The Lymphatic System in Breast Cancer: Anatomical and Molecular Approaches
Abstract
:1. Introduction
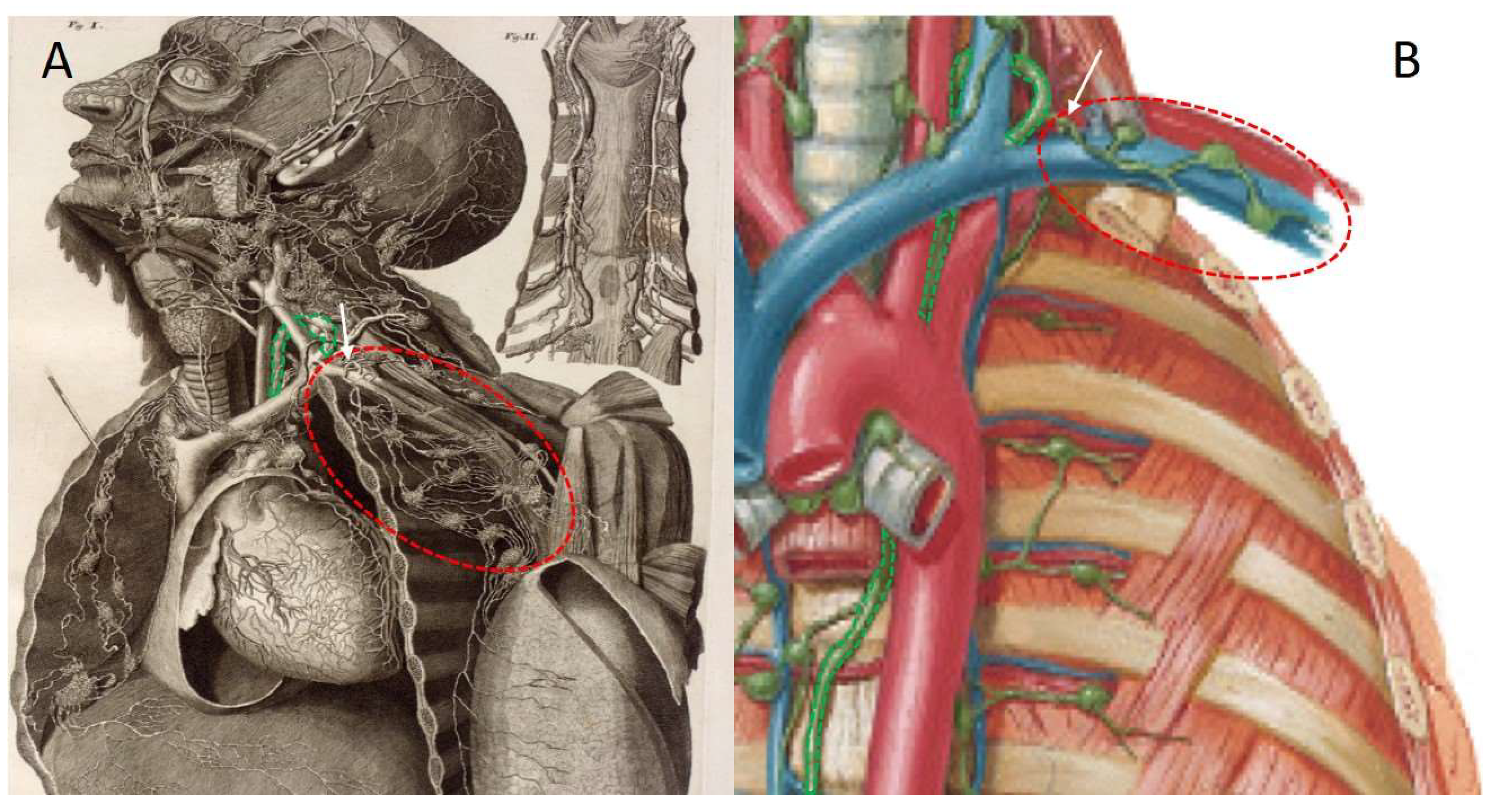
2. Techniques to Characterise Breast Lymphatic Anatomy
3. Molecular Insights in Breast Lymphatics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.S. Epidemiology of Breast Cancer in Women. Adv. Exp. Med. Biol. 2019, 1152, 9–29. [Google Scholar] [PubMed]
- Solanki, M.; Visscher, D. Pathology of breast cancer in the last half century. Hum. Pathol. 2020, 95, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Ekmektzoglou, K.A.; Xanthos, T.; German, V.; Zografos, G.C. Breast cancer: From the earliest times through to the end of the 20th century. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 145, 3–8. [Google Scholar] [CrossRef]
- Budh, D.P.; Sapra, A. Cancer Breast Screening; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Padera, T.P.; Meijer, E.F.; Munn, L.L. The Lymphatic System in Disease Processes and Cancer Progression. Annu. Rev. Biomed. Eng. 2016, 18, 125–158. [Google Scholar] [CrossRef] [Green Version]
- Aselli, G. De Lactibus Sive Lacteis Venis, Quarto Vasorum Mesaraicorum Genere Novo Invento Dissertatio Qua Sententiae Anatomicae Multae, Vel Perperam Receptae Convelluntur, Vel Parum Perceptae Illustrantu; Apud Jo. Baptistam Bidellium: Milan, Italy, 1627. [Google Scholar]
- Santambrogio, L. The Lymphatic Fluid. Int. Rev. Cell. Mol. Biol. 2018, 337, 111–133. [Google Scholar]
- Natale, G.; Bocci, G.; Ribatti, D. Scholars and scientists in the history of the lymphatic system. J. Anat. 2017, 231, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Irschick, R.; Siemon, C.; Brenner, E. The history of anatomical research of lymphatics—From the ancient times to the end of the European Renaissance. Ann. Anat. 2019, 223, 49–69. [Google Scholar] [CrossRef]
- Nuck, A. Adenographia Curiosa et Uteri Foeminei Anatome Nova; Apud Jord. Luchtmans: Ludgduni, Batavorum, 1696. [Google Scholar]
- Cruickshank, W.C. The Anatomy of the Absorbing Vessels of the Human Body; G. Nicol: London, UK, 1786. [Google Scholar]
- Mascagni, P. Vasorum Lymphaticorum Corporis Humani Historia et Ichnographia; Pazzini Carli: Siena, Italy, 1787. [Google Scholar]
- Mascagni, P. Anatomiae Universae Icones; Presso Nicola Capurro: Pisa, Italy, 1823–1831. [Google Scholar]
- Sappey, P.C. Anatomie, Physiologie, Pathologie des Vaisseaux Lymphatiques; Adrien Delahaye: Paris, France, 1874. [Google Scholar]
- Suami, H.; Pan, W.R.; Taylor, G.I. Historical review of breast lymphatic studies. Clin. Anat. 2009, 22, 531–536. [Google Scholar] [CrossRef]
- Gerota, D. Zur Technik der Lymphgefässinjektion. Eine neue Injektionsmasse für Lymphgefässe. Polychrome Injektion. Anat. Anzeiger. 1896, 12, 216–224. [Google Scholar]
- Poirier, P.; Cuneo, B. Les lymphatiques. In Traité D’Anatomie Humaine; Poirier, P., Charpy, A., Eds.; Libraires De L’Academie De Medecine: Paris, France, 1902. [Google Scholar]
- Bartels, P. Das lymphgefasssystem. In Handbuch der Anatomie des Menschen; Bardeleben, K., Ed.; G. Fisher: Jena, Germany, 1909; Volume 4. [Google Scholar]
- Suami, H.; Taylor, G.I.; Pan, W.R. A new radiographic cadaver injection technique for investigating the lymphatic system. Plast. Reconstr. Surg. 2005, 115, 2007–2013. [Google Scholar] [CrossRef]
- Suami, H.; Taylor, G.I.; O’Neill, J.; Pan, W.R. Refinements of the radiographic cadaver injection technique for investigating minute lymphatic vessels. Plast. Reconstr. Surg. 2007, 120, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Suami, H.; Pan, W.R.; Mann, G.B.; Taylor, G.I. The lymphatic anatomy of the breast and its implications for sentinel lymph node biopsy: A human cadaver study. Ann. Surg. Oncol. 2008, 15, 863–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suami, H.; O’Neill, J.K.; Pan, W.R.; Taylor, G.I. Superficial lymphatic system of the upper torso: Preliminary radiographic results in human cadavers. Plast. Reconstr. Surg. 2008, 121, 1231–1239. [Google Scholar] [CrossRef]
- Heydon-White, A.; Suami, H.; Boyages, J.; Koelmeyer, L.; Peebles, K.C. Assessing breast lymphoedema following breast cancer treatment using indocyanine green lymphography. Breast Cancer Res. Treat. 2020, 181, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Shinaoka, A.; Koshimune, S.; Yamada, K.; Kumagishi, K.; Suami, H.; Kimata, Y.; Ohtsuka, A. A Fresh Cadaver Study on Indocyanine Green Fluorescence Lymphography: A New Whole-Body Imaging Technique for Investigating the Superficial Lymphatics. Plast. Reconstr. Surg. 2018, 141, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.J. Axillary anatomy and history. Curr. Probl. Cancer. 2012, 36, 234–244. [Google Scholar] [CrossRef]
- Natale, G.; Bocci, G. Cardiovascular and Central Nervous System Toxicity by Anticancer Drugs in Breast Cancer Patients. In Brain and Heart Dynamics; Govoni, S., Politi, P., Vanoli, E., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 1–25. [Google Scholar]
- Louveau, A.; Plog, B.A.; Antila, S.; Alitalo, K.; Nedergaard, M.; Kipnis, J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J. Clin. Investig. 2017, 127, 3210–3219. [Google Scholar] [CrossRef] [Green Version]
- Natale, G.; Limanaqi, F.; Busceti, C.L.; Mastroiacovo, F.; Nicoletti, F.; Puglisi-Allegra, S.; Fornai, F. Glymphatic System as a Gateway to Connect Neurodegeneration from Periphery to CNS. Front. Neurosci. 2021, 15, 639140. [Google Scholar] [CrossRef]
- Suami, H.; Scaglioni, M.F. Anatomy of the Lymphatic System and the Lymphosome Concept with Reference to Lymphedema. Semin. Plast. Surg. 2018, 32, 5–11. [Google Scholar]
- Gashev, A.A.; Davis, M.J.; Zawieja, D.C. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J. Physiol. 2002, 540, 1023–1037. [Google Scholar] [CrossRef]
- Zawieja, D.C. Contractile physiology of lymphatics. Lymphat. Res. Biol. 2009, 7, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Kunert, C.; Baish, J.W.; Liao, S.; Padera, T.P.; Munn, L.L. Mechanobiological oscillators control lymph flow. Proc. Natl. Acad. Sci. USA 2015, 112, 10938–10943. [Google Scholar] [CrossRef] [Green Version]
- Scallan, J.P.; Zawieja, S.D.; Castorena-Gonzalez, J.A.; Davis, M.J. Lymphatic pumping: Mechanics, mechanisms and malfunction. J. Physiol. 2016, 594, 5749–5768. [Google Scholar] [CrossRef]
- Zhou, H.; Lei, P.J.; Padera, T.P. Progression of Metastasis through Lymphatic System. Cells 2021, 10, 627. [Google Scholar] [CrossRef]
- Nathanson, S.D.; Krag, D.; Kuerer, H.M.; Newman, L.A.; Brown, M.; Kerjaschki, D.; Pereira, E.R.; Padera, T.P. Breast cancer metastasis through the lympho-vascular system. Clin. Exp. Metastasis. 2018, 35, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Natale, G.; Bocci, G. Tumor Dormancy, Angiogenesis and Metronomic Chemotherapy. In Tumor Dormancy and Recurrence; Series: Cancer Drug Discovery and Development; Wang, Y., Crea, F., Eds.; Umana Press: New York, NY, USA, 2017; pp. 31–49. [Google Scholar]
- Natale, G.; Bocci, G.; Lenzi, P. Looking for the Word “Angiogenesis” in the History of Health Sciences: From Ancient Times to the First Decades of the Twentieth Century. World. J. Surg. 2017, 41, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Natale, G.; Bocci, G. Does metronomic chemotherapy induce tumor angiogenic dormancy? A review of available preclinical and clinical data. Cancer Lett. 2018, 432, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Karthik, G.M.; Alkodsi, A.; Kjällquist, U.; Stålhammar, G.; Lövrot, J.; Martinez, N.F.; Lagergren, J.; Hautaniemi, S.; Hartman, J.; et al. Evolutionary history of metastatic breast cancer reveals minimal seeding from axillary lymph nodes. J. Clin. Investig. 2018, 128, 1355–1370. [Google Scholar] [CrossRef] [Green Version]
- Natale, G.; Bocci, G. Discovery and development of the cardiovascular system with a focus on angiogenesis: A historical overview. It. J. Anat. Embryol. 2019, 124, 247–270. [Google Scholar]
- Lee, E.; Pandey, N.B.; Popel, A.S. Crosstalk between cancer cells and blood endothelial and lymphatic endothelial cells in tumour and organ microenvironment. Expert Rev. Mol. Med. 2015, 17, e3. [Google Scholar] [CrossRef]
- Gerull, W.D.; Puri, V.; Kozower, B.D. The epidemiology and biology of pulmonary metastases. J. Thorac. Dis. 2021, 13, 2585–2589. [Google Scholar] [CrossRef]
- Castle, J.; Shaker, H.; Morris, K.; Tugwood, J.D.; Kirwan, C.C. The significance of circulating tumour cells in breast cancer: A review. Breast 2014, 23, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Kalinkova, L.; Zmetakova, I.; Smolkova, B.; Minarik, G.; Sedlackova, T.; Horvathova Kajabova, V.; Cierna, Z.; Mego, M.; Fridrichova, I. Decreased methylation in the SNAI2 and ADAM23 genes associated with de-differentiation and haematogenous dissemination in breast cancers. BMC Cancer 2018, 18, 875. [Google Scholar] [CrossRef]
- Sharma, U.; Medina-Saenz, K.; Miller, P.C.; Troness, B.; Spartz, A.; Sandoval-Leon, A.; Parke, D.N.; Seagroves, T.N.; Lippman, M.E.; El-Ashry, D. Heterotypic clustering of circulating tumor cells and circulating cancer-associated fibroblasts facilitates breast cancer metastasis. Breast Cancer Res. Treat. 2021, 189, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Miura, H.; Seino, H.; Ono, S.; Nishi, T.; Nishimura, A.; Hakamada, K.; Aoki, M. Anatomical classification of breast sentinel lymph nodes using computed tomography-lymphography. Anat. Sci. Int. 2018, 93, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yi, S.; Zhang, D.; Gong, M.; Cai, Y.; Zou, L. Intratumoral and peritumoral lymphatic vessel density both correlate with lymph node metastasis in breast cancer. Sci. Rep. 2017, 7, 40364. [Google Scholar] [CrossRef] [PubMed]
- Skobe, M.; Hawighorst, T.; Jackson, D.G.; Prevo, R.; Janes, L.; Velasco, P.; Riccardi, L.; Alitalo, K.; Claffey, K.; Detmar, M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat. Med. 2001, 7, 192–198. [Google Scholar] [CrossRef]
- Mohammed, R.A.A.; Green, A.; El-Shikh, S.; Paish, E.C.; Ellis, I.O.; Martin, S.G. Prognostic significance of vascular endothelial cell growth factors -A, -C and -D in breast cancer and their relationship with angio- and lymphangiogenesis. Br. J. Cancer. 2007, 96, 1092–1100. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Qi, X.; Guo, S. Lymphangiogenesis induced by VEGF-C and VEGF-D promotes metastasis and a poor outcome in breast carcinoma: A retrospective study of 61 cases. Clin. Exp. Metastasis 2008, 25, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Isaac, D.; Andrechek, E.R. Studying Lymphatic Metastasis in Breast Cancer: Current Models, Strategies, and Clinical Perspectives. J. Mammary Gland Biol. Neoplasia 2020, 25, 191–203. [Google Scholar]
- Uren, R.F.; Howman-Giles, R.; Renwick, S.B.; Gillett, D. Lymphatic mapping of the breast: Locating the sentinel lymph nodes. World J. Surg. 2001, 25, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, H.; Wang, S.; Cao, Y.; Liu, M.; Xie, F.; Liu, P.; Zhou, B.; Tong, F.; Cheng, L.; et al. Comparison of sentinel lymph node biopsy guided by indocyanine green, blue dye, and their combination in breast cancer patients: A prospective cohort study. World J. Surg. Oncol. 2017, 15, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballardini, B.; Santoro, L.; Sangalli, C.; Gentilini, O.; Renne, G.; Lissidini, G.; Pagani, G.M.; Toesca, A.; Blundo, C.; del Castillo, A.; et al. The indocyanine green method is equivalent to the (9)(9)mTc-labeled radiotracer method for identifying the sentinel node in breast cancer: A concordance and validation study. Eur. J. Surg. Oncol. 2013, 39, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.W.; Masia, J.; Garza, R., 3rd; Skoracki, R.; Neligan, P.C. Lymphedema: Surgical and Medical Therapy. Plast. Reconstr. Surg. 2016, 138, 209s–218s. [Google Scholar] [CrossRef]
- Turner-Warwick, R.T. The lymphatics of the breast. Br. J. Surg. 1959, 46, 574–582. [Google Scholar] [CrossRef]
- Cloquet, J. Manuel D’Anatomie Descriptive du Corps Humain, Représentée en Planches Litographiées; Chez Béchet Jeune: Paris, France, 1825. [Google Scholar]
- The Lymphatic System, Considered in Relation to Its Anatomy, Physiology, and Pathology. Br. Foreign Med. Rev. 1837, 4, 325–349.
- Stouthandel, M.E.J.; Veldeman, L.; Van Hoof, T. Call for a Multidisciplinary Effort to Map the Lymphatic System with Advanced Medical Imaging Techniques: A Review of the Literature and Suggestions for Future Anatomical Research. Anat. Rec. 2019, 302, 1681–1695. [Google Scholar] [CrossRef]
- Martinez-Monge, R.; Fernandes, P.S.; Gupta, N.; Gahbauer, R. Cross-sectional nodal atlas: A tool for the definition of clinical target volumes in three-dimensional radiation therapy planning. Radiology 1999, 211, 815–828. [Google Scholar] [CrossRef]
- Madu, C.N.; Quint, D.J.; Normolle, D.P.; Marsh, R.B.; Wang, E.Y.; Pierce, L.J. Definition of the supraclavicular and infraclavicular nodes: Implications for three-dimensional CT-based conformal radiation therapy. Radiology 2001, 221, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Verhoeven, K.; Weltens, C.; Remouchamps, V.; Mahjoubi, K.; Veldeman, L.; Lengele, B.; Hortobagyi, E.; Kirkove, C. Vessel based delineation guidelines for the elective lymph node regions in breast cancer radiation therapy—PROCAB guidelines. Radiother. Oncol. 2015, 114, 11–16. [Google Scholar] [CrossRef]
- Offersen, B.V.; Boersma, L.J.; Kirkove, C.; Hol, S.; Aznar, M.C.; Biete Sola, A.; Kirova, Y.M.; Pignol, J.P.; Remouchamps, V.; Verhoeven, K.; et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early-stage breast cancer. Radiother. Oncol. 2015, 114, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Budach, W.; Kammers, K.; Boelke, E.; Matuschek, C. Adjuvant radiotherapy of regional lymph nodes in breast cancer—A meta-analysis of randomized trials. Radiat. Oncol. 2013, 8, 267. [Google Scholar] [CrossRef] [Green Version]
- Poortmans, P.M.; Weltens, C.; Fortpied, C.; Kirkove, C.; Peignaux-Casasnovas, K.; Budach, V.; van der Leij, F.; Vonk, E.; Weidner, N.; Rivera, S.; et al. Internal mammary and medial supraclavicular lymph node chain irradiation in stage I-III breast cancer (EORTC 22922/10925): 15-year results of a randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1602–1610. [Google Scholar] [CrossRef]
- Ozcan, L.C.; Giuliano, A.E. Is Axillary Lymph Node Dissection Necessary After a Positive Sentinel Lymph Node Biopsy? Adv. Surg. 2017, 51, 165–178. [Google Scholar] [CrossRef]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grantzau, T.; Thomsen, M.S.; Væth, M.; Overgaard, J. Risk of second primary lung cancer in women after radiotherapy for breast cancer. Radiother. Oncol. 2014, 111, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.R. A novel approach to slow down putrefaction of unembalmed cadaveric tissue during lymphatic dissection: A preliminary study. Lymphat. Res. Biol. 2009, 7, 17–20. [Google Scholar] [CrossRef]
- Dobson, J.; Tompsett, D.H. Museum specimens of the main superficial and deep lymphatics of the leg in man. Ann. R. Coll. Surg. Engl. 1968, 43, 111–117. [Google Scholar] [PubMed]
- Stouthandel, M.E.J.; Veldeman, L.; Achten, E.; Van Hoof, T. The use of Thiel embalmed human cadavers for retrograde injection and visualization of the lymphatic system. Anat. Rec. 2020, 303, 2392–2401. [Google Scholar] [CrossRef]
- Augur, A.M.R.; Dalley, A.F.; Grant, J.C.B. Grant’s Atlas of Anatomy, 11th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Netter, F.H. Netter’s Atlas of Human Anatomy, 5th ed.; Saunders Elsevier: Philadelphia, PA, USA, 2011. [Google Scholar]
- Putz, R.; Pabst, R. Sobotta Atlas of Human Anatomy: Head, Neck, Upper Limb, Thorax, Abdomen, Pelvis, Lower Limb, 14th ed.; Elsevier GmbH: Munich, Germany, 2009. [Google Scholar]
- Standring, S. Cardiovascular topography of lymph nodes and vessels. In Gray’s Anatomy: The Anatomical Basis of Clinical Practice; Ellis, H., Ed.; Chuchill Livingstone: Edinburgh, UK, 2004. [Google Scholar]
- Stouthandel, M.E.J.; Debbaut, C.; Deviche, J.; Truyens, B.; Veldeman, L.; Van Hoof, T. Using the venous angle as a pressure reservoir to retrogradely fill the subclavian lymphatic trunk with contrast agent for lymphatic mapping. Ann. Anat. 2020, 232, 151562. [Google Scholar] [CrossRef] [PubMed]
- Peeters, G.; Debbaut, C.; Laleman, W.; Monbaliu, D.; Vander Elst, I.; Detrez, J.R.; Vandecasteele, T.; De Schryver, T.; Van Hoorebeke, L.; Favere, K.; et al. A multilevel framework to reconstruct anatomical 3D models of the hepatic vasculature in rat livers. J. Anat. 2017, 230, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Cornillie, P.; Casteleyn, C.; von Horst, C.; Henry, R. Corrosion casting in anatomy: Visualizing the architecture of hollow structures and surface details. Anat. Histol. Embryol. 2019, 48, 591–604. [Google Scholar] [CrossRef] [Green Version]
- Thiel, W. The preservation of the whole corpse with natural color. Ann. Anat. 1992, 174, 185–195. [Google Scholar] [CrossRef]
- Ruiz-Tovar, J.; Prieto-Nieto, I.; García-Olmo, D.; Clascá, F.; Enriquez, P.; Villalonga, R.; Zubiaga, L. Training Courses in Laparoscopic Bariatric Surgery on Cadaver Thiel: Results of a Satisfaction Survey on Students and Professors. Obes. Surg. 2019, 29, 3465–3470. [Google Scholar] [CrossRef]
- Charbonney, E.; Delisle, S.; Savary, D.; Bronchti, G.; Rigollot, M.; Drouet, A.; Badat, B.; Ouellet, P.; Gosselin, P.; Mercat, A.; et al. A new physiological model for studying the effect of chest compression and ventilation during cardiopulmonary resuscitation: The Thiel cadaver. Resuscitation 2018, 125, 135–142. [Google Scholar] [CrossRef] [PubMed]
- De Crop, A.; Bacher, K.; Van Hoof, T.; Smeets, P.V.; Smet, B.S.; Vergauwen, M.; Kiendys, U.; Duyck, P.; Verstraete, K.; D’Herde, K.; et al. Correlation of contrast-detail analysis and clinical image quality assessment in chest radiography with a human cadaver study. Radiology 2012, 262, 298–304. [Google Scholar] [CrossRef]
- Beger, O.; Karagül, M.İ.; Koç, T.; Kayan, G.; Cengiz, A.; Yılmaz, Ş.N.; Olgunus, Z.K. Effects of different cadaver preservation methods on muscles and tendons: A morphometric, biomechanical and histological study. Anat. Sci. Int. 2020, 95, 174–189. [Google Scholar] [CrossRef]
- Stouthandel, M.E.J.; Vanhove, C.; Devriendt, W.; De Bock, S.; Debbaut, C.; Vangestel, C.; Van Hoof, T. Biomechanical comparison of Thiel embalmed and fresh frozen nerve tissue. Anat. Sci. Int. 2020, 95, 399–407. [Google Scholar] [CrossRef]
- Anderson, S.D. Practical light embalming technique for use in the surgical fresh tissue dissection laboratory. Clin. Anat. 2006, 19, 8–11. [Google Scholar] [CrossRef]
- Kingston, M.J.; Perriman, D.M.; Neeman, T.; Smith, P.N.; Webb, A.L. Contrast agent comparison for three-dimensional micro-CT angiography: A cadaveric study. Contrast Media Mol. Imaging 2016, 11, 319–324. [Google Scholar] [CrossRef]
- Stouthandel, M.E.J.; Pullens, P.; Bogaert, S.; Schoepen, M.; Vangestel, C.; Achten, E.; Veldeman, L.; Van Hoof, T. The application of frozen Thiel-embalmed specimens for radiotherapy delineation guideline development: A method to create accurate MRI-enhanced CT datasets. Strahlenther. Onkol. 2021. submitted. [Google Scholar]
- Jana, S.; Muscarella, R.A., Jr.; Jones, D. The Multifaceted Effects of Breast Cancer on Tumor-Draining Lymph Nodes. Am. J. Pathol. 2021, 191, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Cunnick, G.H.; Jiang, W.G.; Douglas-Jones, T.; Watkins, G.; Gomez, K.F.; Morgan, M.J.; Subramanian, A.; Mokbel, K.; Mansel, R.E. Lymphangiogenesis and lymph node metastasis in breast cancer. Mol. Cancer. 2008, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Shields, J.D.; Fleury, M.E.; Yong, C.; Tomei, A.A.; Randolph, G.J.; Swartz, M.A. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell. 2007, 11, 526–538. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Zhang, W.; Wang, C.; Hu, L.; Wang, R.; Wang, C.; Tang, L.; Zhou, G.; Zou, B.; Xie, H.; et al. Integrative analyses of scRNA-seq and scATAC-seq reveal CXCL14 as a key regulator of lymph node metastasis in breast cancer. Hum. Mol. Genet. 2021, 30, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Secker, G.A.; Harvey, N.L. Regulation of VEGFR Signalling in Lymphatic Vascular Development and Disease: An Update. Int. J. Mol. Sci. 2021, 22, 7760. [Google Scholar] [CrossRef]
- Zhang, Y.; Ulvmar, M.H.; Stanczuk, L.; Martinez-Corral, I.; Frye, M.; Alitalo, K.; Makinen, T. Heterogeneity in VEGFR3 levels drives lymphatic vessel hyperplasia through cell-autonomous and non-cell-autonomous mechanisms. Nat. Commun. 2018, 9, 1296. [Google Scholar] [CrossRef] [PubMed]
- García-Caballero, M.; Paupert, J.; Blacher, S.; Van de Velde, M.; Quesada, A.R.; Medina, M.A.; Noël, A. Targeting VEGFR-3/-2 signaling pathways with AD0157: A potential strategy against tumor-associated lymphangiogenesis and lymphatic metastases. J. Hematol. Oncol. 2017, 10, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, L.L.; Yang, N.Z.; Shi, L.H.; Zhao, G.H.; Zhou, W.; Ding, Q.; Wang, M.H.; Zhang, Y.S. The optimum marker for the detection of lymphatic vessels. Mol. Clin. Oncol. 2017, 7, 515–520. [Google Scholar] [CrossRef] [Green Version]
- Lokmic, Z. Utilizing lymphatic cell markers to visualize human lymphatic abnormalities. J. Biophotonics 2018, 11, e201700117. [Google Scholar] [CrossRef] [Green Version]
- Kahn, H.J.; Marks, A. A new monoclonal antibody, D2-40, for detection of lymphatic invasion in primary tumors. Lab. Investig. 2002, 82, 1255–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Q.; Chen, S.; An, Q.; Li, B.; Fu, Y.; Luo, Y. Extracellular Hsp90α Promotes Tumor Lymphangiogenesis and Lymph Node Metastasis in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 7747. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, S.; Tian, Y.; Ju, A.; Hou, Q.; Liu, J.; Fu, Y.; Luo, Y. Lysyl Oxidase-Like Protein 2 Promotes Tumor Lymphangiogenesis and Lymph Node Metastasis in Breast Cancer. Neoplasia 2019, 21, 413–427. [Google Scholar] [CrossRef]
- Kim, M.S.; Lebron, C.; Nagpal, J.K.; Chae, Y.K.; Chang, X.; Huang, Y.; Chuang, T.; Yamashita, K.; Trink, B.; Ratovitski, E.A.; et al. Methylation of the DFNA5 increases risk of lymph node metastasis in human breast cancer. Biochem. Biophys. Res. Commun. 2008, 370, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.P.; Kim, S.; Nam, S.J.; Kim, I.; Bae, J.W. The role of the CDH1 promoter hypermethylation in the axillary lymph node metastasis and prognosis. J. Breast Cancer 2013, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Jones, D. Parallels of Resistance between Angiogenesis and Lymphangiogenesis Inhibition in Cancer Therapy. Cells 2020, 9, 762. [Google Scholar] [CrossRef] [Green Version]
- Qin, T.; Liu, Z.; Wang, J.; Xia, J.; Liu, S.; Jia, Y.; Liu, H.; Li, K. Anlotinib suppresses lymphangiogenesis and lymphatic metastasis in lung adenocarcinoma through a process potentially involving VEGFR-3 signaling. Cancer Biol. Med. 2020, 17, 753–767. [Google Scholar] [CrossRef]
- Tai, H.C.; Lee, T.H.; Tang, C.H.; Chen, L.P.; Chen, W.C.; Lee, M.S.; Chen, P.C.; Lin, C.Y.; Chi, C.W.; Chen, Y.J.; et al. Phomaketide A Inhibits Lymphangiogenesis in Human Lymphatic Endothelial Cells. Mar. Drugs 2019, 17, 215. [Google Scholar] [CrossRef] [Green Version]
- Blumgart, E.I.; Uren, R.F.; Nielsen, P.M.; Nash, M.P.; Reynolds, H.M. Predicting lymphatic drainage patterns and primary tumour location in patients with breast cancer. Breast Cancer Res. Treat. 2011, 130, 699–705. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natale, G.; Stouthandel, M.E.J.; Van Hoof, T.; Bocci, G. The Lymphatic System in Breast Cancer: Anatomical and Molecular Approaches. Medicina 2021, 57, 1272. https://doi.org/10.3390/medicina57111272
Natale G, Stouthandel MEJ, Van Hoof T, Bocci G. The Lymphatic System in Breast Cancer: Anatomical and Molecular Approaches. Medicina. 2021; 57(11):1272. https://doi.org/10.3390/medicina57111272
Chicago/Turabian StyleNatale, Gianfranco, Michael E. J. Stouthandel, Tom Van Hoof, and Guido Bocci. 2021. "The Lymphatic System in Breast Cancer: Anatomical and Molecular Approaches" Medicina 57, no. 11: 1272. https://doi.org/10.3390/medicina57111272
APA StyleNatale, G., Stouthandel, M. E. J., Van Hoof, T., & Bocci, G. (2021). The Lymphatic System in Breast Cancer: Anatomical and Molecular Approaches. Medicina, 57(11), 1272. https://doi.org/10.3390/medicina57111272







