Neuropsychiatric Consequences of Lipophilic Beta-Blockers
Abstract
:1. Introduction
2. Research Strategy
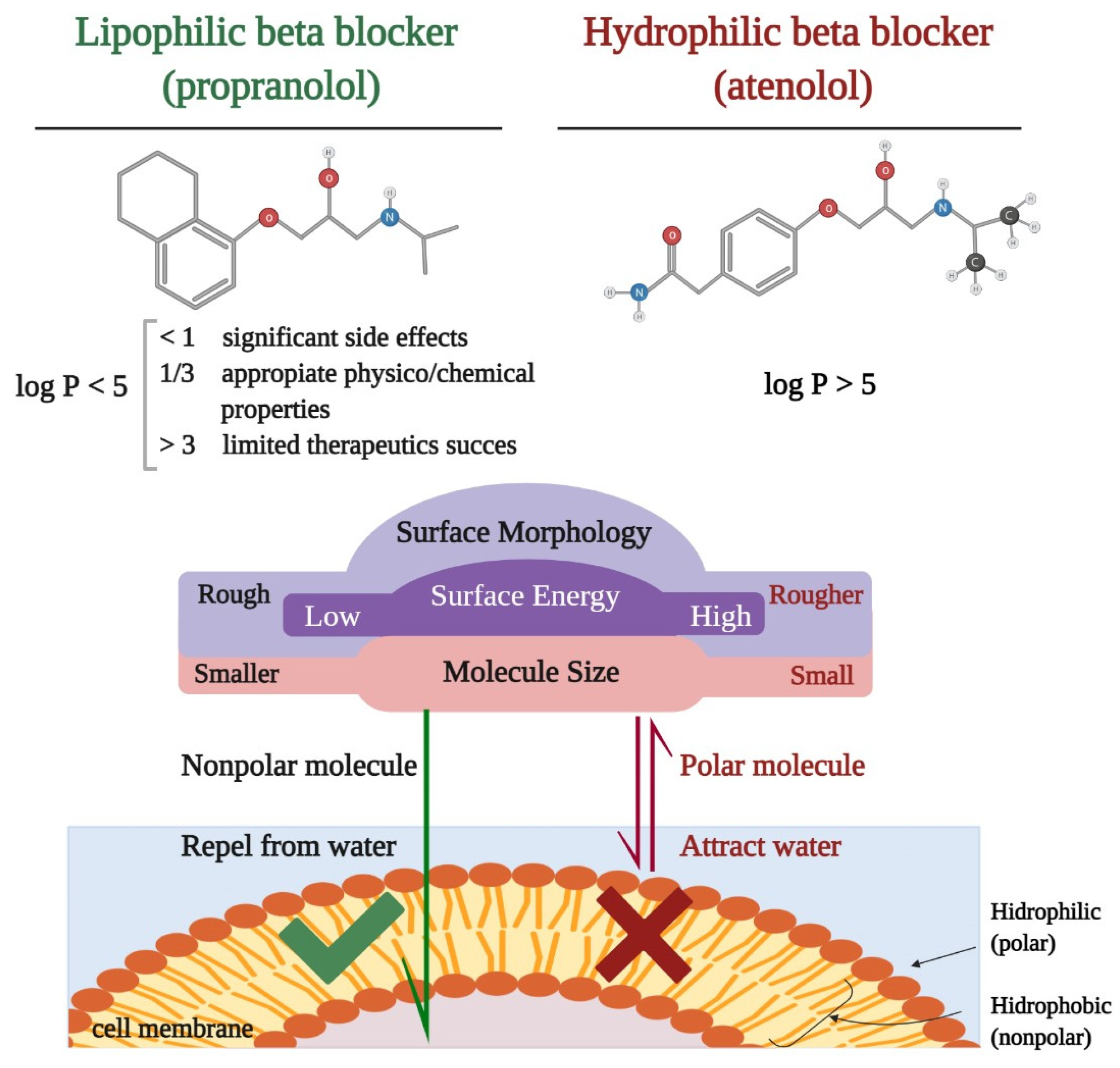
3. Pharmacological Characteristics
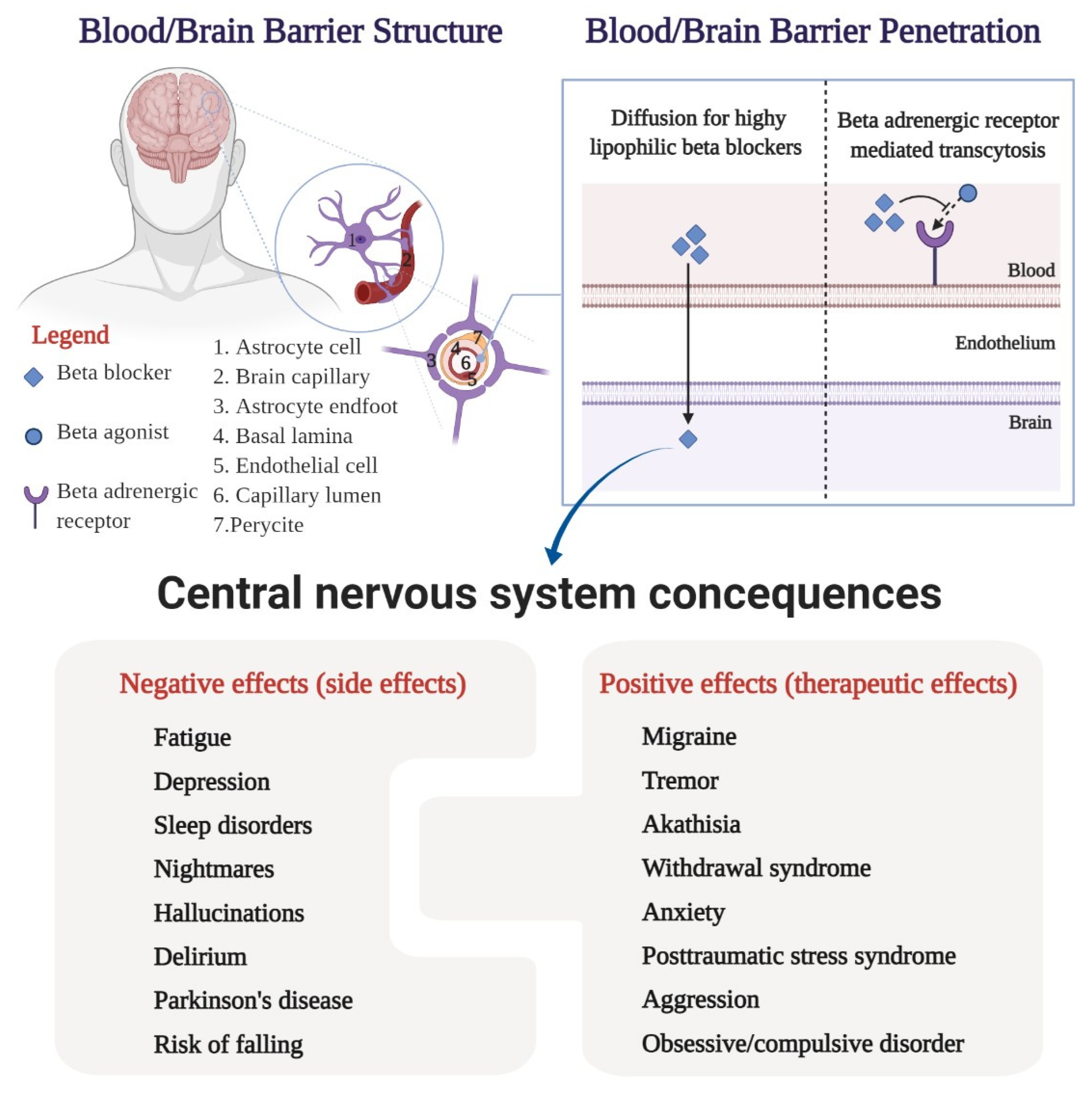
4. Central Nervous System Side Effects
4.1. Fatigue
4.2. Depression
4.3. Sleep Disorders and Nightmares
4.4. Hallucinations and Delirium
4.5. Parkinson’s Disease
4.6. The Risk of Falling
5. Central Nervous System Therapeutic Effects
5.1. Migraine
5.2. Tremor
5.3. Akathisia and Alcohol or Benzodiazepine Withdrawal Syndrome
5.4. Anxiety and Posttraumatic Stress Syndrome
5.5. Aggression
5.6. Obsessive–Compulsive Disorder
6. Limitations and Strengths
7. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- McAinsh, J.; Cruickshank, J.M. Beta-blockers and central nervous system side effects. Pharmacol. Ther. 1990, 46, 163–197. [Google Scholar] [CrossRef]
- Weber, M.A. The role of the new beta-blockers in treating cardiovascular disease. Am. J. Hypertens. 2005, 18, 169S–176S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, R.; Babar, A.; Patel, A.; Dortonne, R.; Jordan, J. Metoprolol-Associated Central Nervous System Complications. Cureus 2020, 12, e8236. [Google Scholar] [CrossRef] [PubMed]
- Márquez, P.H.P.; Torres, O.H.; San-José, A.; Vidal, X.; Agustí, A.; Formiga, F.; López-Soto, A.; Ramírez-Duque, N.; Fernández-Moyano, A.; Ruiz, D.; et al. Potentially Inappropriate Prescription in Older Patients in Spain (PIPOPS) Investigators’ Project. Potentially Inappropriate Antihypertensive Prescriptions to Elderly Patients: Results of a Prospective, Observational Study. Drugs Aging. 2017, 34, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Stuhec, M.; Keuschler, J.; Serra-Mestres, J.; Isetta, M. Effects of different antihypertensive medication groups on cognitive function in older patients: A systematic review. Eur. Psychiatry 2017, 46, 1–15. [Google Scholar] [CrossRef]
- de Quervain, D.J.; Aerni, A.; Roozendaal, B. Preventive effect of beta-adrenoceptor blockade on glucocorticoid-induced memory retrieval deficits. Am. J. Psychiatry 2007, 164, 967–969. [Google Scholar] [CrossRef] [PubMed]
- Fiedorowicz, J.G. Depression and cardiovascular disease: An update on how course of illness may influence risk. Curr. Psychiatry Rep. 2014, 16, 492. [Google Scholar] [CrossRef] [Green Version]
- Lichtman, J.H.; Froelicher, E.S.; Blumenthal, J.A.; Carney, R.M.; Doering, L.V.; Frasure-Smith, N.; Freedland, K.E.; Jaffe, A.S.; Leifheit-Limson, E.C.; Sheps, D.S.; et al. American Heart Association Statistics Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: Systematic review and recommendations: A scientific statement from the American Heart Association. Circulation 2014, 129, 1350–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, S.; Frishman, W.H. Neuropsychiatric effects of cardiovascular drug therapy. Cardiol. Rev. 2003, 11, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.C.; Christian, B.T.; Okonkwo, O.C.; Oh, J.M.; Harding, S.; Xu, G.; Hillmer, A.T.; Wooten, D.W.; Murali, D.; Barnhart, T.E.; et al. Amyloid burden and neural function in people at risk for Alzheimer’s Disease. Neurobiol. Aging 2014, 35, 576–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimi, J.; Kovacs, G.G. Prevalence of mixed pathologies in the aging brain. Alzheimers Res. Ther. 2014, 6, 82. [Google Scholar] [CrossRef] [Green Version]
- Fumagalli, C.; Maurizi, N.; Marchionni, N.; Fornasari, D. β-blockers: Their new life from hypertension to cancer and migraine. Pharmacol. Res. 2020, 151, 104587. [Google Scholar] [CrossRef] [PubMed]
- Brodde, O.E.; Bruck, H.; Leineweber, K. Cardiac adrenoceptors: Physiological and pathophysiological relevance. J. Pharmacol. Sci. 2006, 100, 323–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, L.; Yang, H.; Cai, Y.; Sun, L.; Di, P.; Li, W.; Liu, G.; Tang, Y. ADMET-score–A comprehensive scoring function for evaluation of chemical drug-likeness. MedChemComm 2018, 10, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Frishman, W.H.; Lazar, E.J.; Gorodokin, G. Pharmacokinetic optimisation of therapy with beta-adrenergic blocking agents. Clin. Pharmacokinet. 1991, 20, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Frishman, W. Clinical pharmacology of the new beta-adrenergic blocking drugs. Part 1. Pharmacodynamic and pharmacokinetic properties. Am. Heart J. 1979, 97, 663–670. [Google Scholar] [CrossRef]
- Arnott, J.A.; Planey, S.L. The influence of lipophilicity in drug discovery and design. Expert Opin. Drug Discov. 2012, 7, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Magder, S.; Sami, M.; Ripley, R.; Lisbona, R. Comparison of the effects of pindolol and propranolol on exercise performance in patients with angina pectoris. Am. J. Cardiol. 1987, 59, 1289–1294. [Google Scholar] [CrossRef]
- Lertora, J.J.; Mark, A.L.; Johannsen, J.; Wilson, W.R.; Abboud, F.M. Selective beta-1 receptor blockade with oral practolol in man. A dose-related phenomenon. J. Clin. Investig. 1975, 56, 719–724. [Google Scholar] [CrossRef] [Green Version]
- Leeson, P.D.; Davis, A.M. Time-related differences in the physical property profiles of oral drugs. J. Med. Chem. 2004, 47, 6338–6348. [Google Scholar] [CrossRef]
- Testa, B.; Crivori, P.; Reist, M.; Carrupt, P.A. The influence of lipophilicity on the pharmacokinetic behavior of drugs: Concepts and examples. Perspect. Drug Discov. Des. 2000, 19, 179–211. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Waring, M.J. Lipophilicity in drug discovery. Expert Opin. Drug Discov. 2010, 5, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Krämer, S.D.; Wunderli-Allenspach, H. Physicochemical properties in pharmacokinetic lead optimization. Farmaco 2001, 56, 145–148. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Seelig, A. A general pattern for substrate recognition by P-glycoprotein. Eur. J. Biochem. 1998, 251, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Testa, B.; Fahr, A. Lipophilicity and its relationship with passive drug permeation. Pharm. Res. 2011, 28, 962–977. [Google Scholar] [CrossRef]
- Huffman, J.C.; Stern, T.A. Neuropsychiatric consequences of cardiovascular medications. Dialogues Clin. Neurosci. 2007, 9, 29–45. [Google Scholar] [CrossRef]
- Kaminska, M.; Kimoff, R.J.; Schwartzman, K.; Trojan, D.A. Sleep disorders and fatigue in multiple sclerosis: Evidence for association and interaction. J. Neurol. Sci. 2011, 302, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Braley, T.J.; Chervin, R.D. Fatigue in multiple sclerosis: Mechanisms, evaluation, and treatment. Sleep 2010, 33, 1061–1067. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Behan, P.O. Fatigue in neurological disorders. Lancet 2004, 363, 978–988. [Google Scholar] [CrossRef]
- Hall, P.E.; Kendall, M.J.; Smith, S.R. Beta blockers and fatigue. J. Clin. Hosp. Pharm. 1984, 9, 283–291. [Google Scholar] [CrossRef] [PubMed]
- McKelvie, R.S.; Jones, N.L.; Heigenhauser, G.J. Factors contributing to increased muscle fatigue with beta-blockers. Can. J. Physiol. Pharmacol. 1991, 69, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.T.; Hebert, P.R.; Coffey, C.S.; Sedrakyan, A.; Curtis, J.P.; Krumholz, H.M. Beta-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA 2002, 288, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Fellenius, E. Muscle fatigue and beta-blockers—A review. Int. J. Sports Med. 1983, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Agustini, B.; Mohebbi, M.; Woods, R.L.; McNeil, J.J.; Nelson, M.R.; Shah, R.C.; Murray, A.M.; Ernst, M.E.; Reid, C.M.; Tonkin, A.; et al. on behalf of the ASPREE Investigator Group. The association of antihypertensive use and depressive symptoms in a large older population with hypertension living in Australia and the United States: A cross-sectional study. J. Hum. Hypertens. 2020, 34, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Thiessen, B.Q.; Wallace, S.M.; Blackburn, J.L.; Wilson, T.W.; Bergman, U. Increased prescribing of antidepressants subsequent to beta-blocker therapy. Arch. Intern. Med. 1990, 150, 2286–2290. [Google Scholar] [CrossRef] [PubMed]
- Hallas, J. Evidence of depression provoked by cardiovascular medication: A prescription sequence symmetry analysis. Epidemiology 1996, 7, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Stable, E.J.; Halliday, R.; Gardiner, P.S.; Baron, R.B.; Hauck, W.W.; Acree, M.; Coates, T.J. The effects of propranolol on cognitive function and quality of life: A randomized trial among patients with diastolic hypertension. Am. J. Med. 2000, 108, 359–365. [Google Scholar] [CrossRef]
- Stoudemire, A.; Brown, J.T.; Harris, R.T.; Blessing-Feussner, C.; Roberts, J.H.; Nichols, J.C.; Houpt, J.L. Propranolol and depression: A reevaluation based on a pilot clinical trial. Psychiatr. Med. 1984, 2, 211–218. [Google Scholar]
- Jin, S.; Kostka, K.; Posada, J.D.; Kim, Y.; Seo, S.I.; Lee, D.Y.; Shah, N.H.; Roh, S.; Lim, Y.H.; Chae, S.G.; et al. Prediction of Major Depressive Disorder Following Beta-Blocker Therapy in Patients with Cardiovascular Diseases. J. Pers. Med. 2020, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Yohannes, A.M.; Willgoss, T.G.; Baldwin, R.C.; Connolly, M.J. Depression and anxiety in chronic heart failure and chronic obstructive pulmonary disease: Prevalence, relevance, clinical implications and management principles. Int. J. Geriatr. Psychiatry 2010, 25, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Burkauskas, J.; Noreikaite, A.; Bunevicius, A.; Brozaitiene, J.; Neverauskas, J.; Mickuviene, N.; Bunevicius, R. Beta-1-Selective Beta-Blockers and Cognitive Functions in Patients with Coronary Artery Disease: A Cross-Sectional Study. J. Neuropsychiatry Clin. Neurosci. 2016, 28, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Sateia, M.J. International classification of sleep disorders-third edition: Highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Kostis, J.B.; Rosen, R.C. Central nervous system effects of beta-adrenergic-blocking drugs: The role of ancillary properties. Circulation 1987, 75, 204–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institutes of Health. National Institutes of Health State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults, 13–15 June 2005. Sleep 2005, 28, 1049–1057. [Google Scholar] [CrossRef] [Green Version]
- Ohayon, M.M. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med. Rev. 2002, 6, 97–111. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, F.; Li, W.A.; Geng, X.; Li, C.; Meng, X.; Feng, Y.; Liu, W.; Yu, F. A review of sleep disorders and melatonin. Neurol. Res. 2017, 39, 559–565. [Google Scholar] [CrossRef]
- Thompson, D.F.; Pierce, D.R. Drug-induced nightmares. Ann. Pharmacother. 1999, 33, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.; Larøi, F.; McGuire, P.K.; Aleman, A. The hallucinating brain: A review of structural and functional neuroimaging studies of hallucinations. Neurosci. Biobehav. Rev. 2008, 32, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Goldner, J.A. Metoprolol-induced visual hallucinations: A case series. J. Med. Case Rep. 2012, 6, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirois, F.J. Visual hallucinations and metoprolol. Psychosomatics 2006, 47, 537–538. [Google Scholar] [CrossRef] [PubMed]
- Marcantonio, E.R. Delirium in Hospitalized Older Adults. N. Engl. J. Med. 2017, 377, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Alagiakrishnan, K.; Wiens, C.A. An approach to drug induced delirium in the elderly. Postgrad. Med. J. 2004, 80, 388–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katznelson, R.; Djaiani, G.; Mitsakakis, N.; Lindsay, T.F.; Tait, G.; Friedman, Z.; Wasowicz, M.; Beattie, W.S. Delirium following vascular surgery: Increased incidence with preoperative beta-blocker administration. Can. J. Anaesth. 2009, 56, 793–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, P.J.; Luciano, S.; Colbourne, L. Rates of delirium associated with calcium channel blockers compared to diuretics, renin-angiotensin system agents and beta-blockers: An electronic health records network study. J. Psychopharmacol. 2020, 34, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.A.; Davis, M.; Jeffery, I. Acute delirium induced by metoprolol. Cardiovasc. Drugs Ther. 2002, 16, 161–165. [Google Scholar] [CrossRef]
- Love, J.N.; Handler, J.A. Toxic psychosis: An unusual presentation of propranolol intoxication. Am. J. Emerg. Med. 1995, 13, 536–537. [Google Scholar] [CrossRef]
- Viadero, J.J.; Wong, S.H.; White, W.B. Acute psychotic behavior associated with atenolol. Am. J. Psychiatry 1983, 140, 1382. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Parkinson’s Disease Collaborators. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef] [Green Version]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Braak, H.; Bohl, J.R.; Müller, C.M.; Rüb, U.; de Vos, R.A.; Del Tredici, K. Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov. Disord. 2006, 21, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Bjørnevik, K.; Im, D.S.; Flierl, A.; Dong, X.; Locascio, J.J.; Abo, K.M.; Long, E.; Jin, M.; Xu, B.; et al. β2-Adrenoreceptor is a regulator of the α-synuclein gene driving risk of Parkinson’s disease. Science 2017, 357, 891–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koren, G.; Norton, G.; Radinsky, K.; Shalev, V. Chronic Use of β-Blockers and the Risk of Parkinson’s Disease. Clin. Drug Investig. 2019, 39, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Bellou, V.; Belbasis, L.; Tzoulaki, I.; Evangelou, E.; Ioannidis, J.P. Environmental risk factors and Parkinson’s disease: An umbrella review of meta-analyses. Parkinsonism. Relat. Disord. 2016, 23, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, D.; Nalls, M.A.; Hallgrímsdóttir, I.B.; Hunkapiller, J.; van der Brug, M.; Cai, F.; Kerchner, G.A.; Ayalon, G.; Bingol, B.; Sheng, M.; et al. A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat. Genet. 2017, 49, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Hopfner, F.; Höglinger, G.U.; Kuhlenbäumer, G.; Pottegård, A.; Wod, M.; Christensen, K.; Tanner, C.M.; Deuschl, G. β-adrenoreceptors and the risk of Parkinson’s disease. Lancet Neurol. 2020, 19, 247–254. [Google Scholar] [CrossRef]
- Cumming, R.G. Epidemiology of medication-related falls and fractures in the elderly. Drugs Aging. 1998, 12, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Tinetti, M.E.; Doucette, J.; Claus, E.; Marottoli, R. Risk factors for serious injury during falls by older persons in the community. J. Am. Geriatr. Soc. 1995, 43, 1214–1221. [Google Scholar] [CrossRef]
- Hartholt, K.A.; van Beeck, E.F.; Polinder, S.; van der Velde, N.; van Lieshout, E.M.; Panneman, M.J.; van der Cammen, T.J.; Patka, P. Societal consequences of falls in the older population: Injuries, healthcare costs, and long-term reduced quality of life. J. Trauma. 2011, 71, 748–753. [Google Scholar] [CrossRef] [Green Version]
- Boyle, N.; Naganathan, V.; Cumming, R.G. Medication and falls: Risk and optimization. Clin. Geriatr. Med. 2010, 26, 583–605. [Google Scholar] [CrossRef]
- Reiter, M.J. Cardiovascular drug class specificity: Beta-blockers. Prog. Cardiovasc. Dis. 2004, 47, 11–33. [Google Scholar] [CrossRef]
- Frishman, W.H.; Alwarshetty, M. Beta-adrenergic blockers in systemic hypertension: Pharmacokinetic considerations related to the current guidelines. Clin. Pharmacokinet. 2002, 41, 505–516. [Google Scholar] [CrossRef]
- Ham, A.C.; van Dijk, S.C.; Swart, K.M.; Enneman, A.W.; van der Zwaluw, N.L.; Brouwer-Brolsma, E.M.; van Schoor, N.M.; Zillikens, M.C.; Lips, P.; de Groot, L.C.; et al. Beta-blocker use and fall risk in older individuals: Original results from two studies with meta-analysis. Br. J. Clin. Pharmacol. 2017, 83, 2292–2302. [Google Scholar] [CrossRef] [Green Version]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 954–976. [Google Scholar] [CrossRef] [Green Version]
- Ashina, M. Migraine. N. Engl. J. Med. 2020, 383, 1866–1876. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.D. Practice parameter: Evidence-based guidelines for migraine headache (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000, 55, 754–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byun, Y.J.; Levy, D.A.; Nguyen, S.A.; Brennan, E.; Rizk, H.G. Treatment of Vestibular Migraine: A Systematic Review and Meta-analysis. Laryngoscope 2021, 131, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, K.P.; Bain, P.; Bajaj, N.; Elble, R.J.; Hallett, M.; Louis, E.D.; Raethjen, J.; Stamelou, M.; Testa, C.M.; Deuschl, G. Tremor Task Force of the International Parkinson and Movement Disorder Society. Consensus Statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society. Mov. Disord. 2018, 33, 75–87. [Google Scholar] [CrossRef]
- Haubenberger, D.; Hallett, M. Essential Tremor. N. Engl. J. Med. 2018, 378, 1802–1810. [Google Scholar] [CrossRef] [PubMed]
- Deuschl, G.; Raethjen, J.; Hellriegel, H.; Elble, R. Treatment of patients with essential tremor. Lancet Neurol. 2011, 10, 148–161. [Google Scholar] [CrossRef]
- Heilman, K.M. Orthostatic tremor. Arch. Neurol. 1984, 41, 880–881. [Google Scholar] [CrossRef] [PubMed]
- Musco, S.; McAllister, V.; Caudle, I. Dopamine-receptor blocking agent-associated akathisia: A summary of current understanding and proposal for a rational approach to treatment. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320937575. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.S. Neuroleptic-induced movement disorders: An overview. Psychiatr. Clin. 2005, 28, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Sethuram, K.; Gedzior, J. Akathisia: Case Presentation and Review of Newer Treatment Agents. Psychiatr. Ann. 2014, 44, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Horwitz, R.I.; Gottlieb, L.D.; Kraus, M.L. The efficacy of atenolol in the outpatient management of the alcohol withdrawal syndrome. Results of a randomized clinical trial. Arch. Intern. Med. 1989, 149, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, D.S.; Anderson, I.M.; Nutt, D.J.; Allgulander, C.; Bandelow, B.; den Boer, J.A.; Christmas, D.M.; Davies, S.; Fineberg, N.; Lidbetter, N.; et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: A revision of the 2005 guidelines from the British Association for Psychopharmacology. J. Psychopharmacol. 2014, 28, 403–439. [Google Scholar] [CrossRef] [PubMed]
- Kindt, M.; Soeter, M.; Vervliet, B. Beyond extinction: Erasing human fear responses and preventing the return of fear. Nat. Neurosci. 2009, 12, 256–258. [Google Scholar] [CrossRef]
- Lonergan, M.H.; Olivera-Figueroa, L.A.; Pitman, R.K.; Brunet, A. Propranolol’s effects on the consolidation and reconsolidation of long-term emotional memory in healthy participants: A meta-analysis. J. Psychiatry Neurosci. 2013, 38, 222–231. [Google Scholar] [CrossRef] [Green Version]
- Vaiva, G.; Ducrocq, F.; Jezequel, K.; Averland, B.; Lestavel, P.; Brunet, A.; Marmar, C.R. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol. Psychiatry 2003, 54, 947–949. [Google Scholar] [CrossRef]
- Pitman, R.K.; Sanders, K.M.; Zusman, R.M.; Healy, A.R.; Cheema, F.; Lasko, N.B.; Cahill, L.; Orr, S.P. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol. Psychiatry. 2002, 51, 189–192. [Google Scholar] [CrossRef]
- Steenen, S.A.; van Wijk, A.J.; van der Heijden, G.J.; van Westrhenen, R.; de Lange, J.; de Jongh, A. Propranolol for the treatment of anxiety disorders: Systematic review and meta-analysis. J. Psychopharmacol. 2016, 30, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Thomas, T.; Dooley, T. Treatment of anxiety prior to a medical procedure using an atenolol-scopolamine combination drug. J. Depress. Anxiety 2018, 7, 303. [Google Scholar] [CrossRef]
- Armstrong, C.; Kapolowicz, M.R. A Preliminary Investigation on the Effects of Atenolol for Treating Symptoms of Anxiety. Mil. Med. 2020, 185, e1954–e1960. [Google Scholar] [CrossRef] [PubMed]
- Fleminger, S.; Greenwood, R.J.; Oliver, D.L. Pharmacological management for agitation and aggression in people with acquired brain injury. Cochrane Database Syst. Rev. 2006, 4, CD003299. [Google Scholar] [CrossRef] [PubMed]
- Haspel, T. Beta-blockers and the treatment of aggression. Harv. Rev. Psychiatry 1995, 2, 274–281. [Google Scholar] [CrossRef]
- Fava, M. Psychopharmacologic treatment of pathologic aggression. Psychiatr. Clin. 1997, 20, 427–451. [Google Scholar] [CrossRef]
- Peskind, E.R.; Tsuang, D.W.; Bonner, L.T.; Pascualy, M.; Riekse, R.G.; Snowden, M.B.; Thomas, R.; Raskind, M.A. Propranolol for disruptive behaviors in nursing home residents with probable or possible Alzheimer disease: A placebo-controlled study. Alzheimer Dis. Assoc. Disord. 2005, 19, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Hirschtritt, M.E.; Bloch, M.H.; Mathews, C.A. Obsessive-Compulsive Disorder: Advances in Diagnosis and Treatment. JAMA 2017, 317, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Dannon, P.N.; Sasson, Y.; Hirschmann, S.; Iancu, I.; Grunhaus, L.J.; Zohar, J. Pindolol augmentation in treatment-resistant obsessive compulsive disorder: A double-blind placebo controlled trial. Eur. Neuropsychopharmacol. 2000, 10, 165–169. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, X.; Zhu, D.; Chen, J.; Qin, B.; Zhang, Y.; Wang, X.; Yang, D.; Meng, H.; Luo, Q.; et al. Is pindolol augmentation effective in depressed patients resistant to selective serotonin reuptake inhibitors? A systematic review and meta-analysis. Hum. Psychopharmacol. 2015, 30, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Hirschmann, S.; Dannon, P.N.; Iancu, I.; Dolberg, O.T.; Zohar, J.; Grunhaus, L. Pindolol augmentation in patients with treatment-resistant panic disorder: A double-blind, placebo-controlled trial. J. Clin. Psychopharmacol. 2000, 20, 556–559. [Google Scholar] [CrossRef] [PubMed]
| High Lipophilicity | Moderate Lipophylicity | Low Lipophilicity or Hydrophile | ||
|---|---|---|---|---|
| With ISA | Beta-1 selective | Pindolol Penbutolol | Acebutolol Betaxolol | Carteolol |
| Non-selective | Labetalol | |||
| Without ISA | Beta-1 selective | Metoprolol Bisoprolol Nebivolol | Atenolol Esmolol | |
| Non-selective | Propranolol Timolol | Carvedilol | Nadolol Sotalol | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cojocariu, S.A.; Maștaleru, A.; Sascău, R.A.; Stătescu, C.; Mitu, F.; Leon-Constantin, M.M. Neuropsychiatric Consequences of Lipophilic Beta-Blockers. Medicina 2021, 57, 155. https://doi.org/10.3390/medicina57020155
Cojocariu SA, Maștaleru A, Sascău RA, Stătescu C, Mitu F, Leon-Constantin MM. Neuropsychiatric Consequences of Lipophilic Beta-Blockers. Medicina. 2021; 57(2):155. https://doi.org/10.3390/medicina57020155
Chicago/Turabian StyleCojocariu, Sabina Alexandra, Alexandra Maștaleru, Radu Andy Sascău, Cristian Stătescu, Florin Mitu, and Maria Magdalena Leon-Constantin. 2021. "Neuropsychiatric Consequences of Lipophilic Beta-Blockers" Medicina 57, no. 2: 155. https://doi.org/10.3390/medicina57020155
APA StyleCojocariu, S. A., Maștaleru, A., Sascău, R. A., Stătescu, C., Mitu, F., & Leon-Constantin, M. M. (2021). Neuropsychiatric Consequences of Lipophilic Beta-Blockers. Medicina, 57(2), 155. https://doi.org/10.3390/medicina57020155








