- Peter, A.; Kovarova, M.; Nadalin, S.; Cermak, T.; Königsrainer, A.; Machicao, F.; Stefan, N.; Häring, H.U.; Schleicher, E. PNPLA3 variant I148M is associated with altered hepatic lipid composition in humans. Diabetologia 2014, 57, 2103–2107. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Kojima, T.; Itoh, Y.; Harano, Y.; Fujii, K.; Nakajima, T.; Kato, T.; Takeda, N.; Okuda, J.; Ida, K.; et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am. J. Gastroenterol. 2007, 102, 2708–2715. [Google Scholar] [CrossRef]
- Randrianarisoa, E.; Rietig, R.; Jacob, S.; Blumenstock, G.; Haering, H.U.; Rittig, K.; Balletshofer, B. Normal values for intima-media thickness of the common carotid artery—An update following a novel risk factor profiling. Vasa 2015, 44, 444–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Hazlehurst, J.M.; Woods, C.; Marjot, T.; Cobbold, J.F.; Tomlinson, J.W. Non-alcoholic fatty liver disease and diabetes. Metabolism 2016, 65, 1096–1108. [Google Scholar] [CrossRef] [Green Version]
- Atan, N.A.D.; Koushki, M.; Motedayen, M.; Dousti, M.; Sayehmiri, F.; Vafaee, R.; Norouzinia, M.; Gholami, R. Type 2 diabetes mellitus and non-alcoholic fatty liver disease: A systematic review and meta-analysis. Gastroenterol. Hepatol. Bed. Bench. 2017, 10, S1–S7. [Google Scholar]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Trepo, E.; Romeo, S.; Zucman-Rossi, J.; Nahon, P. PNPLA3 gene in liver diseases. J. Hepatol. 2016, 65, 399–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speliotes, E.K.; Butler, J.L.; Palmer, C.D.; Voight, B.F.; Hirschhorn, J.N. PNPLA3 variants specifically confer increased risk for histologic nonalcoholic fatty liver disease but not metabolic disease. Hepatology 2010, 52, 904–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Touros, A.; Kim, W.R. Nonalcoholic fatty liver disease and metabolic syndrome. Clin. Liver Dis. 2018, 22, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020, 8, 616–627. [Google Scholar] [CrossRef]
| Parameters | Men (n = 44) | Women (n = 48) | Total (n = 92) | p Value * | |
|---|---|---|---|---|---|
| Age (years) [Mean ± SD; 95% CI] | 58.16 ± 11.04; 54.8–61.52 | 62.38 ± 9.27; 59.68–65.07 | 60.36 ± 10.32; 58.22–62.5 | 0.05 | |
| BMI (kg/m2) [Mean ± SD; 95% CI] | 30.43 ± 5.08; 28.89–31.97 | 32.66 ± 5.49; 31.07–34.26 | 31.59 ± 5.39; 30.48–32.71 | 0.046 | |
| WC (cm) [Mean ± SD; 95% CI] | 104.88 ± 11.33; 101.4–108.37 | 104.52 ± 12.56; 100.79–108.25 | 104.7 ± 11.92; 102.19–107.21 | 0.887 | |
| HOMA-IR [Mean ± SD; 95% CI] | 6.33 ± 3.938; 5.036–7.625 | 6.549 ± 3.708; 5.363–7.735 | 6.443 ± 3.799; 5.586–7.299 | 0.801 | |
| Metabolic syndrome present [n; %] | 33; 75 | 42; 87.5 | 75; 81.52 | 0.064 | |
| CIMT (mm) [Mean ± SD; 95% CI] | 0.999 ± 0.133; 0.958–1.04 | 1.001 ± 0.152; 0.957–1.045 | 1 ± 0.143; 0.97–1.03 | 0.942 | |
| Degree of steatosis | none [n; %] | 5; 11.37 | 4; 8.33 | 9; 9.78 | 0.703 |
| mild [n; %] | 9; 20.45 | 15; 31.25 | 24; 26.1 | ||
| moderate [n; %] | 17; 38.64 | 17; 35.42 | 34; 36.96 | ||
| severe [n; %] | 13; 29. 54 | 12; 25 | 25; 27.17 | ||
| Parameters | No Steatosis or Mild Steatosis (n = 33) | Moderate or Severe Steatosis (n = 59) | p Value * |
|---|---|---|---|
| Age (years) [Mean ± SD; 95% CI] | 59.85 ± 8.72; 56.76–62.94 | 60.41 ± 11.13; 57.49–63.34 | 0.802 |
| BMI (kg/m2) [Mean ± SD; 95% CI] | 32.2 ± 4.35; 30.66–33.74 | 31.26 ± 5.95; 29.69–32.82 | 0.151# |
| WC (cm) [Mean ± SD; 95% CI] | 104.97 ± 10.48; 101.12–108.81 | 104.58 ± 12.81; 101.18–107.98 | 0.885 |
| HOMA-IR [Mean ± SD; 95% CI] | 4.21 ± 2.454; 3.219–5.201 | 7.559 ± 3.877; 6.48–8.638 | <0.001 |
| Metabolic syndrome present [n; %] | 22; 66.67 | 53; 89.83 | 0.008 |
| CIMT (mm) [Mean ± SD; 95% CI] | 0.939 ± 0.109; 0.91–0.978 | 1.034 ± 0.149; 0.995–1.074 | 0.003 # |
| Parameters | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Presence of moderate or severe steatosis | 3.23 (1.21–8.63) | 0.019 | 3.06 (1.11–8.45) | 0.031 |
| Age (years) | 1.02 (0.98–1.07) | 0.303 | 1.02 (0.97–1.07) | 0.342 |
| Sex | 0.82 (0.35–1.92) | 0.644 | 1.003 (0.36–2.76) | 0.995 |
| BMI (kg/m2) | 1.03 (0.95–1.11) | 0.508 | 1.06 (0.886–1.27) | 0.53 |
| WC (cm) | 1.005 (0.97–1.042) | 0.776 | 0.99 (0.91–1.07) | 0.736 |
| Genotype | Men (n = 28) | Women (n = 40) | Total (n = 68) |
|---|---|---|---|
| CC [n; %] | 13; 46.4 | 25; 62.5 | 38; 55.9 |
| CG [n; %] | 12; 42.9 | 10; 25.0 | 22; 32.4 |
| GG [n; %] | 3; 10.7 | 5; 12.5 | 8; 11.8 |
| Geno-Type | Degree of Hepatic Steatosis | Total | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No Steatosis | Mild Steatosis | Moderate Steatosis | Severe Steatosis | ||||||||
| n | % | n | % | n | % | n | % | n | % | ||
| CC | 3 | 50 | 9 | 50 | 12 | 48 | 14 | 73.68 | 38 | 55.88 | 0.04 |
| CG | 3 | 50 | 9 | 50 | 6 | 24 | 4 | 21.05 | 22 | 32.35 | >0.05 |
| GG | 0 | 0 | 0 | 0 | 7 | 28 | 1 | 5.26 | 8 | 11.76 | 0.03 |
| Total | 6 | 8.82 | 18 | 26.47 | 25 | 36.76 | 19 | 27.94 | 68 | 100 | |
| Genotype | No or Mild Steatosis | Moderate or Severe Steatosis | Total | p | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| CC | 12 | 50 | 26 | 59,09 | 38 | 55.88 | 0.24 |
| CG | 12 | 50 | 10 | 22.73 | 22 | 32.35 | 0.01 |
| GG | 0 | 0 | 8 | 18.18 | 8 | 11.76 | 0.01 |
| Total | 24 | 35.29 | 44 | 64.71 | 68 | 100 | |
| Parameters | CC (n = 38) | CG (n= 22) | GG (n = 8) | p Value * |
|---|---|---|---|---|
| Age (years) [Mean ± SD; 95% CI] | 61.82 ± 9.85; 58.58–65.05 | 61.36 ± 7.68; 57.96–64.77 | 59.00 ± 10.74; 50.02–67.98 | 0.74 |
| BMI (kg/m2) [Mean ± SD; 95% CI] | 31.69 ± 5.44; 29.9–33.48 | 32.18 ± 4.85; 30.03–34.33 | 31.44 ± 4.91; 27.33–35.54 | 0.918 |
| WC (cm) [Mean ± SD; 95% CI] | 104.03 ± 12.68; 99.74–108.32 | 105.33 ± 10.98; 100.33–110.33 | 105.88 ± 11.02; 96.66–115.09 | 0.883 |
| HOMA-IR [Mean ± SD; 95% CI] | 6.89 ± 3.39; 5.69–8.1 | 6.28 ± 5.074; 3.83–8.73 | 6.72 ± 1.7; 5.3–8.14 | 0.859 |
| Metabolic syndrome present [n; %] | 32; 84.21 | 17; 77.27 | 8; 100 | 0.326 |
| CIMT (mm) [Mean ± SD; 95% CI] | 1.01 ± 0.15; 0.96–1.06 | 0.97 ± 0.12; 0.91–1.02 | 0.99 ± 0.14; 0.88–1.11 | 0.609 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavril, O.I.; Arhire, L.I.; Gavril, R.S.; Zota, M.I.; Gherasim, A.; Nita, O.; Drugescu, A.; Oprescu, A.C.; Esanu, I.M.; Mitu, F.; et al. Correlations between PNPLA3 Gene Polymorphisms and NAFLD in Type 2 Diabetic Patients. Medicina 2021, 57, 1249. https://doi.org/10.3390/medicina57111249
Gavril OI, Arhire LI, Gavril RS, Zota MI, Gherasim A, Nita O, Drugescu A, Oprescu AC, Esanu IM, Mitu F, et al. Correlations between PNPLA3 Gene Polymorphisms and NAFLD in Type 2 Diabetic Patients. Medicina. 2021; 57(11):1249. https://doi.org/10.3390/medicina57111249
Chicago/Turabian StyleGavril, Oana Irina, Lidia Iuliana Arhire, Radu Sebastian Gavril, Madalina Ioana Zota, Andreea Gherasim, Otilia Nita, Andrei Drugescu, Andrei Catalin Oprescu, Irina Mihaela Esanu, Florin Mitu, and et al. 2021. "Correlations between PNPLA3 Gene Polymorphisms and NAFLD in Type 2 Diabetic Patients" Medicina 57, no. 11: 1249. https://doi.org/10.3390/medicina57111249
APA StyleGavril, O. I., Arhire, L. I., Gavril, R. S., Zota, M. I., Gherasim, A., Nita, O., Drugescu, A., Oprescu, A. C., Esanu, I. M., Mitu, F., Graur, M., & Mihalache, L. (2021). Correlations between PNPLA3 Gene Polymorphisms and NAFLD in Type 2 Diabetic Patients. Medicina, 57(11), 1249. https://doi.org/10.3390/medicina57111249







