Long-Term, Single-Centre Observation of Patients with Cardiac Implantable Electronic Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
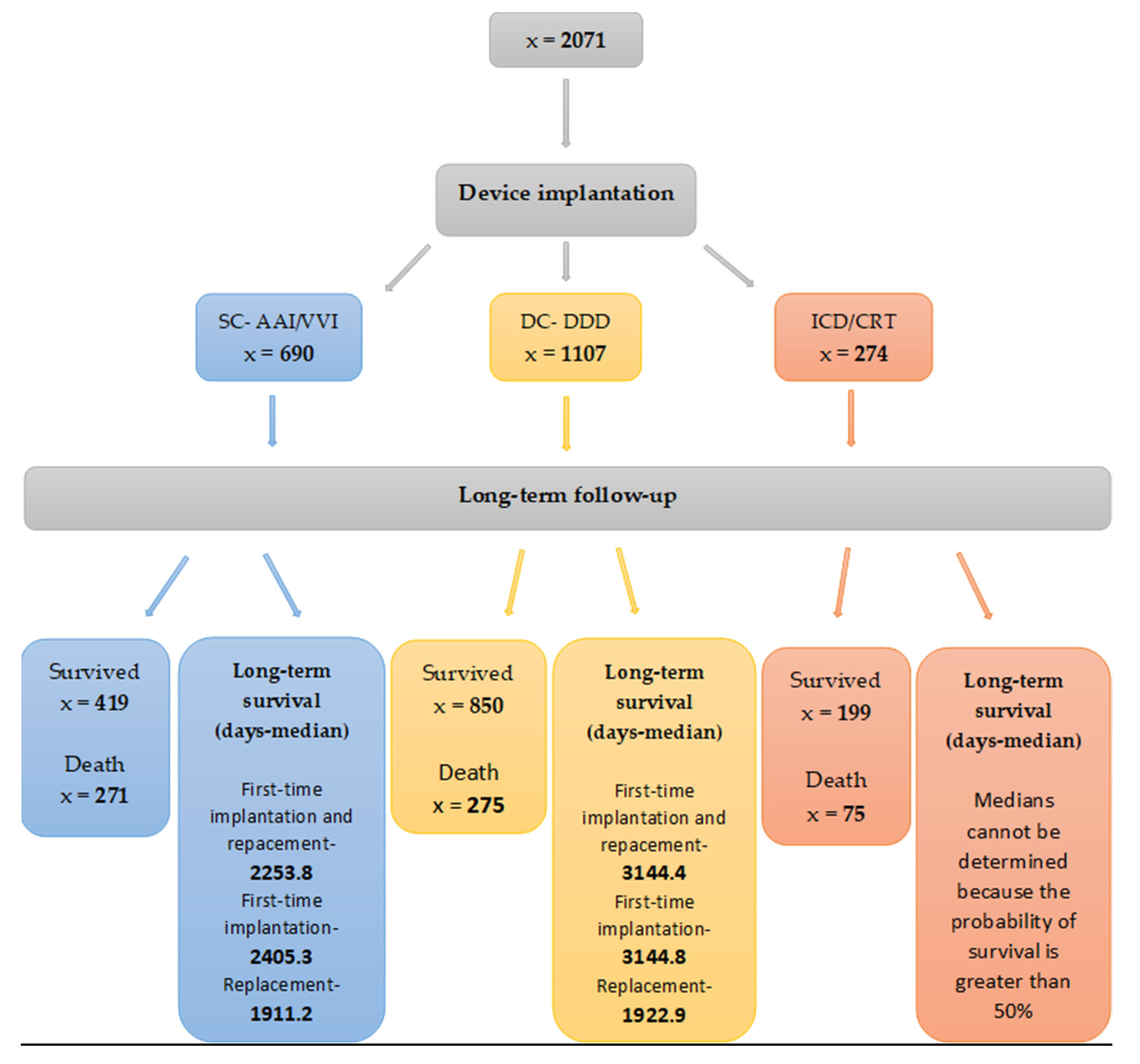
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brignole, M.; Auricchio, A.; Baron-Esquivias, G.; Bordachar, P.; Boriani, G.; Breithardt, O.A.; Cleland, J.; Deharo, J.C.; Delgado, V.; Elliott, P.M.; et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronisation therapy The Task Force on cardiac pacing and resynchronisation therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur. Heart J. 2013, 34, 2281–2329. [Google Scholar] [PubMed] [Green Version]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Dębski, M.; Maniecka–Bryła, I.; Dziankowska–Zaborszczyk, E.; Ulman, M.; Ząbek, A.; Boczar, K.; Haberka, K.; Kuniewicz, M.; Lelakowski, J.; Małecka, B. Years of life lost as a measure of premature death among dual-chamber pacemaker recipients from Małopolska Province. Kardiol. Pol. 2019, 77, 683–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, W.; Grimm, K.; Greene, B.; Parahuleva, M. Predictors of pacing-dependency in patients with cardiovascular implantable electronic devices. Cardiol. J. 2021, 28, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Biffi, M.; Spadotto, A.; Piemontese, G.P.; Toniolo, S.; Bartoli, L.; Sorrentino, S.; Minguzzi, A.; Massaro, G.; Capobianco, C.; Statuto, G. Cardiac Stimulation in the Third Millennium: Where Do We Head from Here? Hearts 2021, 2, 15–35. [Google Scholar] [CrossRef]
- Dębski, M.; Ulman, M.; Ząbek, A.; Boczar, K.; Haberka, K.; Kuniewicz, M.; Lelakowski, J.; Małecka, B. Association of selected factors with long-term prognosis and mortality after dual-chamber pacemaker implant. Cardiol. J. 2019, 26, 717–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elango, K.; Curtis, A.B. Cardiac implantable electrical devices in women. Clin. Cardiol. 2018, 41, 232–238. [Google Scholar] [CrossRef] [Green Version]
- Krzemień-Wolska, K.; Tomasik, A.; Wojciechowska, C.; Barańska-Pawełczak, K.; Nowalany-Kozielska, E.; Jacheć, W. Prognostic Factors in Patients with an Implanted Pacemaker after 80 Years of Age in a 4-Year Follow-Up. Gerontology 2018, 64, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-W.; Wang, C.-H.; Chen, W.-S.; Wang, C.-C.; Cherng, W.-J. Predictors of long-term survival prior to permanent pacemaker implantation in octogenarians or older. Aging Clin. Exp. Res. 2019, 31, 1001–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamas, G.A.; Lee, K.L.; Sweeney, M.O.; Silverman, R.; Yee, A.L.R.; Marinchak, R.A.; Flaker, G.; Schron, E.; Orav, E.J.; Hellkamp, A.S.; et al. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N. Engl. J. Med. 2002, 346, 1854–1862. [Google Scholar] [CrossRef] [Green Version]
- Udo, E.O.; van Hemel, N.M.; Zuithoff, N.P.; Kelder, J.C.; Crommentuijn, H.A.; Koopman-Verhagen, A.M.; Voskuil, T.; Doevendans, P.A.; Moons, K.G. Long-term outcome of cardiac pacing in octogenarians and nonagenarians. Europace 2012, 14, 502–508. [Google Scholar] [CrossRef]
- Bradshaw, P.J.; Stobie, P.; Knuiman, M.; Briffa, T.G.; Hobbs, M.S. Life expectancy after implantation of a first cardiac permanent pacemaker (1995–2008): A population-based study. Int. J. Cardiol. 2015, 190, 42–46. [Google Scholar] [CrossRef] [PubMed]
- McAnulty, J.H.; Rahimtoola, S.H.; Murphy, E.; DeMots, H.; Ritzmann, L.; Kanarek, P.E.; Kauffman, S. Natural history of “high-risk” bundle-branch block: Final report of a prospective study. N. Engl. J. Med. 1982, 307, 137–143. [Google Scholar] [CrossRef]
- Click, R.L.; Gersh, B.J.; Sugrue, D.D.; Holmes, D.R.; Wood, D.L.; Osborn, M.J.; Hammill, S.C. Role of invasive electrophysiologic testing in patients with symptomatic bundle branch block. Am. J. Cardiol. 1987, 59, 817–823. [Google Scholar] [CrossRef]
- Shen, W.-K.; Hayes, D.L.; Hammill, S.C.; Bailey, K.R.; Ballard, D.J.; Gersh, B.J. Survival and Functional Independence after Implantation of a Permanent Pacemaker in Octogenarians and Nonagenarians: A Population-Based Study. Ann. Intern. Med. 1996, 125, 476. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. J. Am. Coll. Cardiol. 2018, 72, e91–e220. [Google Scholar] [CrossRef]
- Fabbian, F.; De Giorgi, A.; Guarino, M.; Malagù, M.; Bertini, M. Impact of chronic kidney disease on mortality in older adults treated with pacemaker implantation. J. Geriatr. Cardiol. 2017, 14, 597–603. [Google Scholar] [PubMed]
- Nishimura, M.; Chong Hsu, J. The Utility of ICDs in Patients With ESRD: Insights from the ICD2 Trial. ACC. 30 August 2019. Available online: https://www.acc.org/latest-in-cardiology/articles/2019/08/30/06/46/the-utility-of-icds-in-patients-with-esr (accessed on 12 December 2021).
- Jankauskiene, E.; Vaskelete, J.J.; Rumbinaite, E. ‘Low—T3 syndrome’ among patients with acute myocardial infarction. Cent. Eur. J. Med. 2014, 9, 10–14. [Google Scholar] [CrossRef]
- Yang, G.; Wang, Y.; Ma, A.; Wang, T. Subclinical thyroid dysfunction is associated with adverse prognosis in heart failure patients with reduced ejection fraction. BMC Cardiovasc. Disord. 2019, 19, 83. [Google Scholar] [CrossRef]
- Wang, W.; Guan, H.; Gerdes, A.M.; Iervasi, G.; Yang, Y.; Tang, Y.-D. Thyroid Status, Cardiac Function, and Mortality in Patients with Idiopathic Dilated Cardiomyopathy. J. Clin. Endocrinol. Metab. 2015, 100, 3210–3218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, L.; Deng, H.; Lin, W.-D.; He, S.-F.; Liu, F.-Z.; Liu, Y.; Zhan, X.-Z.; Fang, X.-H.; Liao, H.-T.; Wei, W.; et al. Association between elevated blood glucose level and non-valvular atrial fibrillation: A report from the Guangzhou heart study. BMC Cardiovasc. Disord. 2019, 19, 270. [Google Scholar] [CrossRef] [PubMed]
- Rautio, E.; Gadler, F.; Gudbjörnsdottir, S.; Franzén, S.; Rydén, L.; Svensson, A.-M.; Mellbin, L.G. Patients with Type 2 Diabetes Have an Increased Demand for Pacemaker Treatment: A Comparison with Age- and Sex-Matched Control Subjects From the General Population. Diabetes Care 2020, 43, 2853–2858. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.; Bernjak, A.; Williams, S.; Fawdry, R.A.; Hibbert, S.; Freeman, J.; Sheridan, P.J.; Heller, S.R. Risk of Cardiac Arrhythmias During Hypoglycemia in Patients with Type 2 Diabetes and Cardiovascular Risk. Diabetes 2014, 63, 1738–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variable | SC AAI/VVI | DC DDD | ICD/CRT | p-Value |
|---|---|---|---|---|
| Age (years) | 80 (74–84) | 78 (71–83) | 66 (59–74) | <0.001 * |
| Male | 357 51.7% | 486 43.9% | 234 85.4% | <0.001 * |
| Heart failure II NYHA class | 217 31.5% | 238 21.6% | 103 37.6% | <0.001 * |
| Heart failure III NYHA class | 119 17.3% | 58 5.2% | 99 36.1% | <0.001 * |
| Hypertension | 456 66.1% | 848 76.6% | 140 51.1% | <0.001 * |
| Diabetes | 192 27.8% | 310 28.0% | 93 33.9% | 0.123 |
| Chronic coronary syndrome | 193 28.0% | 391 35.3% | 204 74.5% | <0.001 * |
| Dilated cardiomyopathy | 6 0.9% | 5 0.5% | 56 20.4% | <0.001 * |
| Hypertrophic cardiomyopathy | 0 0% | 4 0.4% | 1 0.4% | - |
| Atrial fibrillation | 116 16.8% | 350 31.6% | 104 38.0% | <0.001 * |
| History of stroke | 94 13.6% | 97 8.8% | 24 8.8% | 0.003 * |
| Chronic obstructive pulmonary disease | 61 8.84% | 64 5.78% | 36 13.14% | <0.001 * |
| Chronic kidney disease | 116 16.8% | 170 15.4% | 39 14.2% | 0.552 |
| Hyperthyroidism | 29 4.2% | 38 3.4% | 10 3.7% | 0.702 |
| Hypothyroidism | 32 4.6% | 64 5.8% | 8 2.9% | 0.129 |
| LVEF—primary prevention | - | - | 30% | - |
| LVEF—secondary prevention | - | - | 38% | - |
| Type of procedure | N | N | N | |
| First-time implantation | 630 | 1049 | 253 | - |
| Primary Indications | SC AAI/VVI | DC DDD | ICD/CRT |
|---|---|---|---|
| Atrial fibrillation with an AV block * | 488 (70.7%) | 40 (3.6%) | - |
| AV block III | 113 (16.4%) | 285 (25.7%) | - |
| Sick sinus syndrome | 63 (9.1%) | 520 (47.0%) | - |
| AV block II t.2 | 20 (2.9%) | 133 (12.0%) | - |
| AV block 2:1 | 5 (0.7%) | 107 (9.7%) | - |
| Trifascicular block | 1 (0.1%) | 1 (0.1%) | - |
| AV block II t.1 | - | 19 (1.7%) | - |
| Alternating bundle branch block | - | 2 (0.2%) | - |
| Cardiac arrest—primary prevention | - | - | 205 (74.8%) |
| Cardiac arrest—secondary prevention | - | - | 69 (25.2%) |
| Analysed Variable | HR | 95% CI | p-Value |
|---|---|---|---|
| A (SC-AAI/VVI) | |||
| Chronic kidney disease | 1.94 | 1.45–2.59 | <0.001 * |
| Male | 1.62 | 1.25–2.10 | <0.001 * |
| AV block III | 1.59 | 1.18–2.13 | 0.002 * |
| Age (1-year increase) | 1.06 | 1.04–1.08 | <0.001 * |
| Length of hospitalisation | 1.05 | 1.03–1.07 | <0.001 * |
| Stratified by cardiology and urology clinic | |||
| B (DC-DDD) | |||
| Alternating bundle branch block | 39.3 | 5.03–307.78 | <0.001 * |
| Heart failure III NYHA class | 1.78 | 1.19–2.68 | 0.005 * |
| Chronic kidney disease | 1.63 | 1.20–2.22 | 0.002 * |
| Diabetes | 1.56 | 1.19–2.05 | 0.001 * |
| Male | 1.45 | 1.13–1.86 | 0.004 * |
| Age (1-year increase) | 1.06 | 1.04–1.08 | <0.001 * |
| Distance between the place of residence and the implanting centre | 0.99 | 0.99–0.10 | 0.023 * |
| Hypertension | 0.72 | 0.55–0.96 | 0.023 * |
| Stratified by cardiology clinic | |||
| C (ICD/CRT) | |||
| Atrial fibrillation with AV block | 4.95 | 1.51–16.25 | 0.008 * |
| Hypothyroidism | 3.45 | 1.06–11.26 | 0.040 * |
| Age (1-year increase) | 1.04 | 1.02–1.07 | <0.001 * |
| Stratified by cardiology clinic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Załuska, R.; Milewska, A.; Moumtzoglou, A.; Grabowski, M.; Drygas, W. Long-Term, Single-Centre Observation of Patients with Cardiac Implantable Electronic Devices. Medicina 2021, 57, 1357. https://doi.org/10.3390/medicina57121357
Załuska R, Milewska A, Moumtzoglou A, Grabowski M, Drygas W. Long-Term, Single-Centre Observation of Patients with Cardiac Implantable Electronic Devices. Medicina. 2021; 57(12):1357. https://doi.org/10.3390/medicina57121357
Chicago/Turabian StyleZałuska, Roman, Anna Milewska, Anastasius Moumtzoglou, Marcin Grabowski, and Wojciech Drygas. 2021. "Long-Term, Single-Centre Observation of Patients with Cardiac Implantable Electronic Devices" Medicina 57, no. 12: 1357. https://doi.org/10.3390/medicina57121357
APA StyleZałuska, R., Milewska, A., Moumtzoglou, A., Grabowski, M., & Drygas, W. (2021). Long-Term, Single-Centre Observation of Patients with Cardiac Implantable Electronic Devices. Medicina, 57(12), 1357. https://doi.org/10.3390/medicina57121357





