Selected Biochemical Blood Parameters and a Risk of Pressure Ulcers in Patients Receiving Treatment in Intensive Care Units
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
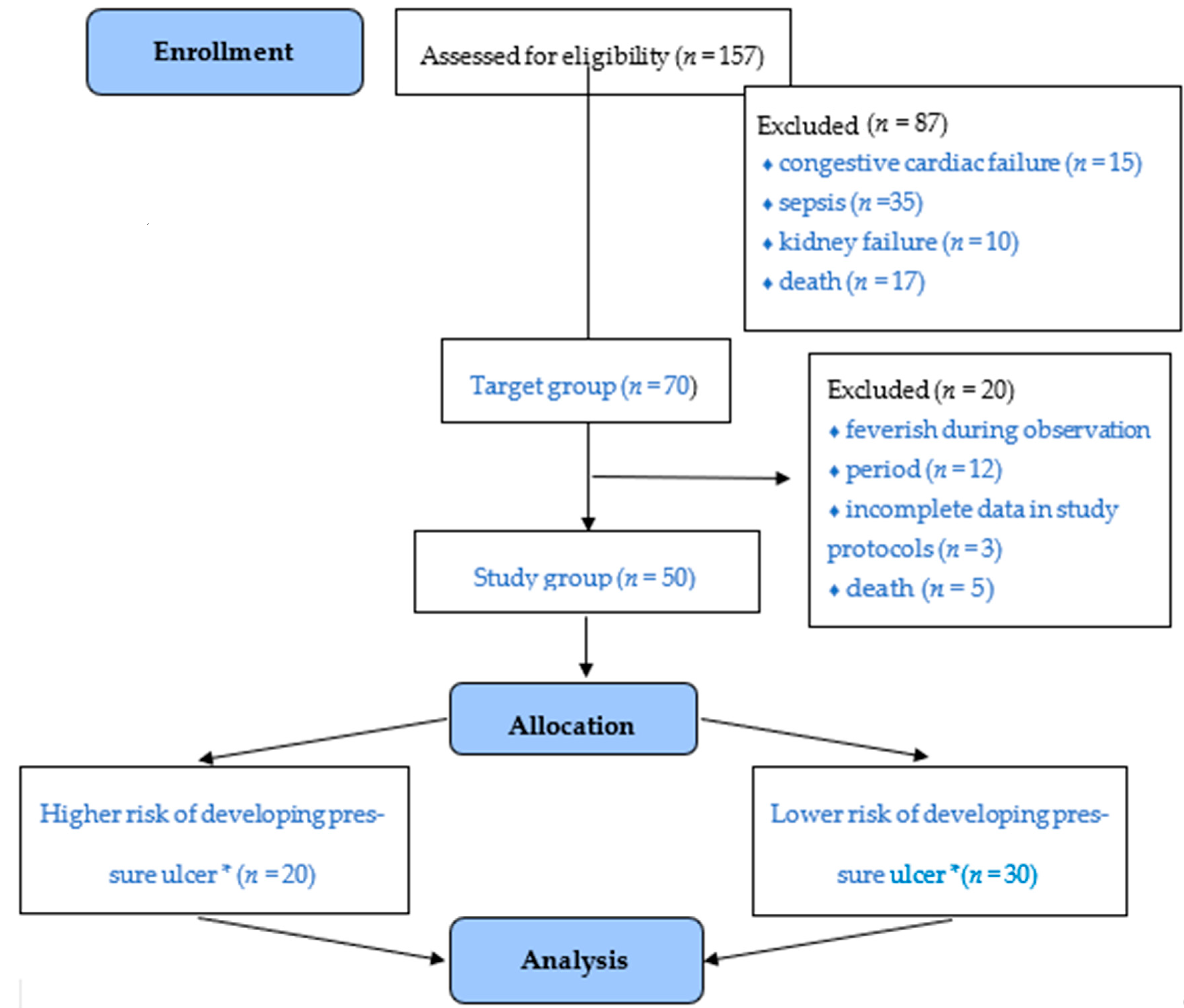
2.2. Setting and Participants
2.3. Study Design
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahin, E.S.; Dassen, T.; Halfens, R.J. Incidence, prevention and treatment of pressure ulcers in intensive care patients: A longitudinal study. Int. J. Nurs. Stud. 2009, 46, 413–421. [Google Scholar] [CrossRef]
- Shahin, E.S.M.; Dassen, T.; Halfens, R.J.G. Pressure ulcer prevention in intensive care patients: Guidelines and practice. J. Eval. Clin. Pr. 2009, 15, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, O.; Preiser, J.-C. Role of Nutrition Support in Inflammatory Conditions. Nutr. Clin. Pr. 2017, 32, 310–317. [Google Scholar] [CrossRef]
- Munoz, N.; Posthauer, M.E.; Cereda, E.; Schols, J.M.G.A.; Haesler, E. The Role of Nutrition for Pressure Injury Prevention and Healing: The 2019. International Clinical Practice Guideline Recommendations. Adv. Ski. Wound Care 2019, 33, 123–136. [Google Scholar] [CrossRef]
- Kottner, J.; Cuddigan, J.; Carville, K.; Balzer, K.; Berlowitz, D.; Law, S.; Litchford, M.; Mitchell, P.; Moore, Z.; Pittman, J.; et al. Prevention and treatment of pressure ulcers/injuries: The protocol for the second update of the international Clinical Practice Guideline 2019. J. Tissue Viability 2019, 28, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Drescher, T.; Singler, K.; Ulrich, A.; Koller, M.; Keller, U.; Christ-Crain, M.; Kressig, R.W. Comparison of two malnutrition risk screening methods (MNA and NRS 2002) and their association with markers of protein malnutrition in geriatric hospi-talized patients. Eur. J. Clin. Nutr. 2010, 64, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Alderden, J.; Rondinelli, J.; Pepper, G.; Cummins, M.; Whitney, J. Risk factors for pressure injures among critical care pa-tients: A systematic review. Int. J. Nurs. Stud. 2017, 71, 91–114. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.; Vermillion, B.; Newton, C.; Fall, M.; Li, X.; Kaewprag, P.; Moffatt-Bruce, S.; Lenz, E.R. Predictive validity of the Braden scale for patients in intensive care units. Am. J. Crit. Care 2013, 22, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-L.; Cao, Y.-J.; Zhang, W.; Wang, J.; Huai, B.-S. Braden scale (ALB) for assessing pressure ulcer risk in hospital patients: A validity and reliability study. Appl. Nurs. Res. 2017, 33, 169–174. [Google Scholar] [CrossRef]
- Jensen, G.L.; Mirtallo, J.; Compher, C.; Dhaliwal, R.; Forbes, A.; Grijalba, R.F.; Hardy, G.; Kondrup, J.; Labadarios, D.; Nyulasi, I.; et al. Adult starvation and disease-related malnutrition: A proposal for etiology-based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. Clin. Nutr. 2010, 29, 151–153. [Google Scholar] [CrossRef]
- Mahmoodpoor, A.; Shadvar, K.; Saghaleini, S.H.; Dehghan, K.; Ostadi, Z. Pressure ulcer and nutrition. Indian J. Crit. Care Med. 2018, 22, 283–289. [Google Scholar] [CrossRef]
- Hanafusa, N.; Nitta, K.; Okazaki, M.; Komatsu, M.; Shiohira, S.; Kawaguchi, H.; Tsuchiya, K. Serum albumin level adjusted with C-reactive protein predicts hemodialysis patient survival. Ren. Replace. Ther. 2017, 3, 9. [Google Scholar] [CrossRef]
- Serra, R.; Caroleo, S.; Buffone, G.; Lugarà, M.; Molinari, V.; Tropea, F.; Amantea, B.; De Franciscis, S. Low serum albumin level as an independent risk factor for the onset of pressure ulcers in intensive care unit patients. Int. Wound J. 2014, 11, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Montalcini, T.; Moraca, M.; Ferrom, Y.; Romeo, S.; Serra, S.; Raso, M.G.; Rossi, F.; Sannita, W.G.; Dolce, G.; Pujia, A. Nutri-tional parameters predicting pressure ulcers and short-term mortality in patients with minimal conscious state as a result of traumatic and non-traumatic acquired brain injury. J. Transl. Med. 2015, 13, 305. [Google Scholar] [CrossRef]
- Kłęk, S.; Jankowski, M.; Kruszewski, W.J.; Fijuth, J.; Kapałą, A.; Kabata, P.; Wysocki, P.; Krzakowski, M.; Rutkowski, P. Standardy leczenia żywieniowego w onkologii. Oncol. Clin. Pract. 2015, 11, 173–190. [Google Scholar]
- Van Bokhorst-de van der Schueren, M.A.E.; Soesters, P.B.; Reijven, P.L.M.; Allison, S.P.; Kondrup, J.; Skowrońska-Piekarska, U.; Matysia, K. Rozpoznanie niedożywienia—Badanie przesiewowe i ocena stanu odżywienia In Podstawy żywienia klin-icznego, 4th ed.; Sobotka, L., Ed.; Scientifica: Kraków, Poland, 2013. [Google Scholar]
- Friedman, A.N.; Fadem, S.Z. Reassessment of Albumin as a Nutritional Marker in Kidney Disease. J. Am. Soc. Nephrol. 2010, 21, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Fontes, D.; Generoso, S.D.V.; Correia, M.I.T.D. Subjective global assessment: A reliable nutritional assessment tool to predict outcomes in critically ill patients. Clin. Nutr. 2014, 33, 291–295. [Google Scholar] [CrossRef]
- Terekeci, H.; Kucukardali, Y.; Top, C.; Onem, Y.; Çelik, S.; Oktenli, C.; Onem, Y. Risk assessment study of the pressure ulcers in intensive care unit patients. Eur. J. Intern. Med. 2009, 20, 394–397. [Google Scholar] [CrossRef]
- Jaul, E.; Barron, J.; Rosenzweig, J.P.; Menczel, J. An overview of co-morbidities and the development of pressure ulcers among older adults. BMC Geriatr. 2018, 18, 305. [Google Scholar] [CrossRef] [PubMed]
- Ninbanphot, S.; Narawong, P.; Theeranut, A.; Sawanyawisuth, K.; Limpawattana, P. Development and validation of CAVE score in predicting presence of pressure ulcer in intensive care patients. Heliyon 2020, 6, e04612. [Google Scholar] [CrossRef]
- Alderden, J.; Cummins, M.R.; Pepper, G.A.; Whitney, J.D.; Zhang, Y.; Butcher, R.; Thomas, D. Midrange Braden Subscale Scores Are Associated With Increased Risk for Pressure Injury Development Among Critical Care Patients. J. Wound Ostomy Cont. Nurs. 2017, 44, 420–428. [Google Scholar] [CrossRef]
- Jentzer, J.C.; Anavekar, N.S.; Brenes-Salazar, J.A.; Wiley, B.; Murphree, D.H.; Bennett, C.; Murphy, J.G.; Keegan, M.T.; Barsness, G.W. Admission Braden Skin Score Independently Predicts Mortality in Cardiac Intensive Care Patients. Mayo Clin. Proc. 2019, 94, 1994–2003. [Google Scholar] [CrossRef] [PubMed]
- Cramer, E.M.; Seneviratne, M.G.; Sharifi, H.; Ozturk, A.; Hernandez-Boussard, T. Predicting the Incidence of Pressure Ulcers in the Intensive Care Unit Using Machine Learning. eGEMs Generating Évid. Methods Improv. Patient Outcomes 2019, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Citty, S.W.; Cowan, L.J.; Wingfield, Z.; Stechmiller, J. Optimizing Nutrition Care for Pressure Injuries in Hospitalized Patients. Adv. Wound Care 2019, 8, 309–322. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]


| Scheme | n | % | |
|---|---|---|---|
| Sex | Women | 18 | 36.0% |
| Men | 32 | 64.0% | |
| Reason for hospitalisation | Conditions due to extensive neurosurgical procedures within the skull (G1) | 21 | 42.0% |
| Injuries of multiple organs (G2) | 10 | 20.0% | |
| Conditions following sudden cardiac arrest (SCA) (G3) | 7 | 14.0% | |
| Inefficiency of organs, incl. respiratory failure due to pneumonia (G4) | 12 | 24.0% | |
| Comorbidities and/or past conditions | Diabetes | 18 | 36.0% |
| Hypotension | 14 | 28.0% | |
| Hypertension | 23 | 46.0% | |
| Atherosclerosis | 29 | 58.0% | |
| Alcohol dependence | 9 | 18.0% | |
| Other | 14 | 28.0% | |
| Age | 58.60 years ± SD 21.22 years (19–96) | ||
| Braden Scale | 8.18 points ± 1.3 points (6–12) | ||
| Parameter | Lower vs. Higher Risk of Developing Pressure Ulcers | ||||
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Effect Size (95% Cl) | F (1. 48) | p Value | |
| Albumins | 2.96 ± 0.49 | 2.48 ± 0.6 | 0.88 (0.64–1.12) | 8.98 | 0.004 * |
| CPR | 104.05 ± 81.62 | 146.57 ± 108.83 | 0.45 (0.32–0.57) | 2.21 | 0.143 |
| PCT | 2.51 ± 7.46 | 7.09 ± 17.33 | 0.37 (0.27–0.47) | 1.24 | 0.271 |
| Haemoglobin | 10.46 ± 2.12 | 10.94 ± 2.05 | 0.23 (0.17–0.29) | 0.64 | 0.428 |
| WBC | 12.6 ± 5.21 | 11.00 ± 3.96 | 0.35 (0.25–0.45) | 1.52 | 0.223 |
| Protein | 5.37 ± 0.87 | 4.91 ± 1.01 | 0.49 (0.35–0.63) | 2.81 | 0.100 |
| Albumins | Descriptive Statistics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | 95.0% Cl | 95.0% Cl | Me | Min. | Max. | Q1 | Q3 | SD | ||
| Group I | 21 | 2.76 | 2.45 | 3.07 | 2.80 | 1.60 | 4.20 | 2.10 | 3.20 | 0.68 |
| Group II | 10 | 2.78 | 2.49 | 3.07 | 2.70 | 2.30 | 3.50 | 2.50 | 3.10 | 0.40 |
| Group III | 6 | 2.58 | 1.93 | 3.24 | 2.75 | 1.40 | 3.10 | 2.50 | 3.00 | 0.62 |
| Group IV | 13 | 2.48 | 2.12 | 2.83 | 2.40 | 1.50 | 3.70 | 2.20 | 2.90 | 0.59 |
| p | F = 0.74 p = 0.528 | |||||||||
| Variables | R | p Value |
|---|---|---|
| Albumins and Braden | 0.55 | <0.001 * |
| CPR and Braden | −0.15 | 0.295 |
| PCT and Braden | −0.18 | 0.219 |
| Haemoglobin and Braden | 0.00 | 0.986 |
| WBC and Braden | 0.16 | 0.282 |
| Protein and Braden | 0.38 | 0.007 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazaliński, D.; Midura, B.; Wójcik, A.; Więch, P. Selected Biochemical Blood Parameters and a Risk of Pressure Ulcers in Patients Receiving Treatment in Intensive Care Units. Medicina 2021, 57, 177. https://doi.org/10.3390/medicina57020177
Bazaliński D, Midura B, Wójcik A, Więch P. Selected Biochemical Blood Parameters and a Risk of Pressure Ulcers in Patients Receiving Treatment in Intensive Care Units. Medicina. 2021; 57(2):177. https://doi.org/10.3390/medicina57020177
Chicago/Turabian StyleBazaliński, Dariusz, Beata Midura, Anna Wójcik, and Paweł Więch. 2021. "Selected Biochemical Blood Parameters and a Risk of Pressure Ulcers in Patients Receiving Treatment in Intensive Care Units" Medicina 57, no. 2: 177. https://doi.org/10.3390/medicina57020177
APA StyleBazaliński, D., Midura, B., Wójcik, A., & Więch, P. (2021). Selected Biochemical Blood Parameters and a Risk of Pressure Ulcers in Patients Receiving Treatment in Intensive Care Units. Medicina, 57(2), 177. https://doi.org/10.3390/medicina57020177






