Unusual Complicated Gastric Ulcers
Abstract
:1. Introduction
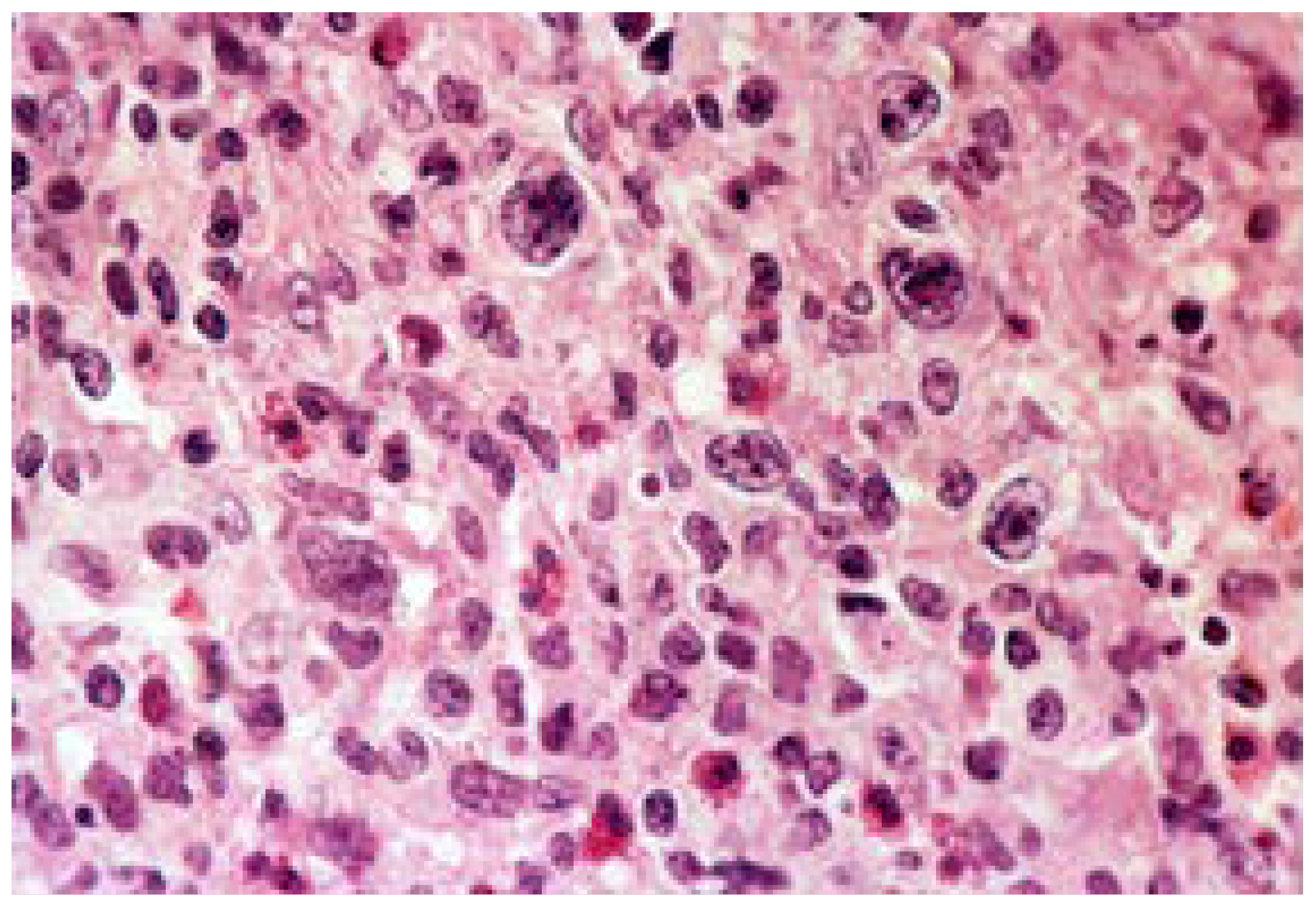
- Deterioration of the mucosa and the entire gastric wall, caused by necrosis, that can progress to the serous membrane, thus generating perforation of the stomach;
- An inflammatory lymphoplasmacytic process at the bottom of the ulcer and its margins;
- Microvascular changes in the ulcer’s perimeter with arteriole hyalinosis.
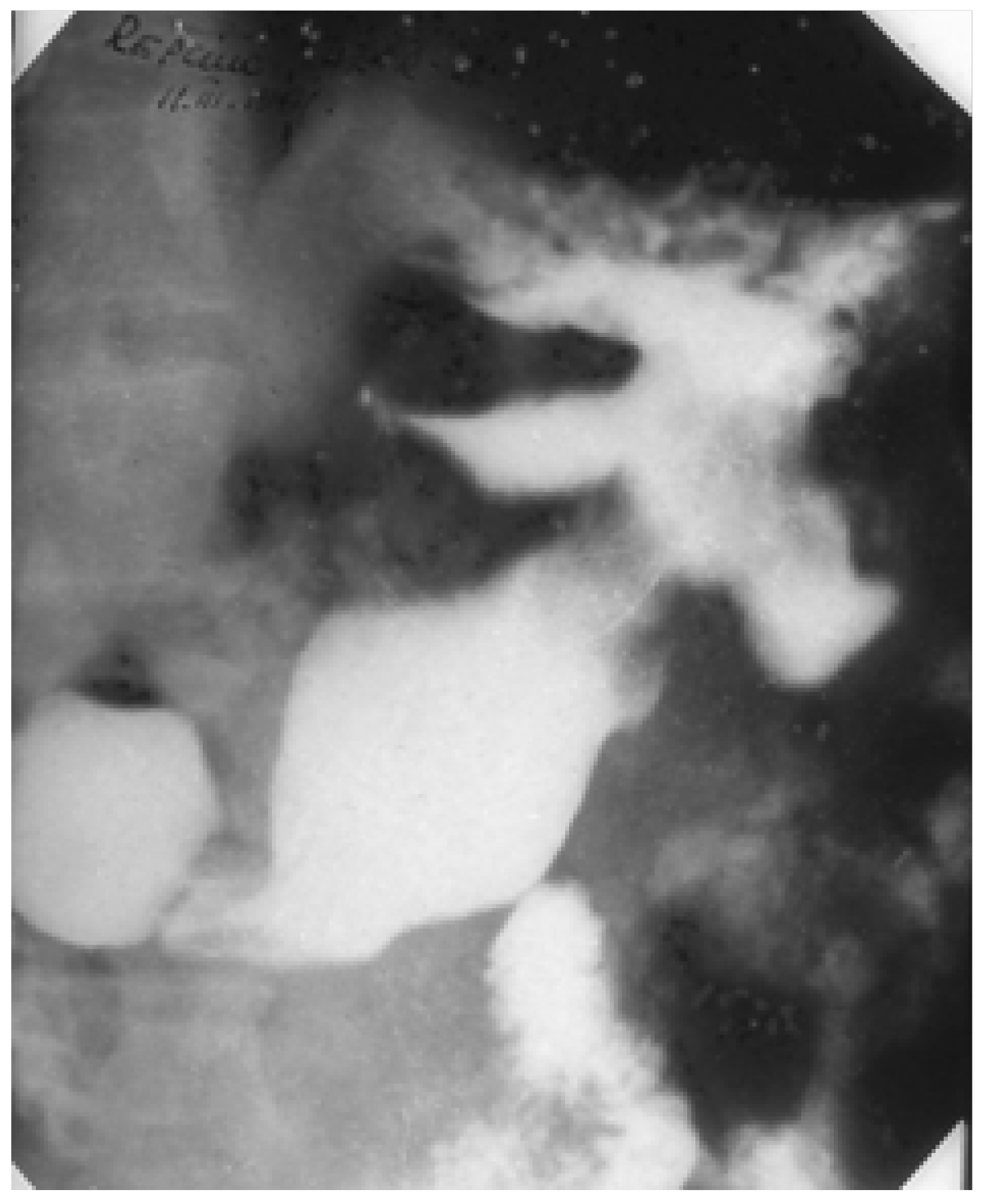
2. Giant Ulcers (over 2–3 cm)
3. Multiple Ulcers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Csendes, A.; Becker, P.; Valenzuela, J.; Braghetto, I.; Csendes, P. Clinical characteristics of patients with multiple or giant peptic ulcers. Rev. Med. Chil. 1991, 119, 38–44. [Google Scholar] [PubMed]
- Palmer, E.D.; Buchanan, D.P. On the ischemic basis of peptic ulcer. I. Historical definition of present status. Intern Med. 1953, 38, 1187–1205. [Google Scholar] [CrossRef]
- Byrd, J.C.; Bresalier, R.S. Alterations in gastric mucin synthesis by Helicobacter pylori. World J. Gastroenterol. 2000, 6, 475–482. [Google Scholar] [CrossRef]
- Boltin, D.; Halpern, M.; Levi, Z.; Vilkin, A.; Morgenstern, S.; Ho, S.B.; Niv, Y. Gastric mucin expression in Helicobacter pylori-related, nonsteroidal anti-inflammatory drug-related and idiopathic ulcers. World J. Gastroenterol. 2012, 18, 4597–4603. [Google Scholar] [CrossRef]
- Soll, A.H. Consensus conference. Medical treatment of peptic ulcer disease. Practice guidelines. Practice Parameters Committee of the American College of Gastroenterology. JAMA 1996, 275, 622–629, Erratum in 1996, 275, 1314. [Google Scholar] [CrossRef]
- Menger, M.D. Microcirculation of gastric mucosa in the pathogenesis of stomach ulcer. Zent. Chir. 1994, 119, 1–10. [Google Scholar]
- Sato, N.; Kawano, S.; Tsuji, S.; Ogihara, T.; Yamada, S. Gastric blood flow in ulcer diseases. Scand. J. Gastroenterol. Suppl. 1995, 208, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Piasecki, C. Evidence for an infarctive pathogenesis of acute and chronic gastroduodenal ulceration. J. Physiol. Pharmacol. 1992, 43, 99–113. [Google Scholar]
- Rau, W.; Hohaus, C.; Jessen, E. A Differential Approach to Form and Site of Peptic Ulcer. Sci. Rep. 2019, 9, 8683. [Google Scholar] [CrossRef] [PubMed]
- Dolphin, J.A.; Smith, L.A.; Waugh, J.M. Multiple gastric ulcers: Their occurrence in benign and malignant lesions. Gastroenterology 1953, 25, 202–217. [Google Scholar] [CrossRef]
- Abbass, A.; Khalid, S.; Boppana, V.; Hanson, J.; Lin, H.; McCarthy, D. Giant Gastric Ulcers: An Unusual Culprit. Dig. Dis. Sci. 2020, 65, 2811–2817. [Google Scholar] [CrossRef]
- West, A.B.; Nolan, N.; O’Briain, D.S. Benign peptic ulcers penetrating pericardium and heart: Clinicopathological features and factors favouring survival. Gastroenterology 1988, 94, 1478–1487. [Google Scholar] [CrossRef]
- Barragry, T.P.; Blatchford, J.W., 3rd; Allen, M.O. Giant gastric ulcers. A review of 49 cases. Ann. Surg. 1986, 203, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Lord, R.; El-Feki, M.; Tomos, L.; Noor, M.; Subramanian, V.; Rembacken, B.J. Giant gastric ulcers: Malignancy yield and predictors from a 10-year retrospective single-center cohort. United Eur. Gastroenterol. J. 2018, 6, 1000–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, A.A.; Yoshida, E.M.; Poulin, M.; Gascoyne, R.D.; Owen, D.A. Massive bleeding from multiple gastric ulcerations in a patient with lymphocytic gastritis and celiac sprue. J. Clin. Gastroenterol. 1997, 25, 354–357. [Google Scholar] [CrossRef]
- Stolte, M.; Panayiotou, S.; Schmitz, J. Can NSAID/ASA-induced erosions of the gastric mucosa be identified at histology? Pathol. Res. Pract. 1999, 195, 137–142. [Google Scholar] [CrossRef]
- Iwamoto, J.; Saito, Y.; Honda, A.; Matsuzaki, Y. Clinical features of gastroduodenal injury associated with long-term low-dose aspirin therapy. World J. Gastroenterol. 2013, 19, 1673–1682. [Google Scholar] [CrossRef]
- Russell, R. Non-steroidal anti-inflammatory drugs and gastrointestinal damage—problems and solutions. Postgrad. Med. J. 2001, 77, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Girvan, D.P.; Passi, R.B. Acute stress ulceration with bleeding or perforation. Arch. Surg. 1971, 103, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Tarasconi, A.; Coccolini, F.; Catena, F. Perforated and bleeding peptic ulcer: WSES guidelines. World J. Emerg. Surg. 2020, 15, 3. [Google Scholar] [CrossRef] [Green Version]
- Vartic, M.; Chilie, A.; Beuran, M. Hemoragia digestivă in terapia intensivă. Chirurgia 2006, 101, 365–374. [Google Scholar] [PubMed]
- Vasilescu, C. Limfomul Gastric Primitiv; MED MUN: Bucharest, Romania, 2002; pp. 1–200. ISBN 973-99260-2-9. [Google Scholar]
- Kodera, Y.; Yamamura, Y.; Nakamura, S.; Shimizu, Y.; Torii, A. The role of radical gastrectomy with systematic lymphadenectomy for the diagnosis and treatment of primary gastric lymphoma. Ann. Surg. 1998, 227, 45–50. [Google Scholar] [CrossRef]
- Roehrkasse, R.L.; Roberts, I.M.; Wald, A.; Talamo, T.S.; Mendelow, H. Celiac sprue complicated by lymphoma presenting with multiple gastric ulcers. Gastroenterology 1986, 91, 740–745. [Google Scholar] [CrossRef]
- Boyle, J.D. The Veterans Administration Cooperative Study on Gastric Ulcer. 8. Multiple gastric ulcers. Gastroenterology 1971, 61 (Suppl. 2), 628–631. [Google Scholar] [CrossRef]
- Aste, H.; Maggiolo, P.; Avendaño, S.; Medina, L.A.; Lucchini, A. Multiple gastric ulcers. Endoscopic study. Rev. Med. Chil. 1972, 100, 1348–1351. [Google Scholar] [PubMed]
- Winans, C.S.; Yoshii, Y.; Kobayashi, S. Endoscopic diagnosis of multiple benign gastric ulcers. Gastrointest. Endosc. 1972, 19, 63–66. [Google Scholar] [CrossRef]
- Mathieu, A.; Moutier, F. L′ulcus geant de l′estomac. Arch. Med. Appl. Dig. 1919, 10, 257–262. [Google Scholar]
- Alvarez, W.C.; Maccarty, W.C. Sizes of Resected Gastric Ulcers and Gastric Carcinomas. JAMA 1928, 91, 226–231. [Google Scholar] [CrossRef]
- Cohn, I.J.; Sartin, J. Giant gastric ulcers. Ann. Surg. 1958, 147, 749–758; discussion 758–759. [Google Scholar] [CrossRef]
- Raju, G.S.; Bardhan, K.D.; Royston, C.; Beresford, J. Giant gastric ulcer: Its natural history and outcome in the H2RA era. Am. J. Gastroenterol. 1999, 94, 3478–3486. [Google Scholar] [CrossRef] [PubMed]
- Rembacken, B. Which Way to Go with a Giant Gastric Ulcer? 2017. Available online: http://ueg.eu/a/218 (accessed on 22 November 2021).






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brătucu, M.N.; Prunoiu, V.-M.; Strâmbu, V.; Brătucu, E.; Răvaş, M.-M.; Simion, L.; Petre, R. Unusual Complicated Gastric Ulcers. Medicina 2021, 57, 1345. https://doi.org/10.3390/medicina57121345
Brătucu MN, Prunoiu V-M, Strâmbu V, Brătucu E, Răvaş M-M, Simion L, Petre R. Unusual Complicated Gastric Ulcers. Medicina. 2021; 57(12):1345. https://doi.org/10.3390/medicina57121345
Chicago/Turabian StyleBrătucu, Mircea Nicolae, Virgiliu-Mihail Prunoiu, Victor Strâmbu, Eugen Brătucu, Maria-Manuela Răvaş, Laurenţiu Simion, and Radu Petre. 2021. "Unusual Complicated Gastric Ulcers" Medicina 57, no. 12: 1345. https://doi.org/10.3390/medicina57121345
APA StyleBrătucu, M. N., Prunoiu, V.-M., Strâmbu, V., Brătucu, E., Răvaş, M.-M., Simion, L., & Petre, R. (2021). Unusual Complicated Gastric Ulcers. Medicina, 57(12), 1345. https://doi.org/10.3390/medicina57121345






