Serum Fibroblast Growth Factor 21 Levels in Children and Adolescents with Hashimoto’s Thyroiditis before and after l-Thyroxin Medication: A Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical and Biochemical Data
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of All Studied Groups
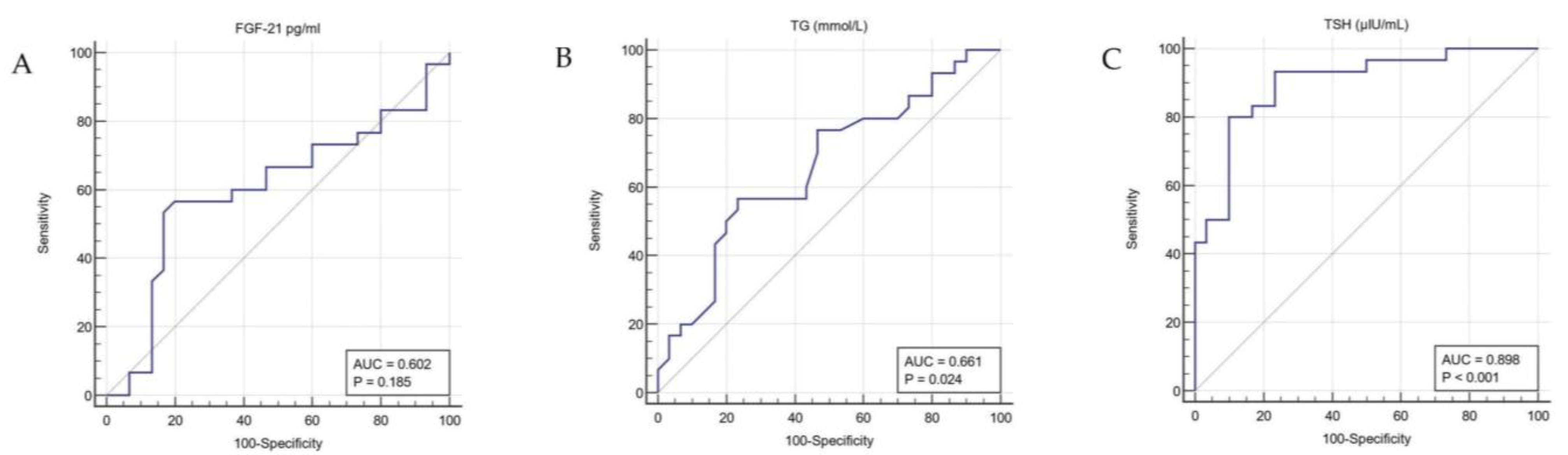
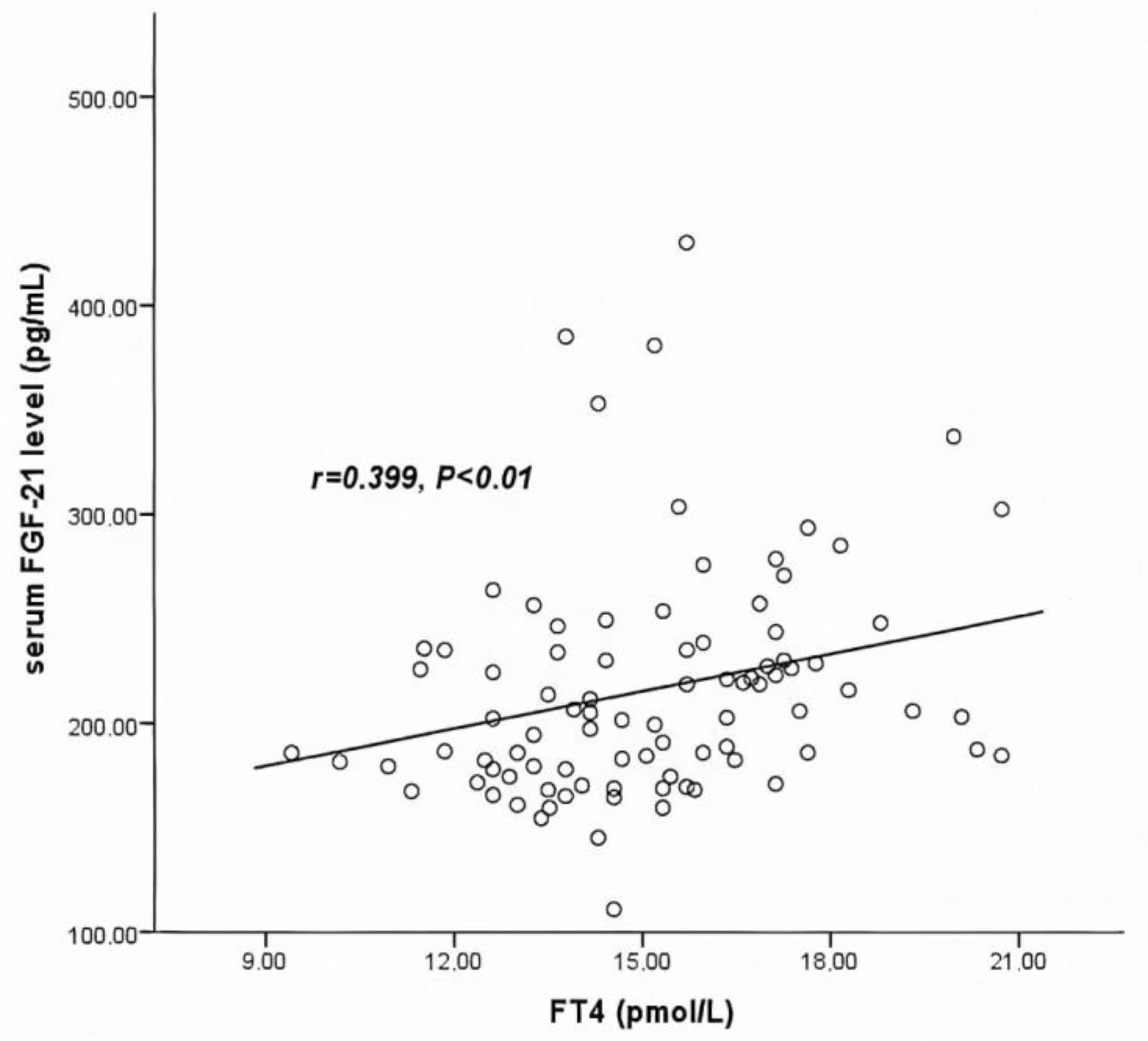
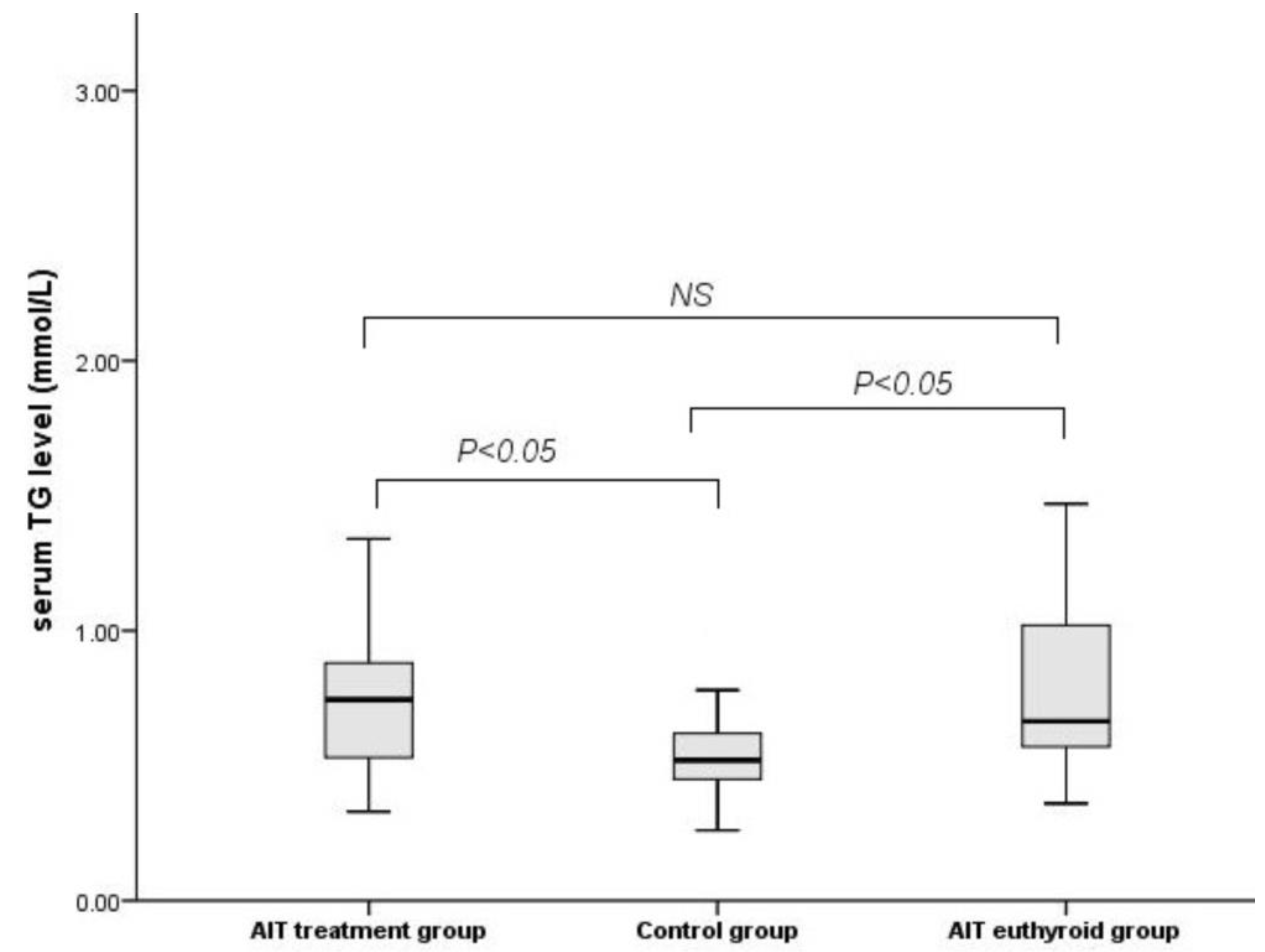
3.2. Characteristics after LT4 Medication
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vukovic, R.; Zeljkovic, A.; Bufan, B.; Spasojevic-Kalimanovska, V.; Milenkovic, T.; Vekic, J. Hashimoto Thyroiditis and Dyslipidemia in Childhood: A Review. Front. Endocrinol. 2019, 10, 868. [Google Scholar] [CrossRef]
- Kaličanin, D.; Brčić, L.; Ljubetić, K.; Barić, A.; Gračan, S.; Brekalo, M.; Lovrić, V.T.; Kolčić, I.; Polašek, O.; Zemunik, T.; et al. Differences in food consumption between patients with Hashimoto’s thyroiditis and healthy individuals. Sci. Rep. 2020, 10, 10670. [Google Scholar] [CrossRef]
- Özen, S.; Berk, Ö.; Şimşek, D.G.; Darcan, Ş. Clinical Course of Hashimoto’s Thyroiditis and Effects of Levothyroxine Therapy on the Clinical Course of the Disease in Children and Adolescents. J. Clin. Res. Pediatric Endocrinol. 2011, 3, 192–197. [Google Scholar] [CrossRef]
- Skarpa, V.; Κousta, E.; Tertipi, A.; Anyfandakis, K.; Vakaki, M.; Dolianiti, M.; Fotinou, A.; Papathanasiou, A. Epidemiological characteristics of children with autoimmune thyroid disease. Hormones 2011, 10, 207–214. [Google Scholar] [CrossRef] [PubMed]
- De Luca, F.; Santucci, S.; Corica, D.; Pitrolo, E.; Romeo, M.; Aversa, T. Hashimoto’s thyroiditis in childhood: Presentation modes and evolution over time. Ital. J. Pediatrics 2013, 39, 8. [Google Scholar] [CrossRef]
- Jonklaas, J.; Bianco, A.; Bauer, A.J.; Burman, K.D.; Cappola, A.R.; Celi, F.S.; Cooper, D.S.; Kim, B.W.; Peeters, R.P.; Rosenthal, M.S.; et al. Guidelines for the treatment of hypothyroidism: Prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid 2014, 24, 1670–1751. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, U.; Tekin, S.; Demir, M.; Cigremis, Y.; Sandal, S. Effects of central FGF21 infusion on the hypothalamus–pituitary–thyroid axis and energy metabolism in rats. J. Physiol. Sci. 2018, 68, 781–788. [Google Scholar] [CrossRef]
- Fisher, F.M.; Maratos-Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Zeng, J.; Huang, P.; Yan, B.; Zeng, X.; Liu, C.; Shi, X.; Wang, L.; Song, H.; Lin, M.; et al. Independent association of serum fibroblast growth factor 21 levels with impaired liver enzymes in hyperthyroid patients. Front. Endocrinol. 2019, 9, 800. [Google Scholar] [CrossRef]
- Keuper, M.; Häring, H.U.; Staiger, H. Circulating FGF21 Levels in Human Health and Metabolic Disease. Exp. Clin. Endocrinol. Diabetes 2020, 128, 752–770. [Google Scholar] [CrossRef]
- Adams, A.C.; Cheng, C.C.; Coskun, T.; Kharitonenkov, A. FGF21 Requires βklotho to Act In Vivo. PLoS ONE 2012, 7, e49977. [Google Scholar] [CrossRef]
- Lee, Y.; Park, Y.J.; Ahn, H.Y.; Lim, J.A.; Park, K.U.; Choi, S.H.; Park, D.J.; Oh, B.-C.; Jang, H.C.; Yi, K.H. Plasma FGF21 levels are increased in patients with hypothyroidism independently of lipid profile. Endocr. J. 2013, 60, 977–983. [Google Scholar] [CrossRef]
- Wang, G.; Liu, J.; Yang, N.; Hu, Y.; Zhang, H.; Miao, L.; Yao, Z.; Xu, Y. Levothyroxine treatment restored the decreased circulating fibroblast growth factor 21 levels in patients with hypothyroidism. Eur. J. Intern. Med. 2016, 31, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Bande, A.; Kalra, P.; Dharmalingam, M.; Selvan, C.; Suryanarayana, K. Serum fibroblast growth factor 21 levels in patients with hyperthyroidism and its association with body fat percentage. Indian J. Endocrinol. Metab. 2019, 23, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Bonde, Y.; Breuer, O.; Lütjohann, D.; Sjöberg, S.; Angelin, B.; Rudling, M. Thyroid hormone reduces PCSK9 and stimulates bile acid synthesis in humans. J. Lipid Res. 2014, 55, 2408–2415. [Google Scholar] [CrossRef]
- Domouzoglou, E.; Fisher, F.M.; Astapova, I.; Fox, E.C.; Kharitonenkov, A.; Flier, J.S.; Hollenberg, A.N.; Maratos-Flier, E. Fibroblast growth factor 21 and thyroid hormone show mutual regulatory dependency but have independent actions in vivo. Endocrinology 2014, 155, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sieglaff, U.H.; York, J.P.; Suh, J.H.; Ayers, S.D.; Winnier, G.E.; Kharitonenkov, A.; Pin, C.; Zhang, P.; Webb, P.; et al. Thyroid hormone receptor regulates most genes independently of fibroblast growth factor 21 in liver. J. Endocrinol. 2015, 224, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Caturegli, P.; De Remigis, A.; Rose, N.R. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Vigone, M.C.; Capalbo, D.; Weber, G.; Salerno, M. Mild Hypothyroidism in Childhood: Who, When, and How Should Be Treated? J. Endocr. Soc. 2018, 2, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, M.H.; Lohman, T.G.; Boileau, R.A.; Horswill, C.A.; Stillman, R.J.; Van Loan, M.D.; Bemben, D.A. Skinfold equations for estimation of body fatness in children and youths. Hum. Biol. 1988, 60, 709–723. [Google Scholar]
- Al Shammari, E.; Bano, R.; Suneetha, E.; Alshammri, A.R.H. FFM Index, FM Index and PBF in Subjects with Normal, Overweight, and Obese BMI in Saudi Arabia Female Population. J. Food Res. 2015, 5, 40–48. [Google Scholar] [CrossRef][Green Version]
- Wendel, D.; Weber, D.; Leonard, M.B.; Magge, S.N.; Kelly, A.; Stallings, V.A.; Pipan, M.; Stettler, N.; Zemel, B.S. Body composition estimation using skinfolds in children with and without health conditions affecting growth and body composition. Ann. Hum. Biol. 2017, 44, 108–120. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in pattern of pubertal changes in girls. Obstet. Gynecol. Surv. 1969, 25, 694–696. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in pattern of pubertal changes in boys. Arch. Diesease Child. 1970, 45, 13–23. [Google Scholar] [CrossRef]
- Nieman, D.C.; Austin, M.D.; Benezra, L.; Pearce, S.; McInnis, T.; Unick, J.; Gross, S.J. Validation of cosmed’s FitMateTM in measuring oxygen consumption and estimating resting metabolic rate. Res. Sport Med. 2006, 14, 89–96. [Google Scholar] [CrossRef]
- Fullmer, S.; Benson-Davies, S.; Earthman, C.P.; Frankenfield, D.C.; Gradwell, E.; Lee, P.S.; Piemonte, T.; Trabulsi, J. Evidence Analysis Library Review of Best Practices for Performing Indirect Calorimetry in Healthy and Non-Critically Ill Individuals. J. Acad. Nutr. Diet 2015, 115, 1417–1446.e2. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Ribas, L.; Ngo, J.; Ortega, R.M.; García, A.; Pérez-Rodrigo, C.; Aranceta, J. Food, youth and the Mediterranean diet in Spain. Development of KIDMED, Mediterranean Diet Quality Index in children and adolescents. Public Health Nutr. 2004, 7, 931–935. [Google Scholar] [CrossRef]
- Štefan, L.; Prosoli, R.; Juranko, D.; Čule, M.; Milinović, I.; Novak, D.; Sporiš, G. The reliability of the mediterranean diet quality index (KIDMED) questionnaire. Nutrients 2017, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, A.; Sørensen, K.; Johannsen, T.; Helge, J.; Andersson, A.-M.; Juul, A. Significant gender difference in serum levels of fibroblast growth factor 21 in Danish children and adolescents. Int. J. Pediatric Endocrinol. 2014, 2014, 7. [Google Scholar] [CrossRef][Green Version]
- Giannini, C.; Feldstein, A.E.; Santoro, N.; Kim, G.; Kursawe, R.; Pierpont, B.; Caprio, S. Circulating levels of FGF-21 in obese youth: Associations with liver fat content and markers of liver damage. J. Clin. Endocrinol. Metab. 2013, 98, 2993–3000. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Nam, H.-K.; Rhie, Y.-J.; Lee, K.-H. Serum FGF21 Levels in Obese Korean Children and Adolescents. J. Obes. Metab. Syndr. 2017, 26, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Yang, J.; Li, H.; Zhong, H.; Wan, Q. Changes in glucose-lipid metabolism, insulin resistance, and inflammatory factors in patients with autoimmune thyroid disease. J. Clin. Lab. Anal. 2019, 33, e22929. [Google Scholar] [CrossRef]
- Çatlı, G.; Anık, A.; Tuhan, H.; Böber, E.; Abacı, A. The Effect of l-Thyroxine Treatment on Hypothyroid Symptom Scores and Lipid Profile in Children with Subclinical Hypothyroidism. J. Clin. Res. Pediatric Endocrinol. 2014, 6, 238–244. [Google Scholar] [CrossRef]
- Xiao, F.; Lin, M.; Huang, P.; Zeng, J.; Zeng, X.; Zhang, H.; Li, X.; Yang, S.; Li, Z.; Li, X. Elevated serum fibroblast growth factor 21 levels in patients with hyperthyroidism. J. Clin. Endocrinol. Metab. 2015, 100, 3800–3805. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hanks, L.J.; Gutiérrez, O.M.; Bamman, M.M.; Ashraf, A.; McCormick, K.L.; Casazza, K. Circulating levels of fibroblast growth factor-21 increase with age independently of body composition indices among healthy individuals. J. Clin. Transl. Endocrinol. 2015, 2, 77–82. [Google Scholar] [CrossRef]
- Mcaninch, E.A.; Bianco, A.C. Thyroid hormone signaling in energy homeostasis and energy metabolism. Ann. N. Y. Acad. Sci. 2014, 1311, 77–87. [Google Scholar] [CrossRef]
- Hu, S.; Rayman, M.P. Multiple Nutritional Factors and the Risk of Hashimoto’s Thyroiditis. Thyroid 2017, 27, 597–610. [Google Scholar] [CrossRef]
| Parameter | Control Group (n = 30) | AIT Treatment Group (n = 30) | AIT Euthyroid Group (n = 30) | p-Value |
|---|---|---|---|---|
| Age (yrs) | 10.89 ± 2.29 | 10.99 ± 1.85 | 11.01 ± 1.93 | 0.970 |
| SDS BMI | 0.88 (−0.01–1.22) | 0.73 (0.12–1.64) | 0.36 (−1.07–1.16) | 0.046 |
| WAIST C. (cm) | 69.33 ± 9.99 | 67.73 ± 9.32 | 65.65 ± 8.59 | 0.313 |
| HIP C. (cm) | 80.63 ± 10.58 | 81.38 ± 9.58 | 77.41 ± 9.69 | 0.267 |
| MUAC (cm) | 23.00 (20.00–25.25) | 22.00 (20.00–25.25) | 21.00 (19.75–23.62) | 0.195 |
| %BF | 22.6 (18.53–31.8) | 24.95 (21.15–32.07) | 21.65 (16.47–29.83) | 0.482 |
| FMI (kg/ht2) | 4.42 (3.17–5.98) | 4.49 (3.43–7.06) | 4.12 (2.64–5.63) | 0.313 |
| FFMI (kg/ht2) | 14.66 (13.43–15.31) | 14.27 (13.50–14.63) | 13.47 (12.69–14.58) | 0.103 |
| TSH (μIU/L) | 2.40 (1.95–3.06) | 4.82 (3.99–9.66) | 2.63 (2.13–3.09) | 0.002 |
| FT3 (pmol/L) | 6.28 (5.71–6.79) | 5.96 (5.44–6.40) | 6.27 (5.91–6.71) | 0.295 |
| FT4 (pmol/L) | 15.19 ± 1.93 | 14.29 ± 2.45 | 15.19 ± 2.57 | 0.313 |
| Glucose (mmol/L) | 4.88 (4.65–5.11) | 4.86 (4.66–5.12) | 4.74 (4.49–5.05) | 0.302 |
| Insulin (pmol/L) | 48.12 (37.36–81.32) | 64.58 (46.46–86.87) | 54.65 (33.96–81.87) | 0.472 |
| HOMA-IR | 1.50 (1.11–2.54) | 1.93 (1.39–2.72) | 1.62 (1.02–2.52) | 0.143 |
| TC (mmol/L) | 3.88 ± 0.75 | 4.22 ± 0.84 | 4.22 ± 0.69 | 0.144 |
| TG (mmol/L) | 0.52 (0.45–0.65) | 0.74 (0.52–0.89) | 0.66 (0.56–1.02) | 0.042 |
| HDL (mmol/L) | 1.38 (1.23–1.63) | 1.43 (1.18–1.65) | 1.54 (1.24–1.99) | 0.157 |
| LDL (mmol/L) | 2.07 (1.65–2.56) | 2.30 (2.01–2.92) | 2.33 (2.04–2.64) | 0.501 |
| AST (IU/L) | 24.50 (18.75–28.25) | 23.00 (19.00–25.25) | 27.50 (23.75–29.00) | 0.253 |
| ALT (IU/L) | 14.50 (13.00–18.00) | 15.00 (13.00–19.50) | 16.50 (13.75–19.00) | 0.410 |
| γ-GT (IU/L) | 12.00 (10.00–13.25) | 12.00 (10.75–15.00) | 12.00 (11.00–13.25) | 0.284 |
| ALP (IU/L) | 217.00 (149.5–275.50) | 200.00 (157.75–291.50) | 217.00 (183.25–275.00) | 0.475 |
| RMR/Weight (kJ/kg per d) | 150.46 (122.09–190.58) | 131.08 (108.62–165.10) | 168.74 (133.26–193.51) | 0.089 |
| FGF-21 (pg/mL) | 217.36 (193.60–235.21) | 182.71 (169.32–234.55) | Na | 0.717 |
| Parameter | Correlation Coefficient (r) | p-Value |
|---|---|---|
| %BF | 0.015 | 0.887 |
| FMI (kg/ht2) | −0.017 | 0.871 |
| FFMI (kg/ht2) | −0.036 | 0.738 |
| TSH (μIU/L) | −0.096 | 0.367 |
| FT3 (pmol/L) | 0.177 | 0.094 |
| FT4 (pmol/L) | 0.399 | 0.001 * |
| Glucose (mmol/L) | −0.035 | 0.741 |
| Insulin (pmol/L) | 0.078 | 0.463 |
| HOMA-IR | 0.083 | 0.436 |
| TC (mmol/L) | −0.025 | 0.812 |
| TG (mmol/L) | 0.17 | 0.872 |
| HDL (mmol/L) | 0.089 | 0.405 |
| LDL (mmol/L) | −0.23 | 0.833 |
| AST (IU/L) | 0.31 | 0.771 |
| ALT (IU/L) | −0.035 | 0.742 |
| γ-GT (IU/L) | −1.05 | 0.324 |
| ALP (IU/L) | −0.060 | 0.576 |
| RMR/FFM (kJ/kg per d) | 0.088 | 0.411 |
| RMR/Weight (kJ/kg per d) | 0.064 | 0.551 |
| KIDMED score | −0.200 | 0.126 |
| Parameters | Before Treatment (n = 30) | After Treatment (n = 30) | p-Value |
|---|---|---|---|
| SDS BMI | 0.73 (0.12–1.64) | 0.88 (0.10–1.50) | 0.915 |
| WAIST C. (cm) | 67.73 ± 9.32 | 69.2 ± 8.61 | 0.529 |
| HIP C. (cm) | 81.38 ± 9.58 | 81.50 ± 14.71 | 0.971 |
| MUAC (cm) | 22.00 (20.00–25.25) | 23.00 (22.00–26.00) | 0.215 |
| %BF | 24.95 (21.15–32.07) | 24.06 (19.27–31.16) | 0.955 |
| FMI (kg/ht2) | 4.49 (3.43–7.06) | 8.98 (4.87–13.44) | 0.001 |
| FFMI (kg/ht2) | 14.27 (13.50–14.63) | 24.90 (18.52–31.31) | 0.000 |
| TSH (μIU/L) | 4.82 (3.99–9.66) | 4.03 (2.43–6.20) | 0.054 |
| FT3 (pmol/L) | 5.96 (5.44–6.40) | 5.90 (5.22–6.21) | 0.055 |
| FT4 (pmol/L) | 14.29 ± 2.45 | 15.70 ± 2.45 | 0.037 |
| Anti-TPOAb (IU/mL) | 979.50 (135.27–2383.72) | 800.00 (73.47–1687.20) | 0.400 |
| Anti-TgAb (IU/mL) | 283.90 (69.40–500.00) | 236.20 (87.95–500.00) | 0.401 |
| Glucose (mmol/L) | 4.86 (4.66–5.12) | 4.97 (4.72–5.19) | 0.374 |
| Insulin (pmol/L) | 64.58 (46.46–86.87) | 65.07 (47.85–87.43) | 0.346 |
| HOMA-IR | 1.93 (1.39–2.72) | 1.90 (1.57–2.65) | 0.405 |
| TC (mmol/L) | 4.22 ± 0.84 | 4.19 ± 0.86 | 0.913 |
| TG (mmol/L) | 0.74 (0.52–0.89) | 0.73 (0.56–0.93) | 0.517 |
| HDL (mmol/L) | 1.43 (1.18–1.65) | 1.46 (1.26–1.61) | 0.865 |
| LDL (mmol/L) | 2.30 (2.01–2.92) | 2.30 (1.93–2.82) | 0.781 |
| AST (IU/L) | 23.00 (19.00–25.25) | 22.00 (18.00–25.00) | 0.096 |
| ALT (IU/L) | 15.00 (13.00–19.50) | 15.50 (13.75–19.25) | 0.315 |
| γ-GT (IU/L) | 12.00 (10.75–15.00) | 11.00 (10.00–14.00) | 0.652 |
| ALP (IU/L) | 200.00 (157.75–291.50) | 224.50 (162.50–270.25) | 0.414 |
| RMR/Weight (kJ/kg per d) | 131.08 (108.62–165.10) | 142.51 (116.61–168.53) | 0.517 |
| FGF-21 (pg/mL) | 182.71 (169.32–234.55) | 198.43 (183.86–248.42) | 0.734 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drongitis, P.; Kotanidou, E.P.; Serbis, A.; Tsinopoulou, V.R.; Gerou, S.; Galli-Tsinopoulou, A. Serum Fibroblast Growth Factor 21 Levels in Children and Adolescents with Hashimoto’s Thyroiditis before and after l-Thyroxin Medication: A Prospective Study. Medicina 2021, 57, 1374. https://doi.org/10.3390/medicina57121374
Drongitis P, Kotanidou EP, Serbis A, Tsinopoulou VR, Gerou S, Galli-Tsinopoulou A. Serum Fibroblast Growth Factor 21 Levels in Children and Adolescents with Hashimoto’s Thyroiditis before and after l-Thyroxin Medication: A Prospective Study. Medicina. 2021; 57(12):1374. https://doi.org/10.3390/medicina57121374
Chicago/Turabian StyleDrongitis, Pavlos, Eleni P Kotanidou, Anastasios Serbis, Vasiliki Rengina Tsinopoulou, Spyridon Gerou, and Assimina Galli-Tsinopoulou. 2021. "Serum Fibroblast Growth Factor 21 Levels in Children and Adolescents with Hashimoto’s Thyroiditis before and after l-Thyroxin Medication: A Prospective Study" Medicina 57, no. 12: 1374. https://doi.org/10.3390/medicina57121374
APA StyleDrongitis, P., Kotanidou, E. P., Serbis, A., Tsinopoulou, V. R., Gerou, S., & Galli-Tsinopoulou, A. (2021). Serum Fibroblast Growth Factor 21 Levels in Children and Adolescents with Hashimoto’s Thyroiditis before and after l-Thyroxin Medication: A Prospective Study. Medicina, 57(12), 1374. https://doi.org/10.3390/medicina57121374








