Nonbacterial Thrombotic Endocarditis Associated with Acute Promyelocytic Leukemia: An Autopsy Case Report
Abstract
:1. Introduction
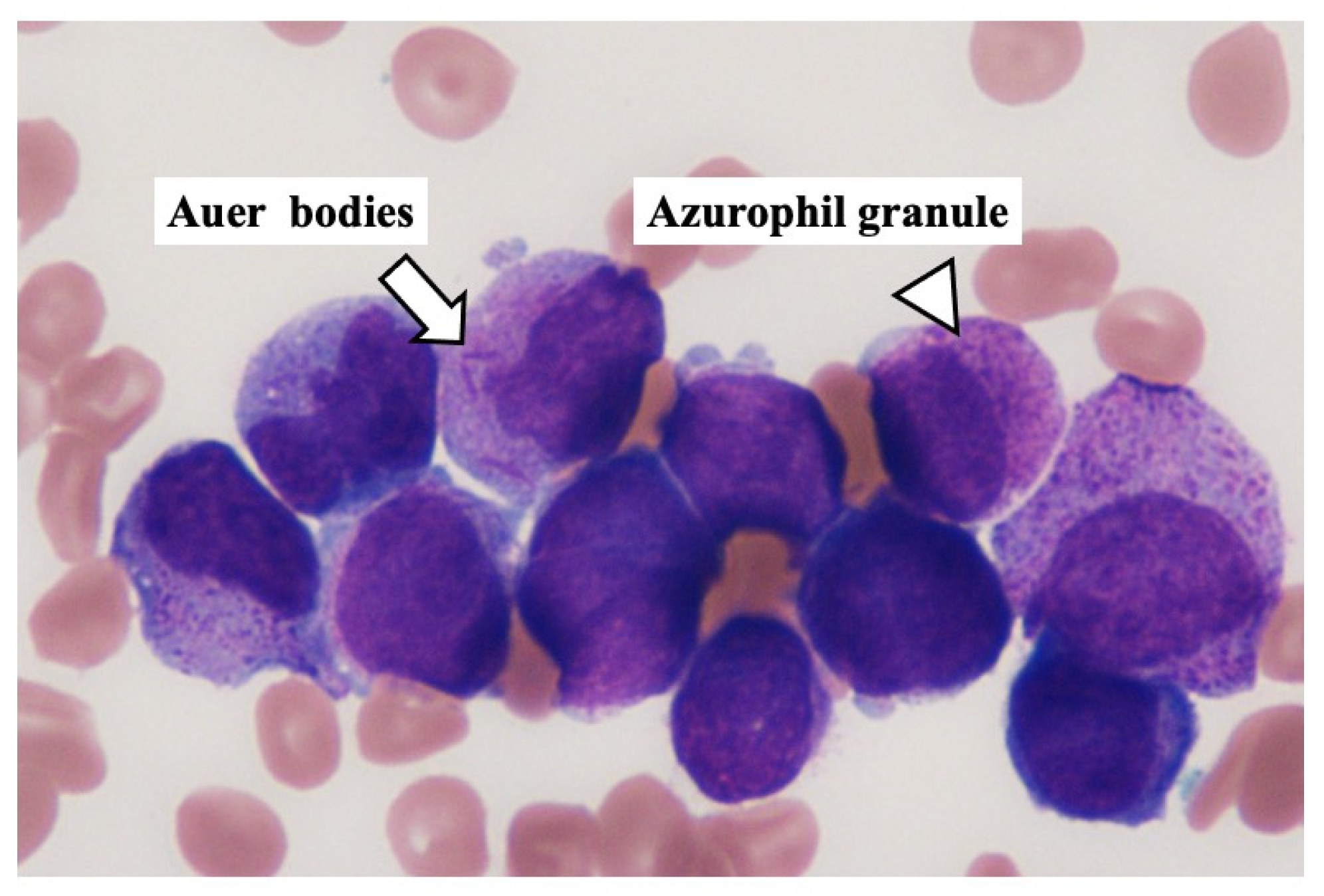
2. Case Presentation
3. Autopsy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asopa, S.; Patel, A.; Khan, O.A.; Sharma, R.; Ohri, S.K. Non-bacterial Thrombotic Endocarditis. Eur. J. Cardio-Thorac. Surg. 2007, 32, 696–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurrell, H.; Roberts-Thomson, R.; Prendergast, B.D. Non-infective Endocarditis. Heart 2020, 106, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.A.; Ross, R.R.; Fishbein, M.C.; Siegel, R.J. Nonbacterial Thrombotic Endocarditis: A Review. Am. Heart J. 1987, 113, 773–784. [Google Scholar] [CrossRef]
- Edoute, Y.; Haim, N.; Rinkevich, D.; Brenner, B.; Reisner, S.A. Cardiac Valvular Vegetations in Cancer Patients: A Prospective Echocardiographic Study of 200 patients. Am. J. Med. 1997, 102, 252–258. [Google Scholar] [CrossRef]
- Zhen, C.; Wang, Y.; Wang, H.; Li, D.; Wang, X. Multiple Cerebral Infarction Linked to Underlying Cancer: A Review of Trousseau Syndrome-related Cerebral Infarction. Br. J. Hosp. Med. 2021, 82, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.D.; Topcuoglu, M.A.; Buonanno, F.S. Acute Ischemic Stroke Patterns in Infective and Nonbacterial Thrombotic Endocarditis: A Diffusion-weighted Magnetic Resonance Imaging Study. Stroke 2002, 33, 1267–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Shami, K.; Griffiths, E.; Streiff, M. Nonbacterial Thrombotic Endocarditis in Cancer Patients: Pathogenesis, Diagnosis, and Treatment. Oncologist 2007, 12, 518–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zmaili, M.A.; Alzubi, J.M.; Kocyigit, D.; Bansal, A.; Samra, G.C.; Grimm, R.; Griffin, B.P.; Xu, B. A Contemporary 20-Year Cleveland Clinic Experience of Nonbacterial Thrombotic Endocarditis: Etiology, Echocardiographic Imaging, Management, and Outcomes. Am. J. Med. 2021, 134, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.G.; Jung, M.H.; Youn, H.J. Histologically Confirmed Nonbacterial Thrombotic Endocarditis in a Febrile Leukemic Patient. Korean J. Intern. Med. 2018, 33, 632–633. [Google Scholar] [CrossRef] [PubMed]
- Parker, N.; Atallah, R.; Ojile, N.; Chamoun, K.; Nehme, F.; Vindhyal, M. Nonbacterial Thrombotic Endocarditis. Kansas J. Med. 2020, 13, 61–62. [Google Scholar] [CrossRef]
- De Serna, J.; Montesinos, P.; Vellenga, E.; Rayón, C.; Parody, R.; León, A.; Esteve, J.; Bergua, J.M.; Milone, G.; Debén, G.; et al. Causes and Prognostic Factors of Remission Induction Failure in Patients with Acute Promyelocytic Leukemia Treated with All-trans retinoic acid and Idarubicin. Blood J. Am. Soc. Hematol. 2008, 111, 3395–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz, M.A.; Fenaux, P.; Tallman, M.S.; Estey, E.H.; Löwenberg, B.; Naoe, T.; Lengfelder, E.; Döhner, H.; Burnett, A.K.; Chen, S.; et al. Management of Acute Promyelocytic Leukemia: Updated Recommendations From an Expert Panel of the European LeukemiaNet. Blood 2019, 133, 1630–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuffer, L.; Koerber, M.I.; Nettersheim, F.; Tichelbaecker, T.; Hohmann, C.; Schaetzle, A.K.; Kabbasch, K.; Borrega, J.G.; Boell, B.; Kohle, F.; et al. Recovery of Non-bacterial Thrombotic Endocarditis and Severe Aortic Regurgitation in a Young Patient with Acute Promyelocytic Leukemia. Eur. Heart J. Cardiovasc. Imaging 2020, 21, jez319.314. [Google Scholar] [CrossRef]
- Kapelushnik, J.; Kafka, M.; Moser, A.; Yermiahu, T. Automated Differential Counts in Acute Promyelocytic Leukemia Patients May be Misleading. Med. Pediatr. Oncol. 2002, 38, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Graber, M.L.; Franklin, N.; Gordon, R. Diagnostic Error in Internal Medicine. Arch. Intern. Med. 2005, 165, 1493–1499. [Google Scholar] [CrossRef] [PubMed]


| Complete Blood Count | Results | (Normal Range) |
|---|---|---|
| White blood cells | 6.8 × 109/L | (3.59.9) |
| Hemoglobin | 11.0 g/dL | (11.0–14.8) |
| Mean corpuscular volume | 96.4/fL | (83–99) |
| Platelets | 100 × 109/L | (120–400) |
| Blood Coagulation Tests | ||
| PT% | 57% | (80–120) |
| APTT | 33.2 s | (24.0–39.0) |
| Biochemistry | ||
| Total protein | 6.7 g/dL | (6.5–8.2) |
| Albumin | 3.8 g/dL | (3.5–5.0) |
| Total bilirubin | 0.34 mg/dL | (0.2–1.2) |
| Glutamic oxaloacetic transaminase | 21 IU/L | (5–40) |
| Glutamic pyruvic transaminase | 12 IU/L | (3–35) |
| Lactate dehydrogenase | 401 IU/L | (124–222) |
| Blood urea nitrogen | 15.8 mg/dL | (8.0–23.0) |
| Creatinine | 0.75 mg/dL | (0.62–1.10) |
| Sodium | 140 mEq/L | (139–146) |
| Potassium | 3.7 mEq/L | (3.7–4.8) |
| Chloride | 106 mEq/L | (101–109) |
| Alkaline phosphatase | 203 IU/L | (38–113) |
| C-reactive protein | 3.09 mg/dL | (0.0–0.20) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimoto, T.; Aoki, T.; Kawabata, Y.; Owai, Y.; Matsuda, Y.; Tamura, S. Nonbacterial Thrombotic Endocarditis Associated with Acute Promyelocytic Leukemia: An Autopsy Case Report. Medicina 2021, 57, 1264. https://doi.org/10.3390/medicina57111264
Hashimoto T, Aoki T, Kawabata Y, Owai Y, Matsuda Y, Tamura S. Nonbacterial Thrombotic Endocarditis Associated with Acute Promyelocytic Leukemia: An Autopsy Case Report. Medicina. 2021; 57(11):1264. https://doi.org/10.3390/medicina57111264
Chicago/Turabian StyleHashimoto, Tadayuki, Tatsuya Aoki, Yoshitaka Kawabata, Yoshihiro Owai, Yoshikazu Matsuda, and Shinobu Tamura. 2021. "Nonbacterial Thrombotic Endocarditis Associated with Acute Promyelocytic Leukemia: An Autopsy Case Report" Medicina 57, no. 11: 1264. https://doi.org/10.3390/medicina57111264
APA StyleHashimoto, T., Aoki, T., Kawabata, Y., Owai, Y., Matsuda, Y., & Tamura, S. (2021). Nonbacterial Thrombotic Endocarditis Associated with Acute Promyelocytic Leukemia: An Autopsy Case Report. Medicina, 57(11), 1264. https://doi.org/10.3390/medicina57111264






