Accuracy of Opportunistic Bone Mineral Density Assessment on Staging Computed Tomography for Gynaecological Cancers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Radiological Methodology
2.3. Statistical Analyses
3. Results
3.1. Cohort Demographics
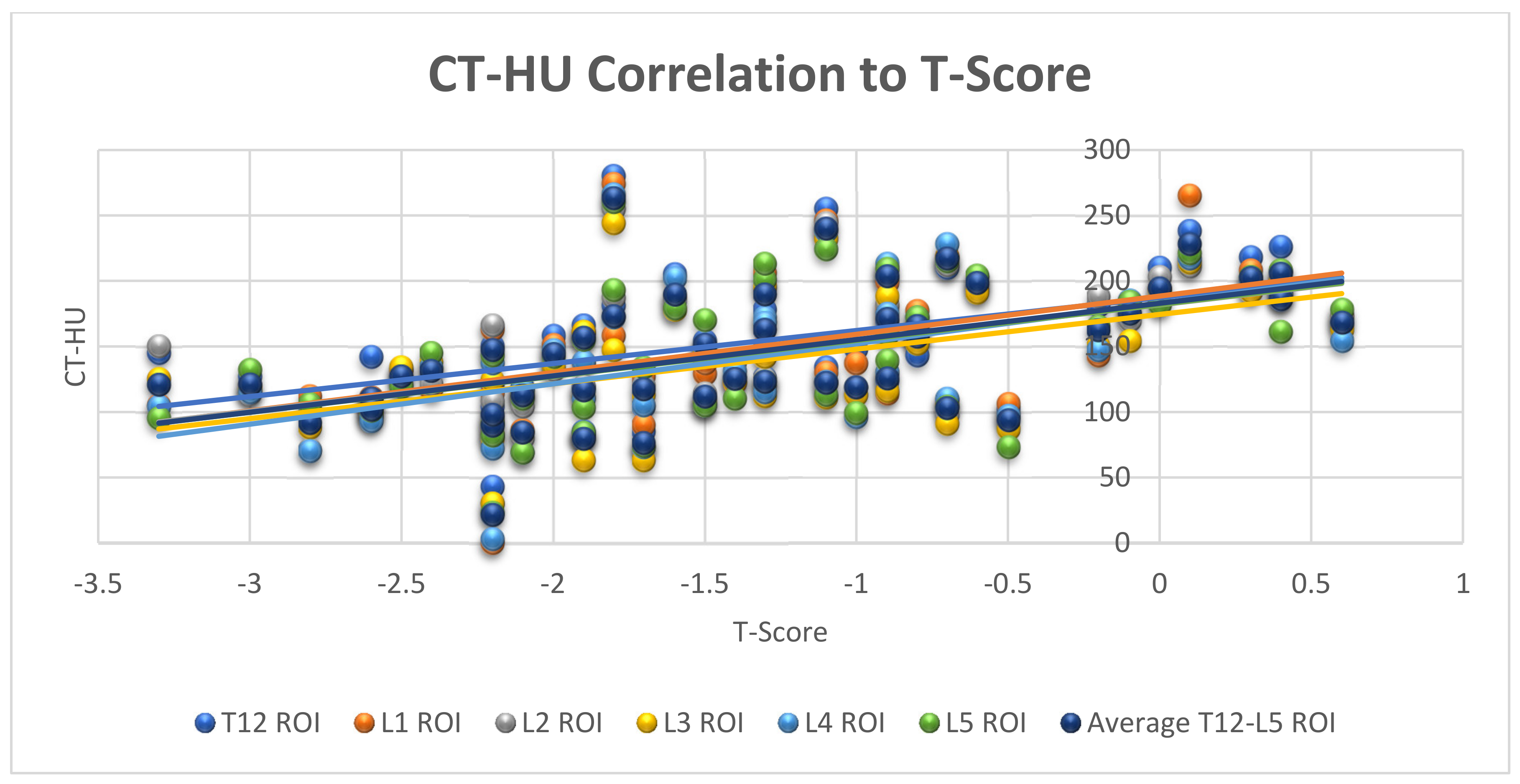
3.2. Correlation between CT and DXA in Measurement of Bone Mineral Density
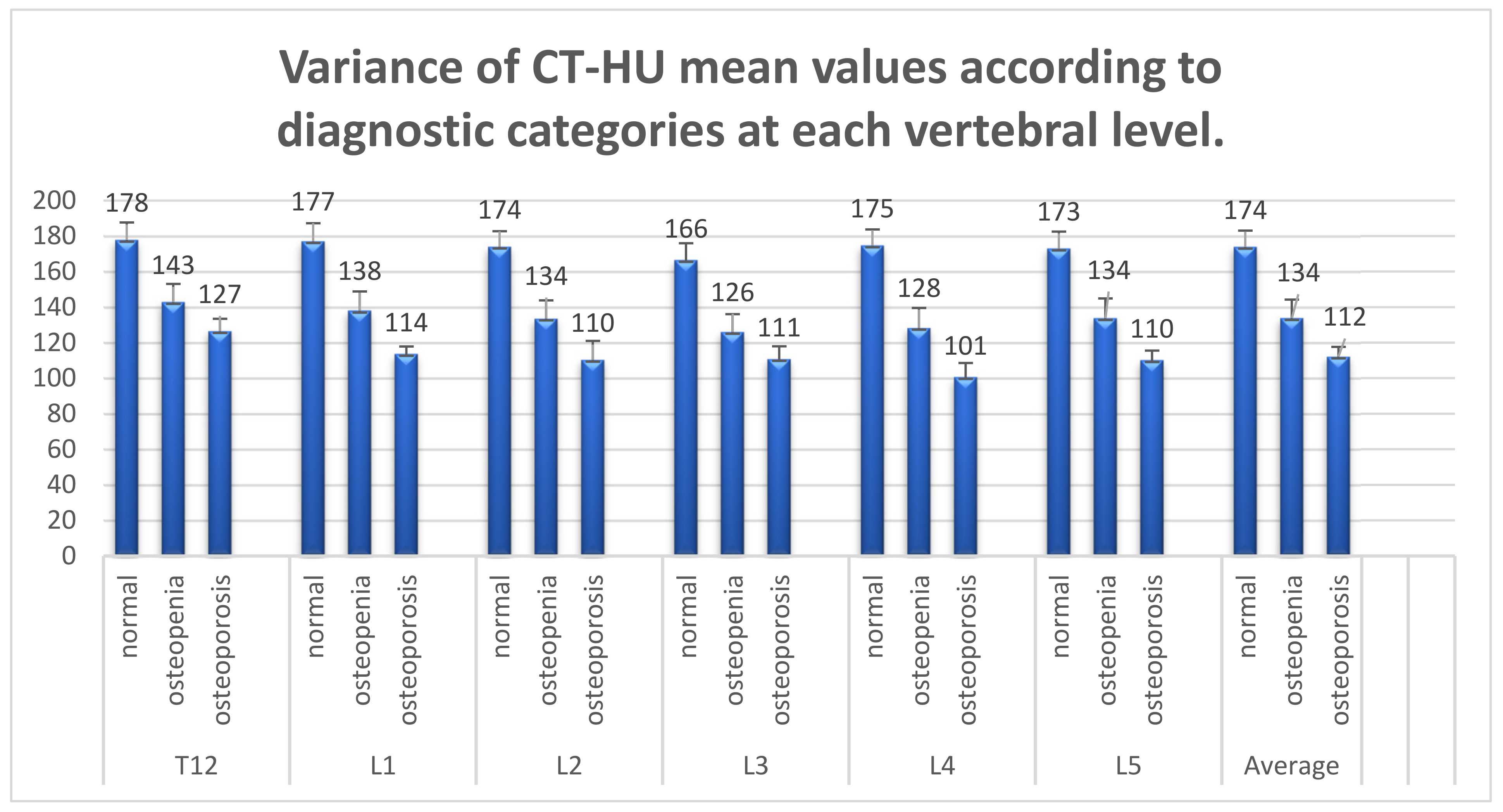
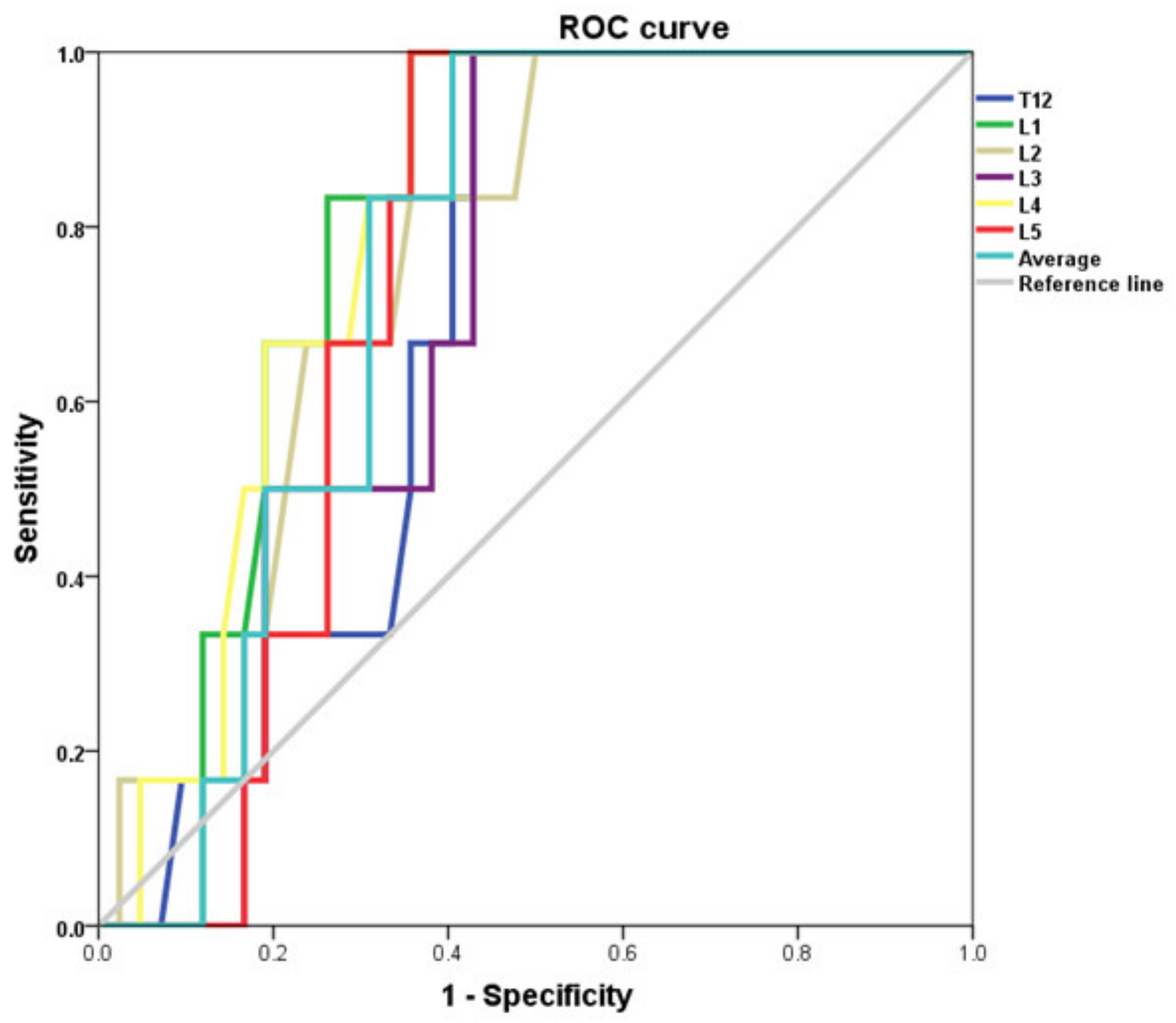
3.3. Analysis of CT Diagnostic Capabilities in Bone Densitometry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Lumbar Levels | Areal BMD (Mean) | CT-HU (Mean) |
|---|---|---|
| T12 | - | 153 HU |
| L1 | 1.07 g/cm2 | 149 HU |
| L2 | 1.15 g/cm2 | 145 HU |
| L3 | 1.19 g/cm2 | 139 HU |
| L4 | 1.22 g/cm2 | 141 HU |
| L5 | - | 145 HU |
| Vertebral Level | ANOVA (p Value) | Normal—Osteopaenia (p Value) | Normal—Osteoporosis (p Value) | Osteopaenia-Osteoporosis (p Value) |
|---|---|---|---|---|
| T12 | 0.02 * | |||
| Hochberg GT2 | 0.049 * | 0.059 | 0.809 | |
| Games-Howell | 0.046 * | 0.001 * | 0.399 | |
| Dunnett t | 0.016 * | 0.019 * | N/A | |
| L1 | 0.008 * | |||
| Hochberg GT2 | 0.033 * | 0.02 * | 0.59 | |
| Games-Howell | 0.032 * | 0.0 * | 0.11 | |
| Dunnett t | 0.011 * | 0.007 * | N/A | |
| L2 | 0.004 * | |||
| Hochberg GT2 | 0.016 * | 0.012 * | 0.574 | |
| Games-Howell | 0.013 * | 0.0028 | 0.285 | |
| Dunnett t | 0.005 * | 0.004 * | N/A | |
| L3 | 0.006 * | |||
| Hochberg GT2 | 0.015 * | 0.03 * | 0.826 | |
| Games-Howell | 0.015 * | 0.0 * | 0.44 | |
| Dunnett t | 0.005 * | 0.01 * | N/A | |
| L4 | 0.002 * | |||
| Hochberg GT2 | 0.009 * | 0.006 * | 0.488 | |
| Games-Howell | 0.007 * | 0.0 * | 0.129 | |
| Dunnett t | 0.003 * | 0.002 * | N/A | |
| L5 | 0.008 * | |||
| Hochberg GT2 | 0.033 * | 0.022 * | 0.615 | |
| Games-Howell | 0.029 * | 0.0 * | 0.154 | |
| Dunnett t | 0.011 * | 0.007* | N/A | |
| Average | 0.005 * | |||
| Hochberg GT2 | 0.018 * | 0.015 * | 0.631 | |
| Games-Howell | 0.016 * | 0.0 * | 0.17 | |
| Dunnett t | 0.006 * | 0.005 * | N/A |
| Threshold (HU) | SENS | SPEC | PPV | NPV | ACCURACY |
|---|---|---|---|---|---|
| 90 | 12.9 | 100 | 100 | 38.64 | 43.75 |
| 100 | 16.13 | 100 | 100 | 39.53 | 45.83 |
| 110 | 22.58 | 88.24 | 77.78 | 38.46 | 45.83 |
| 120 | 41.94 | 82.35 | 81.25 | 43.75 | 56.25 |
| 130 | 54.84 | 82.35 | 85 | 50 | 64.58 |
| 140 | 67.74 | 82.35 | 87.5 | 58.33 | 72.92 |
| 150 | 74.19 | 76.47 | 85.19 | 61.9 | 75 |
| 160 | 80.65 | 70.59 | 83.33 | 66.67 | 77.08 |
| 170 | 87.1 | 70.59 | 84.38 | 75 | 81.25 |
| 180 | 87.1 | 58.82 | 79.41 | 71.43 | 77.08 |
| 190 | 90.3 | 47.1 | 75.7 | 72.7 | 75 |
| 200 | 90.3 | 23.5 | 68.3 | 57.1 | 66 |
| Threshold (HU) | SENS | SPEC | PPV | NPV | ACCURACY |
|---|---|---|---|---|---|
| 90 | 14.3 | 92.7 | 25 | 86.4 | 81.25 |
| 100 | 14.3 | 90.24 | 20 | 86.1 | 79.2 |
| 110 | 42.9 | 85.4 | 33.3 | 89.74 | 79.2 |
| 120 | 85.7 | 75.6 | 37.5 | 96.9 | 77.08 |
| 130 | 85.7 | 65.9 | 30 | 96.4 | 68.8 |
| 140 | 100 | 58.5 | 29.2 | 100 | 64.6 |
| 150 | 100 | 51.2 | 25.9 | 100 | 58.33 |
| 160 | 100 | 43.9 | 23.33 | 100 | 52.1 |
| 170 | 100 | 39 | 21.9 | 100 | 47.9 |
| 180 | 100 | 34 | 20.6 | 100 | 43.8 |
References
- Van Staa, T.P.; Dennison, E.M.; Leufkens, H.G.M.; Cooper, C. Epidemiology of fractures in England and Wales. Bone 2001, 29, 517–522. [Google Scholar] [CrossRef] [Green Version]
- McGowan, B.; Kanis, J.A.; Johansson, H.; Silke, C.; Whelan, B. Development and application of FRAX in the management of osteoporosis in Ireland. Arch. Osteoporos. 2013, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.M.; Moon, R.J.; Harvey, N.C.; Cooper, C. The impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Bone 2017, 104, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borgström, F.; Karlsson, L.; Ortsäter, G.; Norton, N.; Halbout, P.; Cooper, C.; Lorentzon, M.; McCloskey, E.V.; Harvey, N.C.; Javaid, M.K. Fragility fractures in Europe: Burden, management and opportunities. Arch. Osteoporos. 2020, 15, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Sernbo, I.; Johnell, O. Consequences of a hip fracture: A prospective study over 1 year. Osteoporos. Int. 1993, 3, 148–153. [Google Scholar] [CrossRef]
- Menéndez-Colino, R.; Alarcon, T.; Gotor, P.; Queipo, R.; Ramírez-Martín, R.; Otero, A.; González-Montalvo, J.I. Baseline and pre-operative 1-year mortality risk factors in a cohort of 509 hip fracture patients consecutively admitted to a co-managed orthogeriatric unit (FONDA Cohort). Injury 2018, 49, 656–661. [Google Scholar] [CrossRef]
- Hadjidakis, D.; Kokkinakis, E.; Sfakianakis, M.; Raptis, S.A. The type and time of menopause as decisive factors for bone mass changes. Eur. J. Clin. Investig. 1999, 29, 877–885. [Google Scholar] [CrossRef]
- Guise, T.A. Bone loss and fracture risk associated with cancer therapy. Oncologist 2006, 11, 1121–1131. [Google Scholar] [CrossRef]
- Hadji, P.; Gnant, M.; Body, J.-J.; Bundred, N.; Brufsky, A.; Coleman, R.; Guise, T.; Lipton, A.; Aapro, M. Cancer treatment-induced bone loss in premenopausal women: A need for therapeutic intervention? Cancer Treat. Rev. 2012, 38, 798–806. [Google Scholar] [CrossRef]
- Shapiro, C.L.; Van Poznak, C.; Lacchetti, C.; Kirshner, J.; Eastell, R.; Gagel, R.; Smith, S.; Edwards, B.J.; Frank, E.; Lyman, G.H.; et al. Management of Osteoporosis in Survivors of Adult Cancers With Nonmetastatic Disease: ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 2916–2946. [Google Scholar] [CrossRef]
- Res Sapienza, L.G.; Salcedo, M.P.; Ning, M.S.; Jhingran, A.; Klopp, A.H.; Calsavara, V.F.; Schmeler, K.M.; Leite Gomes, M.J.; de Freitas Carvalho, E.; Baiocchi, G. Pelvic Insufficiency Fractures After External Beam Radiation Therapy for Gynecologic Cancers: A Meta-analysis and Meta-regression of 3929 Patients. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 475–484. [Google Scholar] [CrossRef] [Green Version]
- Coleman, R.; Hadji, P.; Body, J.-J.; Santini, D.; Chow, E.; Terpos, E.; Oudard, S.; Bruland, Ø.; Flamen, P.; Kurth, A. Bone health in cancer: ESMO clinical practice guidelines. Ann. Oncol. 2020, 31, 1650–1663. [Google Scholar] [CrossRef]
- Shuhart, C.R.; Yeap, S.S.; Anderson, P.A.; Jankowski, L.G.; Lewiecki, E.M.; Morse, L.R.; Rosen, H.N.; Weber, D.R.; Zemel, B.S.; Shepherd, J.A. Executive Summary of the 2019 ISCD Position Development Conference on Monitoring Treatment, DXA Cross-calibration and Least Significant Change, Spinal Cord Injury, Peri-prosthetic and Orthopedic Bone Health, Transgender Medicine, and Pediatrics. J. Clin. Densitom. 2019, 22, 453–471. [Google Scholar] [CrossRef]
- Shepstone, L.; Lenaghan, E.; Cooper, C.; Clarke, S.; Fong-Soe-Khioe, R.; Fordham, R.; Gittoes, N.; Harvey, I.; Harvey, N.; Heawood, A. Screening in the community to reduce fractures in older women (SCOOP): A randomised controlled trial. Lancet 2018, 391, 741–747. [Google Scholar] [CrossRef] [Green Version]
- Rubin, K.H.; Rothmann, M.J.; Holmberg, T.; Hoiberg, M.; Moller, S.; Barkmann, R.; Gluer, C.C.; Hermann, A.P.; Bech, M.; Gram, J.; et al. Effectiveness of a two-step population-based osteoporosis screening program using FRAX: The randomized Risk-stratified Osteoporosis Strategy Evaluation (ROSE) study. Osteoporos. Int. 2018, 29, 567–578. [Google Scholar] [CrossRef]
- O’Gorman, C.; Minnock, S.; Mulhall, J.; Gleeson, N. Attention to Bone Health in Follow Up of Gynecological Cancers in Tertiary Care; Women’s Health. 2021. [Google Scholar]
- Jang, S.; Graffy, P.M.; Ziemlewicz, T.J.; Lee, S.J.; Summers, R.M.; Pickhardt, P.J. Opportunistic osteoporosis screening at routine abdominal and thoracic CT: Normative L1 trabecular attenuation values in more than 20 000 adults. Radiology 2019, 291, 360–367. [Google Scholar] [CrossRef]
- Lee, S.J.; Pickhardt, P.J. Opportunistic screening for osteoporosis using body CT scans obtained for other indications: The UW experience. Clin. Rev. Bone Miner. Metab. 2017, 15, 128–137. [Google Scholar] [CrossRef]
- Pickhardt, P.J.; Pooler, B.D.; Lauder, T.; del Rio, A.M.; Bruce, R.J.; Binkley, N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann. Intern. Med. 2013, 158, 588–595. [Google Scholar] [CrossRef]
- Summers, R.M.; Baecher, N.; Yao, J.; Liu, J.; Pickhardt, P.J.; Choi, J.R.; Hill, S. Feasibility of simultaneous CT colonography and fully-automated bone mineral densitometry in a single examination. J. Comput. Assist. Tomogr. 2011, 35, 212. [Google Scholar] [CrossRef] [Green Version]
- Gerety, E.L.; Hopper, M.A.; Bearcroft, P.W. The reliability of measuring the density of the L1 vertebral body on CT imaging as a predictor of bone mineral density. Clin. Radiol. 2017, 72, 177.e9–177.e15. [Google Scholar] [CrossRef]
- Genant, H.K.; Wu, C.Y.; van Kuijk, C.; Nevitt, M.C. Vertebral fracture assessment using a semiquantitative technique. J. Bone Miner. Res. 1993, 8, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Buckens, C.F.; Dijkhuis, G.; de Keizer, B.; Verhaar, H.J.; de Jong, P.A. Opportunistic screening for osteoporosis on routine computed tomography? An external validation study. Eur. Radiol. 2015, 25, 2074–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenchik, L.; Weaver, A.A.; Ward, R.J.; Boone, J.M.; Boutin, R.D. Opportunistic Screening for Osteoporosis Using Computed Tomography: State of the Art and Argument for Paradigm Shift. Curr. Rheumatol. Rep. 2018, 20, 74. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.J.; Roberts, C.C.; Bencardino, J.T.; Arnold, E.; Baccei, S.J.; Cassidy, R.C.; Chang, E.Y.; Fox, M.G.; Greenspan, B.S.; Gyftopoulos, S.; et al. ACR Appropriateness Criteria((R)) Osteoporosis and Bone Mineral Density. J. Am. Coll. Radiol. 2017, 14, S189–S202. [Google Scholar] [CrossRef] [Green Version]
- Mao, S.S.; Li, D.; Syed, Y.S.; Gao, Y.; Luo, Y.; Flores, F.; Child, J.; Cervantes, M.; Kalantar-Zadeh, K.; Budoff, M.J. Thoracic Quantitative Computed Tomography (QCT) Can Sensitively Monitor Bone Mineral Metabolism: Comparison of Thoracic QCT vs Lumbar QCT and Dual-energy X-ray Absorptiometry in Detection of Age-relative Change in Bone Mineral Density. Acad. Radiol. 2017, 24, 1582–1587. [Google Scholar] [CrossRef]
- Graffy, P.M.; Lee, S.J.; Ziemlewicz, T.J.; Pickhardt, P.J. Prevalence of Vertebral Compression Fractures on Routine CT Scans According to L1 Trabecular Attenuation: Determining Relevant Thresholds for Opportunistic Osteoporosis Screening. AJR Am. J. Roentgenol. 2017, 209, 491–496. [Google Scholar] [CrossRef]
- Fink, H.A.; Harrison, S.L.; Taylor, B.C.; Cummings, S.R.; Schousboe, J.T.; Kuskowski, M.A.; Stone, K.L.; Ensrud, K.E.; Study of Osteoporotic Fractures Group. Differences in site-specific fracture risk among older women with discordant results for osteoporosis at hip and spine: Study of osteoporotic fractures. J. Clin. Densitom. 2008, 11, 250–259. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Binkley, N.; Lubner, M.G.; Bruce, R.J.; Ziemlewicz, T.J.; Pickhardt, P.J. Opportunistic screening for osteoporosis using the sagittal reconstruction from routine abdominal CT for combined assessment of vertebral fractures and density. Osteoporos. Int. 2016, 27, 1131–1136. [Google Scholar] [CrossRef]
- Garner, H.W.; Paturzo, M.M.; Gaudier, G.; Pickhardt, P.J.; Wessell, D.E. Variation in Attenuation in L1 Trabecular Bone at Different Tube Voltages: Caution Is Warranted When Screening for Osteoporosis With the Use of Opportunistic CT. AJR Am. J. Roentgenol. 2017, 208, 165–170. [Google Scholar] [CrossRef]

| Age | Median (range) | 57 | (31–86) |
| BMI | Median (range) | 30.4 | (15.6–57.8) |
| N | (%) | ||
| Smoking Status | Current Smokers | 9 | (18%) |
| Ex-smokers | 15 | (30%) | |
| Never-smokers | 26 | (52%) | |
| Menopausal Status | Premenopausal | 13 | (26%) |
| Post-menopausal | 37 | (74%) | |
| Alcohol intake | <10 units/week | 32 | (64%) |
| 10–20 units/week | 2 | (4%) | |
| >20 units/week | 3 | (6%) | |
| Tumour Site | Endometrium | 29 | (58%) |
| Tubal/ovarian/peritoneal | 7 | (14%) | |
| Cervix | 11 | (22%) | |
| Vulva | 2 | (4%) | |
| Unknown | 1 | (2%) | |
| Treatment | Bilateral Oophorectomy | 40 | (80%) |
| Ext. Beam Radiotherapy | 21 | (42%) | |
| Chemotherapy | 18 | (36%) |
| DXA Categories | T-Score (Mean) | Areal BMD (Mean) | CT-HU (Mean) |
|---|---|---|---|
| Normal (n = 17) | −0.3 SD | 1.3 g/cm2 | 174 HU (95%CI 154–193) |
| Osteopaenia (n = 26) | −1.7 SD | 1.1 g/cm2 | 134 HU (95%CI 112–155) |
| Osteoporosis (n = 7) | −2.8 SD | 1.0 g/cm2 | 112 HU (95%CI 98–126) |
| Vertebral Level | AUC | St. Error | 95% CI | Significance |
|---|---|---|---|---|
| T12 Low BMD | 0.754 | 0.078 | 0.60–0.91 | 0.004 |
| Osteoporosis | 0.698 | 0.079 | 0.54–0.85 | 0.119 |
| L1 Low BMD | 0.771 | 0.075 | 0.62–0.92 | 0.002 |
| Osteoporosis | 0.796 | 0.064 | 0.67–0.92 | 0.020 |
| L2 Low BMD | 0.789 | 0.073 | 0.65–0.93 | 0.001 |
| Osteoporosis | 0.758 | 0.079 | 0.60–0.91 | 0.043 |
| L3 Low BMD | 0.786 | 0.074 | 0.64–0.93 | 0.001 |
| Osteoporosis | 0.702 | 0.077 | 0.55–0.85 | 0.112 |
| L4 Low BMD | 0.811 | 0.065 | 0.68–0.94 | 0.000 |
| Osteoporosis | 0.802 | 0.066 | 0.67–0.93 | 0.018 |
| L5 Low BMD | 0.759 | 0.075 | 0.61–0.91 | 0.003 |
| Osteoporosis | 0.738 | 0.068 | 0.61–0.87 | 0.061 |
| Avg. Low BMD | 0.792 | 0.071 | 0.65–0.93 | 0.001 |
| Osteoporosis | 0.750 | 0.070 | 0.61–0.89 | 0.050 |
| Threshold above which patients are less likely to have low BMD | ||||
| CT-HU | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
| 170 | 87.1 | 70.59 | 84.38 | 75 |
| 180 | 87.1 | 58.82 | 79.41 | 71.43 |
| 190 | 90.3 | 47.1 | 75.7 | 72.7 |
| 200 | 90.3 | 23.5 | 68.3 | 57.1 |
| Threshold below which patients are more likely to have osteoporosis/vertebral fracture | ||||
| CT-HU | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
| 90 | 14.3 | 92.7 | 25 | 86.4 |
| 100 | 14.3 | 90.24 | 20 | 86.1 |
| 110 | 42.9 | 85.4 | 33.3 | 89.74 |
| 120 | 85.7 | 75.6 | 37.5 | 96.9 |
| Youdon Index; statistically optimal CT-HU diagnostic thresholds for L1 | ||||
| CT-HU | Sensitivity (%) | Specificity (%) | Youden’s J stat. | |
| Low BMD | 168 | 87 | 70 | 0.577 |
| Osteoporosis | 134 | 100 | 64 | 0.643 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Gorman, C.A.; Milne, S.; Lambe, G.; Sobota, A.; Beddy, P.; Gleeson, N. Accuracy of Opportunistic Bone Mineral Density Assessment on Staging Computed Tomography for Gynaecological Cancers. Medicina 2021, 57, 1386. https://doi.org/10.3390/medicina57121386
O’Gorman CA, Milne S, Lambe G, Sobota A, Beddy P, Gleeson N. Accuracy of Opportunistic Bone Mineral Density Assessment on Staging Computed Tomography for Gynaecological Cancers. Medicina. 2021; 57(12):1386. https://doi.org/10.3390/medicina57121386
Chicago/Turabian StyleO’Gorman, Catherine Anne, Sarah Milne, Gerard Lambe, Aleksandra Sobota, Peter Beddy, and Noreen Gleeson. 2021. "Accuracy of Opportunistic Bone Mineral Density Assessment on Staging Computed Tomography for Gynaecological Cancers" Medicina 57, no. 12: 1386. https://doi.org/10.3390/medicina57121386
APA StyleO’Gorman, C. A., Milne, S., Lambe, G., Sobota, A., Beddy, P., & Gleeson, N. (2021). Accuracy of Opportunistic Bone Mineral Density Assessment on Staging Computed Tomography for Gynaecological Cancers. Medicina, 57(12), 1386. https://doi.org/10.3390/medicina57121386





