Outcomes and Risk Factors of Critically Ill Patients with Hematological Malignancy. Prospective Single-Centre Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Research Data
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lithuanian Ministry of Health Health Information Centre of Institute of Hygiene. Health Statistics of Lithuania 2019. Available online: https://www.hi.lt/uploads/pdf/leidiniai/Statistikos/LT_sveik_stat_health/la_2019.pdf (accessed on 23 October 2021).
- National Cancer Registry. Newly Diagnosed Malignancies in Lithuania 2015. Male and Female. Available online: https://www.nvi.lt/naujausi-duomenys/ (accessed on 23 October 2021).
- Cetintepe, T.; Cetintepe, L.; Solmaz, S.; Calık, S.; Ugur, M.C.; Gediz, F.; Bilgir, O. Determination of the relationship between mortality and SOFA, qSOFA, MASCC scores in febrile neutropenic patients monitored in the intensive care unit. Support Care Cancer 2021, 29, 4089–4094. [Google Scholar] [CrossRef]
- Hill, Q.A. Intensify, resuscitate or palliate: Decision making in the critically ill patient with haematological malignancy. Blood Rev. 2010, 24, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Oeyen, S.G.; Benoit, D.D.; Annemans, L.; Depuydt, P.O.; Van Belle, S.J.; Troisi, R.I.; Noens, L.A.; Pattyn, P.; Decruyenaere, J.M. Long-term outcomes and quality of life in critically ill patients with hematological or solid malignancies: A single center study. Intensive Care Med. 2013, 39, 889–898. [Google Scholar] [CrossRef]
- Pulte, D.; Jansen, L.; Brenner, H. Changes in long term survival after diagnosis with common hematologic malignancies in the early 21st century. Blood Cancer J. 2020, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Pulte, D.; Jansen, L.; Castro, F.A.; Emrich, K.; Katalinic, A.; Holleczek, B.; Brenner, H.; GEKID Cancer Survival Working Group. Trends in survival of multiple myeloma patients in Germany and the United States in the first decade of the 21st century. Br. J. Haematol. 2015, 171, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Khoury, H.J.; Wang, T.; Hemmer, M.T.; Couriel, D.; Alousi, A.; Cutler, C.; Aljurf, M.; Antin, J.H.; Ayas, M.; Battiwalla, M.; et al. Improved survival after acute graft-versus-host disease diagnosis in the modern era. Haematologica 2017, 102, 958–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darmon, M.; Bourmaud, A.; Georges, Q.; Soares, M.; Jeon, K.; Oeyen, S.; Rhee, C.K.; Gruber, P.; Ostermann, M.; Hill, Q.A.; et al. Changes in critically ill cancer patients’ short-term outcome over the last decades: Results of systematic review with meta-analysis on individual data. Intensive Care Med. 2019, 45, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Kalicińska, E.; Kuszczak, B.; Dębski, J.; Szukalski, Ł.; Wątek, M.; Strzała, J.; Rybka, J.; Czyż, J.; Lech-Marańda, E.; Zaucha, J.; et al. Hematological malignancies in Polish population: What are the predictors of outcome in patients admitted to Intensive Care Unit? Support Care Cancer 2021, 29, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Grgić Medić, M.; Gornik, I.; Gašparović, V. Hematologic malignancies in the medical intensive care unit—Outcomes and prognostic factors. Hematology 2015, 20, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Mounier, M.; Bossard, N.; Remontet, L.; Belot, A.; Minicozzi, P.; de Angelis, R.; Capocaccia, R.; Iwaz, J.; Monnereau, A.; Troussard, X.; et al. Changes in dynamics of excess mortality rates and net survival after diagnosis of follicular lymphoma or diffuse large B-cell lymphoma: Comparison between European population-based data (EUROCARE-5). Lancet Haematol. 2015, 2, e481–e491. [Google Scholar] [CrossRef]
- Minicozzi, P.; Walsh, P.M.; Sánchez, M.-J.; Trama, A.; Innos, K.; Marcos-Gragera, R.; Dimitrova, N.; Botta, L.; Johannesen, T.B.; Rossi, S.; et al. Is low survival for cancer in Eastern Europe due principally to late stage at diagnosis? Eur. J. Cancer 2018, 93, 127–137. [Google Scholar] [CrossRef]
- Organization for Economic Co-Operation and Development Health Spending. 2021. Available online: https://data.oecd.org/healthres/health-spending.htm (accessed on 21 November 2021). [CrossRef]
- Tambor, M.; Klich, J.; Domagała, A. Financing healthcare in Central and Eastern European countries: How far are we from universal health coverage? Int. J. Environ. Res. Public Health 2021, 18, 1382. [Google Scholar] [CrossRef]
- Passweg, J.R.; Baldomero, H.; Chabannon, C.; Basak, G.W.; Corbacioglu, S.; Duarte, R.; Dolstra, H.; Lankester, A.C.; Mohty, M.; Montoto, S.; et al. The EBMT activity survey on hematopoietic-cell transplantation and cellular therapy 2018: CAR-T’s come into focus. Bone Marrow Transplant. 2020, 55, 1604–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Azoulay, E.; Mokart, D.; Pène, F.; Lambert, J.; Kouatchet, A.; Mayaux, J.; Vincent, F.; Nyunga, M.; Bruneel, F.; Laisne, L.-M.; et al. Outcomes of critically ill patients with hematologic malignancies: Prospective multicenter data from France and Belgium—A groupe de recherche respiratoire en réanimation onco-hématologique study. J. Clin. Oncol. 2013, 31, 2810–2818. [Google Scholar] [CrossRef] [PubMed]
- Pichereau, C.; Lengliné, E.; Valade, S.; Michonneau, D.; Ghrenassia, E.; Lemiale, V.; Socié, G.; Azoulay, E. Trajectories of acute graft-versus-host disease and mortality in critically ill allogeneic-hematopoietic stem cell recipients: The Allo-GRRR-OH score. Bone Marrow Transplant. 2020, 55, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. Available online: https://link.springer.com/10.1007/s00134-021-06506-y (accessed on 24 October 2021). [CrossRef] [PubMed]
- Lappalainen, M.; Hämäläinen, S.; Romppanen, T.; Pulkki, K.; Pyörälä, M.; Koivula, I.; Jantunen, E.; Juutilainen, A. Febrile neutropenia in patients with acute myeloid leukemia: Outcome in relation to qSOFA score, C-reactive protein, and blood culture findings. Eur. J. Haematol. 2020, 105, 731–740. [Google Scholar] [CrossRef]
- Koh, T.L.; Canet, E.; Amjad, S.; Bellomo, R.; Taylor, D.; Gan, H.K.; Marhoon, N.; Lim, A.; Ong, W.L.; Krishnan, V.; et al. Prognostic performance of qSOFA in oncology patients admitted to the emergency department with suspected infection. Asia Pac. J. Clin. Oncol. 2021, 17, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Chae, B.-R.; Kim, Y.-J.; Lee, Y.-S. Prognostic accuracy of the sequential organ failure assessment (SOFA) and quick SOFA for mortality in cancer patients with sepsis defined by systemic inflammatory response syndrome (SIRS). Support Care Cancer 2020, 28, 653–659. [Google Scholar] [CrossRef]
- Probst, L.; Schalk, E.; Liebregts, T.; Zeremski, V.; Tzalavras, A.; Von Bergwelt-Baildon, M.; Hesse, N.; Prinz, J.; Vehreschild, J.J.; et al.; for the Working Party on Intensive Care Medicine in Hematologic and Oncologic Patients (iCHOP) of the German Society of Hematology and Medical Oncology (DGHO) Prognostic accuracy of SOFA, qSOFA and SIRS criteria in hematological cancer patients: A retrospective multicenter study. J. Intensive Care 2019, 7, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornet, A.D.; Issa, A.I.; van de Loosdrecht, A.A.; Ossenkoppele, G.J.; Strack van Schijndel, R.J.M.; Groeneveld, A.B.J. Sequential organ failure predicts mortality of patients with a haematological malignancy needing intensive care. Eur. J. Haematol. 2005, 74, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Vandijck, D.M.; Depuydt, P.O.; Offner, F.C.; Nollet, J.; Peleman, R.A.; Steel, E.; Noens, L.A.; Decruyenaere, J.M.; Benoit, D.D. Impact of organ dysfunction on mortality in ICU patients with hematologic malignancies. Intensive Care Med. 2010, 36, 1744–1750. [Google Scholar] [CrossRef]
- Demandt, A.M.; Geerse, D.A.; Janssen, B.J.; Winkens, B.; Schouten, H.C.; Van Mook, W.N. The prognostic value of a trend in modified SOFA score for patients with hematological malignancies in the intensive care unit. Eur. J. Haematol. 2017, 99, 315–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namendys-Silva, S.A.; González-Herrera, M.O.; García-Guillén, F.J.; Texcocano-Becerra, J.; Herrera-Gómez, A. Outcome of critically ill patients with hematological malignancies. Ann. Hematol. 2013, 92, 699–705. [Google Scholar] [CrossRef]
- Domizi, R.; Calcinaro, S.; Harris, S.; Beilstein, C.; Boerma, C.; Chiche, J.-D.; D’Egidio, A.; Damiani, E.; Donati, A.; Koetsier, P.M.; et al. Relationship between norepinephrine dose, tachycardia and outcome in septic shock: A multicentre evaluation. J. Crit. Care 2020, 57, 185–190. [Google Scholar] [CrossRef]
- Castro, R.; Regueira, T.; Aguirre, M.L.; Llanos, O.P.; Bruhn, A.; Bugedo, G.; Dougnac, A.; Castillo, L.; Andresen, M.; Hernández, G. An evidence-based resuscitation algorithm applied from the emergency room to the ICU improves survival of severe septic shock. Minerva Anestesiol. 2008, 74, 223–231. [Google Scholar]
- Auchet, T.; Regnier, M.-A.; Girerd, N.; Levy, B. Outcome of patients with septic shock and high-dose vasopressor therapy. Ann. Intensive Care 2017, 7, 43. [Google Scholar] [CrossRef] [Green Version]
- Hernández, G.; Teboul, J.-L.; Bakker, J. Norepinephrine in septic shock. Intensive Care Med. 2019, 45, 687–689. [Google Scholar] [CrossRef]
- Stolk, R.F.; van der Poll, T.; Angus, D.C.; van der Hoeven, J.G.; Pickkers, P.; Kox, M. Potentially inadvertent immunomodulation: Norepinephrine use in sepsis. Am. J. Respir. Crit. Care Med. 2016, 194, 550–558. [Google Scholar] [CrossRef]
- Al-Zubaidi, N.; Shehada, E.; Alshabani, K.; ZazaDitYafawi, J.; Kingah, P.; Soubani, A.O. Predictors of outcome in patients with hematologic malignancies admitted to the intensive care unit. Hematol. Oncol. Stem Cell Ther. 2018, 11, 206–218. [Google Scholar] [CrossRef]
- Maqsood, S.; Badar, F.; Hameed, A. Characteristics and outcomes of patients with hematological malignancies admitted for intensive care—A single centre experience. Asian Pac. J. Cancer Prev. 2017, 18, 1833–1837. [Google Scholar] [CrossRef]
- Saillard, C.; Mokart, D.; Lemiale, V.; Azoulay, E. Mechanical ventilation in cancer patients. Minerva Anestesiol. 2014, 80, 712–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDowall, K.L.; Hart, A.J.; Cadamy, A.J. The outcomes of adult patients with haematological malignancy requiring admission to the intensive care unit. J. Intensive Care Soc. 2011, 12, 112–125. [Google Scholar] [CrossRef] [Green Version]


| Age, years (mean ± SD). | 59.8 ± 15.38 |
| Male sex (n (%)) | 49 (57.0) |
| Source of admission (n (%)) | |
| Ward | 92 (80.7) |
| Operating theatre | 6 (5.26) |
| Emergency department | 16 (14.04) |
| qSOFA score (mean ± SD) | 1.4 ± 0.91 |
| APACHE II score (mean ± SD) | 21.72 ± 5.68 |
| SAPS 3 score (mean ± SD) | 75.01 ± 13.27 |
| SOFA score on admission to ICU (mean ± SD) | 6.56 ± 3.20 |
| Charlson’s comorbidity index (mean ± SD) | 4.90 ± 2.26 |
| ECOG ≤ 2 (n (%)) | 85 (74.56) |
| Hematological diagnosis (n (%)) | |
| Acute Myeloid Leukaemia | 46 (40.35) |
| Non-Hodgkin’s Lymphoma | 28 (24.6) |
| Multiple Myeloma | 13 (11.40) |
| Chronic Lymphocytic Leukaemia | 11 (9.65) |
| Acute Lymphoblastic Leukaemia | 8 (7.0) |
| Hodgkin’s Lymphoma | 4 (3.5) |
| Other | 4 (3.5) |
| Graft versus host disease | 16 (14.04) |
| Controlled or stable | 7 (6.14) |
| Uncontrolled | 7 (6.14) |
| Refractory | 2 (6.14) |
| Chemotherapy intensive regimen (n (%)) | 56 (49.12) |
| Bone marrow transplantation (n (%)) | 39 (34.21) |
| Autologous | 14 (12.28) |
| Allogenic | 25 (21.93) |
| Reason for ICU admission (n (%)) | |
| Acute respiratory failure | 48 (42.11) |
| Shock | 23 (20.18) |
| Neurological impairment | 14 (12.28) |
| Sepsis | 7 (6.14) |
| Multiple organ failure | 6 (5.26) |
| Observation after surgery | 5 (4.39) |
| Post cardiac arrest | 2 (1.75) |
| Other | 9 (7.89) |
| Length of stay before ICU admission, days (n (%)) | 20.65 ± 34.60 |
| Management during ICU stay (n (%)) | |
| Invasive mechanical ventilation 1st day | 37 (32.46) |
| Invasive mechanical ventilation | 63 (55.26) |
| Vasoactive drugs | 88 (77.19) |
| CVVHDF | 29 (25.4) |
| Length of stay in ICU, days (mean ± SD) | 6.70 ± 5.48 |
| Median follow-up (IQR), days | 539.5 (367) |
| Characteristics | Survivors (n = 63), n (%) 63 (55.26) | Non-Survivors (n = 51), n (%) 51 (44.74) | p-Value |
|---|---|---|---|
| Sex, female | 30 (47.6) | 19 (37.3) | 0.342 |
| Hematological diagnosis | 0.474 | ||
| Acute leukaemia | 30 (47.6) | 24 (47.1) | |
| Non-Hodgkin’s Lymphoma | 14 (22.2) | 14 (27.5) | |
| Multiple Myeloma | 10 (15.9) | 3 (5.9) | |
| Chronic leukaemia | 5 (7.9) | 6 (11.8) | |
| Hodgkin’s Lymphoma | 1 (1.6) | 3 (5.9) | |
| Other | 3 (4.76) | 1 (2.0) | |
| High-risk malignancy | 43 (68.3) | 36 (70.6) | 0.840 |
| Intensive chemotherapy | 30 (47.6) | 26 (51.0) | 0.557 |
| Bone marrow transplant | 22 (34.9) | 17 (33.3) | 1.000 |
| Allogenic | 11 (17.5) | 14 (27.5) | 0.116 |
| Autologous | 11 (17.5) | 3 (5.9) | |
| ECOG group | 0.829 | ||
| 0–2 | 46 (73.0) | 39 (76.5) | |
| ≥3 | 17 (27.0) | 12 (23.5) | |
| qSOFA score | 0.004 | ||
| 0 | 12 (19.0) | 4 (7.8) | |
| 1 | 36 (57.1) | 18 (35.3) | |
| 2 | 10 (15.9) | 17 (33.3) | |
| 3 | 5 (7.9) | 12 (23.5) | |
| Mechanical ventilation day 1 in ICU | 9 (14.3) | 28 (54.9) | <0.001 |
| Mechanical ventilation anytime in ICU | 14 (22.2) | 49 (96.1) | <0.001 |
| Vasoactive drugs anytime in ICU | 35 (55.6) | 51 (100.0) | <0.001 |
| Renal replacement therapy | 11 (17.5) | 21 (41.2) | 0.007 |
| CVVHDF | 9 (14.3) | 20 (39.2) | 0.004 |
| Need for colistin therapy in ICU | 6 (9.5) | 14 (27.5) | 0.024 |
| Neutrophil count < 500/mm3 | 24 (38.1) | 20 (39.2) | 1.000 |
| Source of admission to ICU | 0.783 | ||
| Emergency department | 8 (12.7) | 8 (15.7) | |
| Ward | 44 (69.8) | 33 (64.7) | |
| n.a. | 11 (17.46) | 10 (19.61) |
| Variable | Odds Ratio (95% CI) | p Value |
|---|---|---|
| Age, years | 1.000 (0.976–1.025) | 0.986 |
| Female sex | 1.531 (0.721–3.250) | 0.267 |
| Days in hospital before admission to ICU | 0.994 (0.981–1.006) | 0.327 |
| ECOG | 1.011 (0.730–1.399) | 0.948 |
| Charlson’s comorbidity index | 1.050 (0.891–1.237) | 0.563 |
| High risk haematological malignancy | 1.116 (0.500–2.491) | 0.788 |
| Intensive chemotherapy | 1.277 (0.583–2.798) | 0.541 |
| Bone marrow transplantation | 0.932 (0.428–2.031) | 0.859 |
| Autologous bone marrow transplantation | 0.329 (0.085–1.275) | 0.132 |
| Allogenic bone marrow transplantation | 1.535 (0.617–3.817) | |
| qSOFA ≥ 2 | 4.217 (1.891–9.405) | <0.001 |
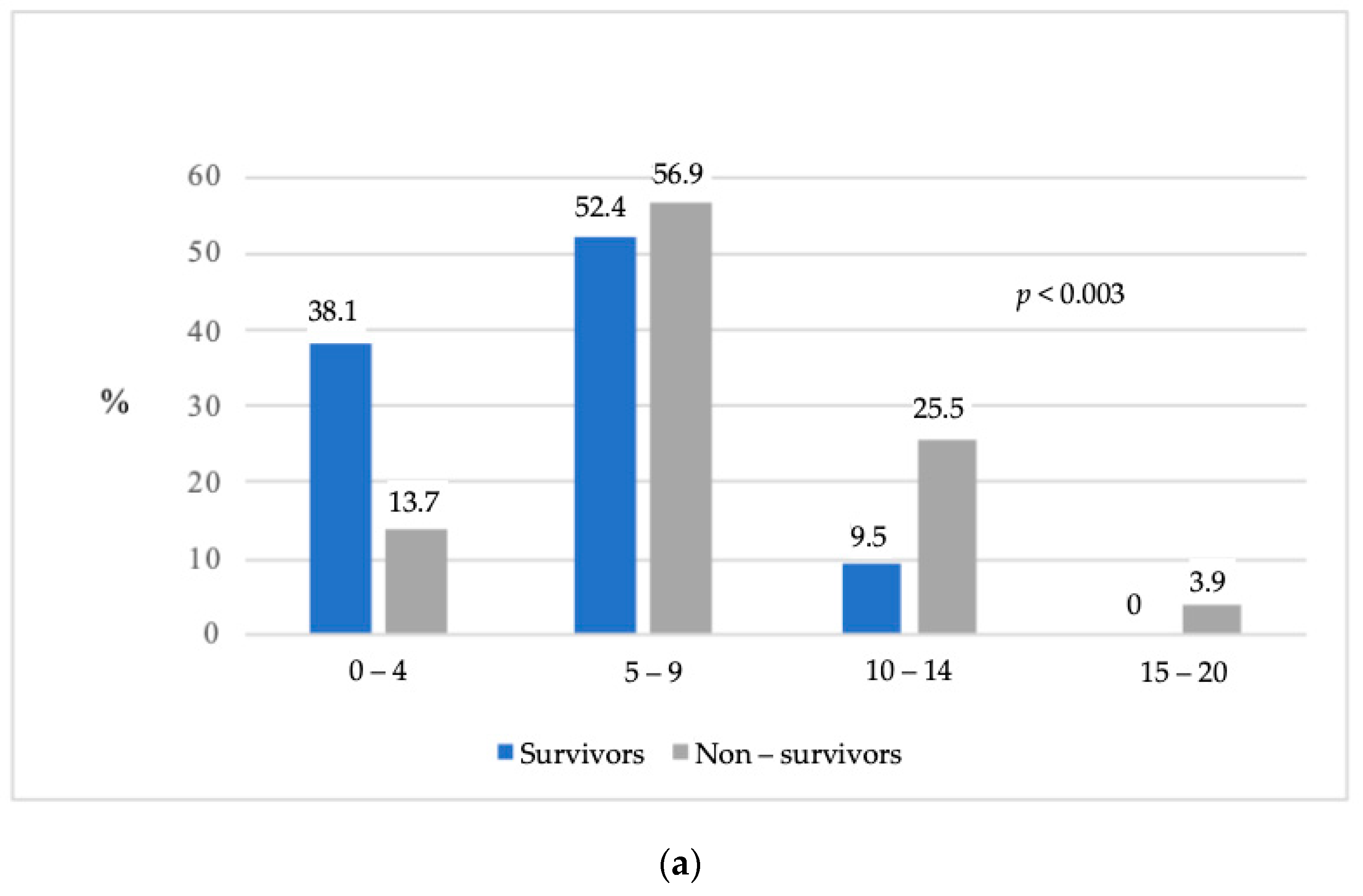
| SOFA score 5–9 on day 1 in ICU | 3.013 (1.132–8.017) | 0.004 |
| SOFA score 10–20 on day 1 in ICU | 8.571 (2.414–30.429) | |
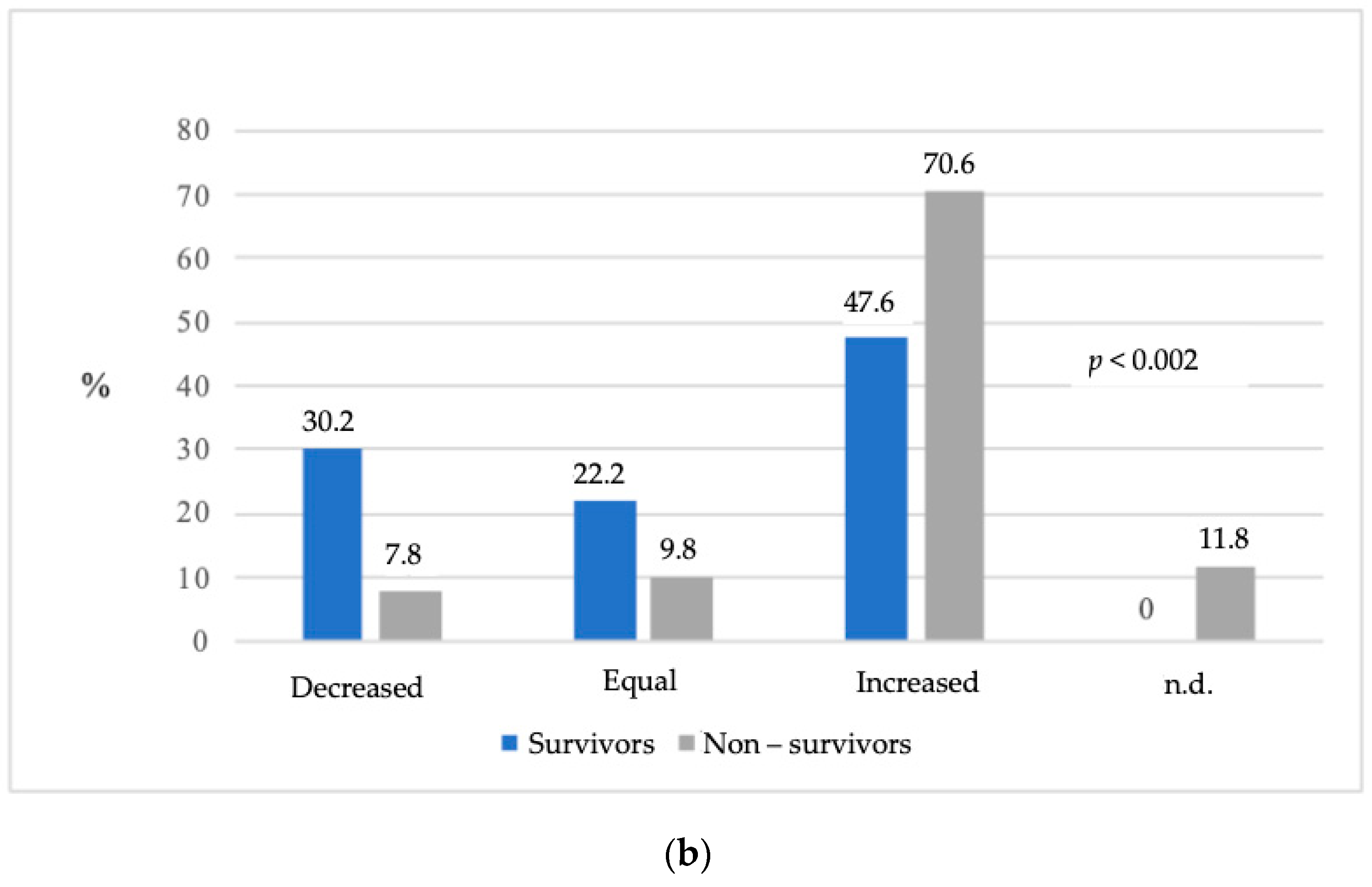
| Equal SOFA score in the first 48 h in ICU | 1.696 (0.384–7.489) | 0.004 |
| Increased SOFA score in the first 48 h in ICU | 5.700 (1.748–18.587) | |
| APACHE II score | 1.092 (1.019–1.171) | 0.013 |
| SAPS 3 score | 1.041 (1.010–1.074) | 0.010 |
| Neutrophil count < 500/mm3 on arrival at the ICU | 0.995 (0.464–2.130) | 0.989 |
| Haemoglobin on arrival at the ICU, g/L | 1.026 (1.002–1.050) | 0.030 |
| Potassium on arrival at the ICU, mmol/L | 2.244 (1.428–3.527) | <0.001 |
| apH on arrival at the ICU, units | <0.001 (<0.001–0.031) | <0.001 |
| Lactate on arrival at the ICU, mmol/L | 1.314 (1.084–1.592) | 0.005 |
| Base excess on arrival at the ICU, units | 0.918 (0.869–0.970) | 0.002 |
| Bicarbonate on arrival at the ICU, units | 1.102 (1.020–1.190) | 0.014 |
| Need for colistin therapy in the ICU | 3.531 (1.245–10.014) | 0.018 |
| Mechanical ventilation day 1 in the ICU | 7.304 (2.983–17.888) | <0.001 |
| Mechanical ventilation anytime in the ICU | 85.749 (18.501–397.43) | <0.001 |
| FiO2, % | 1.032 (1.001–1.064) | 0.042 |
| Vasoactive drugs in ICU | 2.213 (1.511–3.243) | <0.001 |
| CVVHDF | 3.870 (1.570–9.540) | 0.003 |
| Variable | Odds Ratio (95% CI) | p Value |
|---|---|---|
| qSOFA ≥ 2 | 4.403 (1.376–14.081) | 0.0125 |
| Equal SOFA score first 48 h in the ICU | 4.903 (0.643–37.397) | 0.0156 |
| Increased SOFA score first 48 h in the ICU | 11.171 (2.072–60.226) | |
| Invasive mechanical ventilation day 1 in the ICU | 6.157 (1.867–20.308) | 0.0028 |
| Need for colistin therapy in the ICU | 11.037 (2.673–45.572) | 0.0009 |
| Arterial pH on arrival to the ICU, units | 0.392 (0.201–0.7620) | 0.0058 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Judickas, Š.; Stasiūnaitis, R.; Žučenka, A.; Žvirblis, T.; Šerpytis, M.; Šipylaitė, J. Outcomes and Risk Factors of Critically Ill Patients with Hematological Malignancy. Prospective Single-Centre Observational Study. Medicina 2021, 57, 1317. https://doi.org/10.3390/medicina57121317
Judickas Š, Stasiūnaitis R, Žučenka A, Žvirblis T, Šerpytis M, Šipylaitė J. Outcomes and Risk Factors of Critically Ill Patients with Hematological Malignancy. Prospective Single-Centre Observational Study. Medicina. 2021; 57(12):1317. https://doi.org/10.3390/medicina57121317
Chicago/Turabian StyleJudickas, Šarūnas, Raimundas Stasiūnaitis, Andrius Žučenka, Tadas Žvirblis, Mindaugas Šerpytis, and Jūratė Šipylaitė. 2021. "Outcomes and Risk Factors of Critically Ill Patients with Hematological Malignancy. Prospective Single-Centre Observational Study" Medicina 57, no. 12: 1317. https://doi.org/10.3390/medicina57121317
APA StyleJudickas, Š., Stasiūnaitis, R., Žučenka, A., Žvirblis, T., Šerpytis, M., & Šipylaitė, J. (2021). Outcomes and Risk Factors of Critically Ill Patients with Hematological Malignancy. Prospective Single-Centre Observational Study. Medicina, 57(12), 1317. https://doi.org/10.3390/medicina57121317






