Effects of Antithrombotic Treatment on Bleeding Complications of EBUS-TBNA
Abstract
1. Introduction
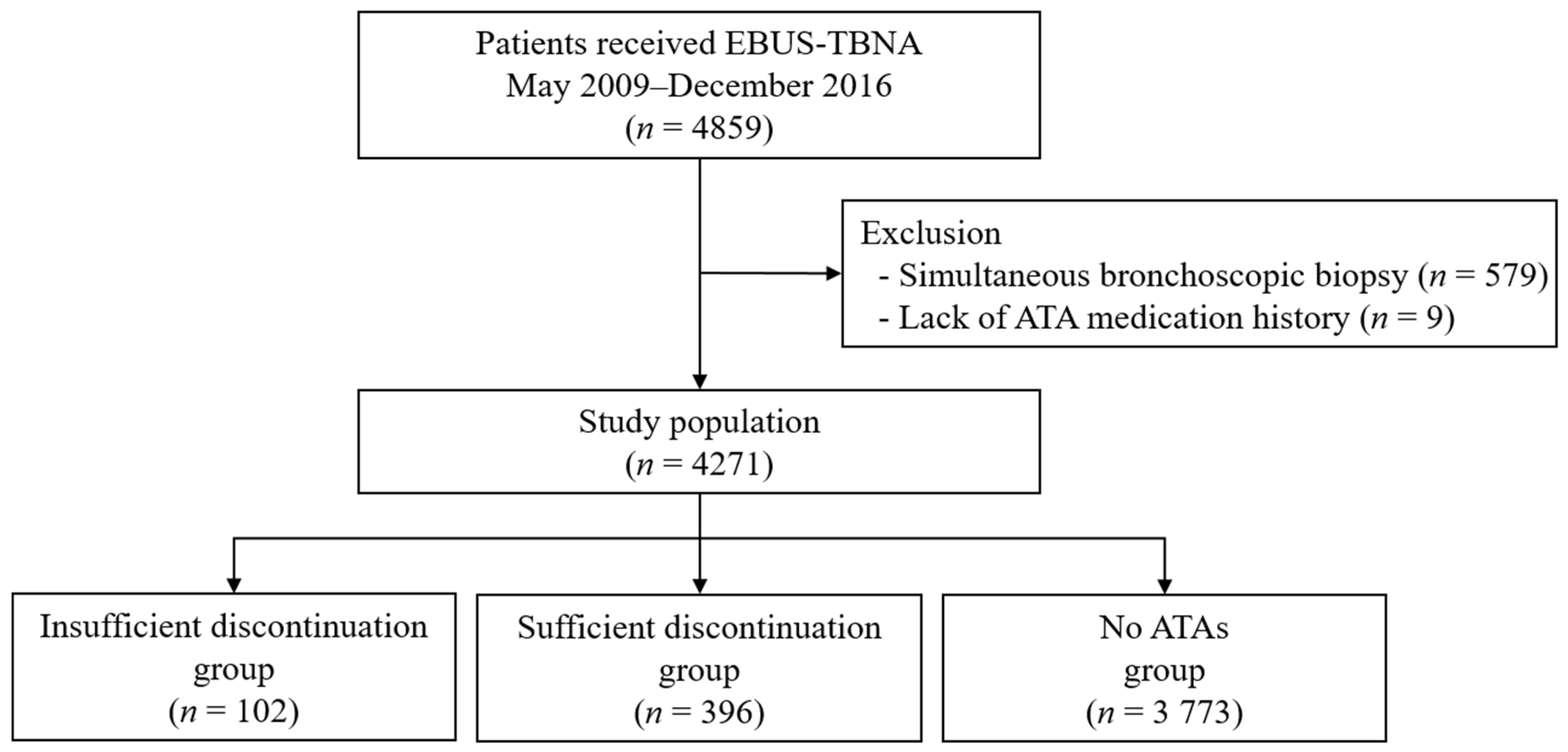
2. Materials and Methods
2.1. Patients
2.2. Data Collection and Definitions
2.3. EBUS-TBNA Procedures
2.4. Statistical Analysis
3. Results
3.1. Basline Characteristics
3.2. Antithrombotic Medication
3.3. Bleeding Complications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, K.; Um, S.-W. Minimally invasive mediastinal staging of non-small cell lung cancer. Precis Future Med. 2018, 2, 18–26. [Google Scholar] [CrossRef]
- Adams, K.; Shah, P.L.; Edmonds, L.; Lim, E. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: Systematic review and meta-analysis. Thorax 2009, 64, 757–762. [Google Scholar] [CrossRef] [PubMed]
- De Leyn, P.; Dooms, C.; Kuzdzal, J.; Lardinois, D.; Passlick, B.; Rami-Porta, R.; Turna, A.; Van Schil, P.; Venuta, F.; Waller, D.; et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 2014, 45, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Um, S.W.; Kim, H.K.; Jung, S.H.; Han, J.; Lee, K.J.; Park, H.Y.; Choi, Y.S.; Shim, Y.M.; Ahn, M.J.; Park, K.; et al. Endobronchial ultrasound versus mediastinoscopy for mediastinal nodal staging of non-small-cell lung cancer. J. Thorac. Oncol. 2015, 10, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Facciolongo, N.; Patelli, M.; Gasparini, S.; Lazzari Agli, L.; Salio, M.; Simonassi, C.; Del Prato, B.; Zanoni, P. Incidence of complications in bronchoscopy. Multicentre prospective study of 20,986 bronchoscopies. Monaldi Arch. Chest Dis. 2009, 71, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.; Eberhardt, R.; Wahidi, M.; Becker, H.D.; Herth, F.J. Effect of routine clopidogrel use on bleeding complications after transbronchial biopsy in humans. Chest 2006, 129, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Du Rand, I.A.; Blaikley, J.; Booton, R.; Chaudhuri, N.; Gupta, V.; Khalid, S.; Mandal, S.; Martin, J.; Mills, J.; Navani, N.; et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: Accredited by NICE. Thorax 2013, 68 (Suppl. S1), i1–i44. [Google Scholar] [CrossRef] [PubMed]
- Abuqayyas, S.; Raju, S.; Bartholomew, J.R.; Abu Hweij, R.; Mehta, A.C. Management of antithrombotic agents in patients undergoing flexible bronchoscopy. Eur. Respir. Rev. 2017, 26, 170001. [Google Scholar] [CrossRef] [PubMed]
- Meena, N.; Abouzgheib, W.; Patolia, S.; Rosenheck, J.; Boujaoude, Z.; Bartter, T. EBUS-TBNA and EUS-FNA: Risk Assessment for Patients Receiving Clopidogrel. J. Bronchol. Interv. Pulmonol. 2016, 23, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Webb, T.N.; Flenaugh, E.; Martin, R.; Parks, C.; Bechara, R.I. Effect of Routine Clopidogrel Use on Bleeding Complications After Endobronchial Ultrasound-guided Fine Needle Aspiration. J. Bronchol. Interv. Pulmonol. 2019, 26, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Stather, D.R.; MacEachern, P.; Chee, A.; Tremblay, A. Safety of endobronchial ultrasound-guided transbronchial needle aspiration for patients taking clopidogrel: A report of 12 consecutive cases. Respiration 2012, 83, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Ko, R.E.; Jeong, B.H.; Chon, H.R.; Huh, H.J.; Han, J.; Lee, H.; Lee, K.; Kim, H.; Kwon, O.J.; Um, S.W. Clinical usefulness of routine AFB culture and MTB PCR of EBUS-TBNA needle rinse fluid. Respirology 2019, 24, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Jeong, D.Y.; Lee, K.S.; Cho, J.H.; Choi, Y.S.; Lee, K.; Um, S.W.; Kim, H.; Jeong, B.H. Which definition of a central tumour is more predictive of occult mediastinal metastasis in nonsmall cell lung cancer patients with radiological N0 disease? Eur. Respir. J. 2019, 53, 1801508. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Morimoto, T.; Natsuaki, M.; Furukawa, Y.; Nakagawa, Y.; Kadota, K.; Yamaji, K.; Ando, K.; Shizuta, S.; Shiomi, H.; et al. Antiplatelet therapy discontinuation and the risk of serious cardiovascular events after coronary stenting: Observations from the CREDO-Kyoto Registry Cohort-2. PLoS ONE 2015, 10, e0124314. [Google Scholar] [CrossRef] [PubMed]
- Gil, H.I.; Choe, J.; Jeong, B.H.; Um, S.W.; Jeon, K.; Hahn, J.Y.; Kim, H.; Kwon, O.J.; Chang, Y.S.; Lee, K. Safety of endobronchial ultrasound-guided transbronchial needle aspiration in patients with lung cancer within a year after percutaneous coronary intervention. Thorac. Cancer 2018, 9, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Weimar, C.; Cotton, D.; Sha, N.; Sacco, R.L.; Bath, P.M.; Weber, R.; Diener, H.C. Discontinuation of antiplatelet study medication and risk of recurrent stroke and cardiovascular events: Results from the PRoFESS study. Cerebrovasc. Dis. 2013, 35, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.L.; Diener, H.C.; Yusuf, S.; Cotton, D.; Ounpuu, S.; Lawton, W.A.; Palesch, Y.; Martin, R.H.; Albers, G.W.; Bath, P.; et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N. Engl. J. Med. 2008, 359, 1238–1251. [Google Scholar] [CrossRef] [PubMed]
- Eapen, G.A.; Shah, A.M.; Lei, X.; Jimenez, C.A.; Morice, R.C.; Yarmus, L.; Filner, J.; Ray, C.; Michaud, G.; Greenhill, S.R.; et al. Complications, consequences, and practice patterns of endobronchial ultrasound-guided transbronchial needle aspiration: Results of the AQuIRE registry. Chest 2013, 143, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
| Variables | Insufficient Discontinuation Group (n = 102) | Sufficient Discontinuation Group (n = 396) | p |
|---|---|---|---|
| Age, years | 69.7 ± 7.2 | 68.6 ± 7.9 | 0.201 |
| Sex, male | 84 (82.4) | 316 (79.8) | 0.661 |
| Pre-procedure diagnosis | 0.526 | ||
| Primary lung cancer | 91 (89.2) | 342 (86.4) | |
| Other cancer | 8 (7.8) | 37 (9.3) | |
| Other benign disease | 3 (2.9) | 17 (4.3) | |
| Laboratory tests | |||
| Platelet count, ×103/µL | 252.6 ± 101.3 | 247.1 ± 81.3 | 0.924 |
| Prothrombin time, INR | 1.1 ± 0.3 | 1.0 ± 0.1 | 0.026 |
| aPTT, seconds | 37.7 ± 7.7 | 36.9 ± 6.5 | 0.459 |
| Blood urea nitrogen, mg/dL | 17.6 ± 7.2 | 17.0 ± 6.5 | 0.850 |
| Creatinine, mg/dL | 1.1 ± 0.7 | 1.0 ± 0.8 | 0.174 |
| Total bilirubin, mg/dL | 0.5 ± 0.3 | 0.5 ± 0.2 | 0.547 |
| Aspartate aminotransferase, U/L | 22.7 ± 12.2 | 21.6 ± 8.4 | 0.946 |
| Alanine aminotransferase, U/L | 20.2 ± 14.8 | 19.7 ± 11.7 | 0.932 |
| Variables | Insufficient Discontinuation Group (n = 284) | Sufficient Discontinuation Group (n = 999) | p |
|---|---|---|---|
| Examined site | 0.897 | ||
| Mediastinal LN | 231 (81.3) | 822 (82.3) | |
| Hilar, interlobar, and lobar LN | 42 (14.8) | 137 (13.7) | |
| Others * | 11 (3.9) | 40 (4.0) | |
| Size of examined lesion, mm † | |||
| Short-axis diameter | 11.2 ± 6.7 | 12.0 ± 6.9 | 0.093 |
| Long-axis diameter | 16.2 ± 9.6 | 17.0 ± 9.8 | 0.211 |
| Number of needle passes per lesion | 1.9 ± 0.8 | 1.9 ± 1.1 | 0.389 |
| Number of obtained core tissues per lesion | 1.5 ± 0.7 | 1.6 ± 1.1 | 0.128 |
| Procedure time, minutes | 21.7 ± 12.1 | 20.8 ± 11.7 | 0.640 |
| Variables | Insufficient Discontinuation Group (n = 102) | Sufficient Discontinuation Group (n = 396) | p |
|---|---|---|---|
| Reason for antithrombotic medication | |||
| Ischemic heart disease | 49 (48.0) | 121 (30.6) | 0.001 |
| Cerebral vascular disease | 29 (28.4) | 80 (20.2) | 0.090 |
| Prevention | 12 (11.8) | 141 (35.6) | <0.001 |
| Thrombotic arrhythmia | 7 (6.9) | 39 (9.8) | 0.474 |
| Peripheral artery occlusive disease | 4 (3.9) | 6 (1.5) | 0.125 |
| Other * | 1 (1.0) | 9 (2.3) | 0.695 |
| Antithrombotic medication | |||
| Aspirin | 45 (44.1) † | 329 (83.1) | <0.001 |
| Discontinued days | 1 [0–4] | 6 [5–8] | - |
| Clopidogrel | 86 (84.3) | 57 (14.4) | <0.001 |
| Discontinued days | 1 [0–5] | 7 [6–7] | - |
| Cilostazol | 13 (12.7) | 22 (5.6) | 0.019 |
| Discontinued days | 1 [0–2] | 6 [3–6] | - |
| Warfarin | 5 (4.9) | 22 (5.6) | 0.807 |
| Discontinued days | 2 [1–4] | 6 [6–7] | - |
| LMWH | 4 (3.9) | 7 (1.8) | 0.246 |
| Discontinued hours | 19 [17–26] ‡ | 30 [27–37] | - |
| Direct oral anticoagulants | 5 (4.9) | 5 (1.3) | 0.034 |
| Discontinued days | 1 [1–1] | 5 [2–7] | - |
| More than two drugs | 52 (51.0) | 49 (12.4) | <0.001 |
| Case # | Group | Age/ Sex | Reason for Procedure | Platelets, ×103/µL | PT, INR | No. of Examined Lesions, n | Singularity on the Examined Lesions | Procedure Time, min | Management for Bleeding | Final Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|
| #1 * | Insuff | 68/M | Suspected meta of CRC | 363 | 1.18 | 3 | - | 28 | TF | Meta of CRC |
| #2 † | Suff | 74/M | Suspected LC | 296 | 1.00 | 1 | Necrotic | 20 | Epi | NSCLC (cT4N1M0) |
| #3 | No ATAs | 68/F | Suspected LC | 251 | 0.95 | 4 | Necrotic | 40 | Epi | Benign granuloma |
| #4 | No ATAs | 66/M | Suspected LC | 308 | 1.03 | 3 | Mucosal fragility | 14 | Epi | NSCLC (cT2aN2M0) |
| #5 | No ATAs | 74/F | Suspected recurrence of LC | 125 | 1.01 | 2 | - | 17 | Epi | No recurrence |
| #6 | No ATAs | 49/M | Suspected LC | 387 | 1.09 | 2 | Mucosal fragility | 19 | Epi | NSCLC (pT3N1M0) |
| #7 | No ATAs | 66/F | Suspected LC | 266 | 1.07 | 3 | Necrotic | 14 | Epi | NSCLC (cT3N2M0) |
| #8 | No ATAs | 60/F | Suspected meta of EMC or HCC | 163 | 1.04 | 3 | Necrotic | 10 | ICU | Meta of EMC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, H.-I.; Ko, R.-E.; Lee, K.; Um, S.-W.; Kim, H.; Jeong, B.-H. Effects of Antithrombotic Treatment on Bleeding Complications of EBUS-TBNA. Medicina 2021, 57, 142. https://doi.org/10.3390/medicina57020142
Gil H-I, Ko R-E, Lee K, Um S-W, Kim H, Jeong B-H. Effects of Antithrombotic Treatment on Bleeding Complications of EBUS-TBNA. Medicina. 2021; 57(2):142. https://doi.org/10.3390/medicina57020142
Chicago/Turabian StyleGil, Hyun-Il, Ryoung-Eun Ko, Kyungjong Lee, Sang-Won Um, Hojoong Kim, and Byeong-Ho Jeong. 2021. "Effects of Antithrombotic Treatment on Bleeding Complications of EBUS-TBNA" Medicina 57, no. 2: 142. https://doi.org/10.3390/medicina57020142
APA StyleGil, H.-I., Ko, R.-E., Lee, K., Um, S.-W., Kim, H., & Jeong, B.-H. (2021). Effects of Antithrombotic Treatment on Bleeding Complications of EBUS-TBNA. Medicina, 57(2), 142. https://doi.org/10.3390/medicina57020142








