The Role of Complement System and the Immune Response to Tuberculosis Infection
Abstract
:1. Introduction
2. Widely Accepted Paradigm of Infection
3. Classical Pathway and Mycobacteria

4. Alternative Pathway and Mycobacteria
5. Collectins, Lectin Pathway, and Mycobacteria
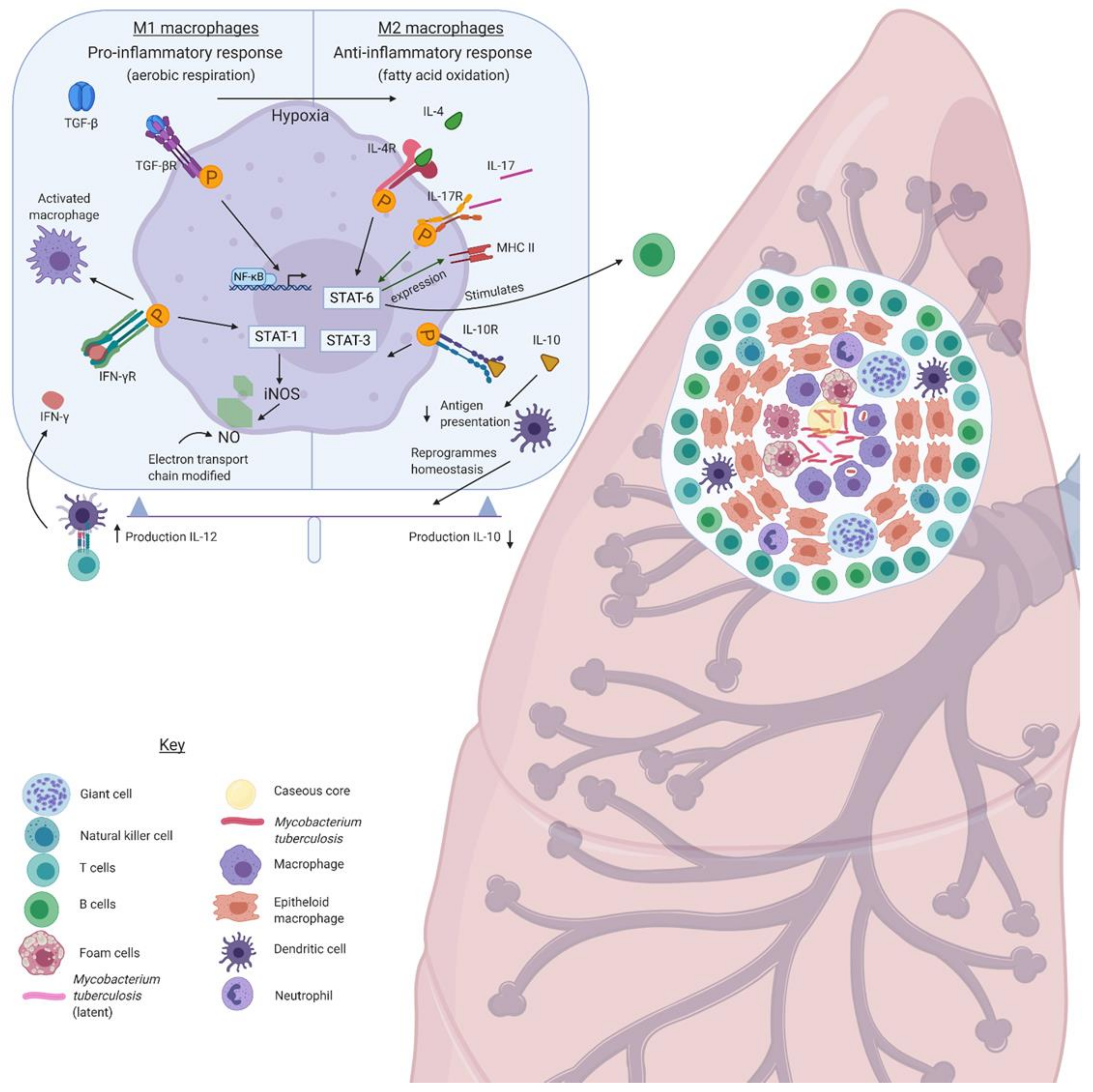
6. Granuloma Formation and the Complement System
7. Th1/Th2 Switch in the Granuloma and the Involvement of Complement System
8. Anti-Tuberculosis Drugs and Immune Modulation
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McMurray, D.N. Mycobacteria and Nocardia. In Medical Microbiology; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. Available online: https://www.ncbi.nlm.nih.gov/books/NBK7812/ (accessed on 20 January 2021).
- WHO. WHO Global Tuberculosis Report 2017; World Health Organization Press: Geneva, Switzerland, 2017; Available online: http://www.who.int/tb/publications/global_report/en/ (accessed on 20 January 2021).
- Public Health England. Tuberculosis in England 2017 Report; Version 1.:173; Public Health England: London, UK, 2017.
- Gao, B.; Gupta, R.S. Phylogenetic Framework and Molecular Signatures for the Main Clades of the Phylum Actinobacteria. Microbiol. Mol. Biol. Rev. 2012, 76, 66–112. Available online: http://mmbr.asm.org/cgi/doi/10.1128/MMBR.05011-11 (accessed on 20 January 2021). [CrossRef] [PubMed] [Green Version]
- Bermudez, L.E.; Goodman, J. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect. Immun. 1996, 64, 1400–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlesinger, L.S. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J. Immunol. 1993, 150, 2920–2930. [Google Scholar] [PubMed]
- Echeverria-Valencia, G.; Flores-Villalva, S.; Espitia, C.I. Virulence Factors and Pathogenicity of Mycobacterium. In Mycobacterium—Research and Development; Tech: Rijeka, Croatia, 2018; pp. 231–255. [Google Scholar]
- Smith, I. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin. Microbiol. Rev. 2003, 16, 463–496. [Google Scholar] [CrossRef] [Green Version]
- Orme, I.M. A new unifying theory of the pathogenesis of tuberculosis. Tuberculosis 2014, 94, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Bajic, G.; Degn, S.E.; Thiel, S.; Andersen, G.R. Complement activation, regulation, and molecular basis for complement-related diseases. EMBO J. 2015, 34, 2735–2757. [Google Scholar] [CrossRef] [Green Version]
- Tascon, R.E.; Soares, C.S.; Ragno, S.; Stavropoulos, E.; Hirst, E.M.A.; Colston, M.J. Mycobacterium tuberculosis-activated dendritic cells induce protective immunity in mice. Immunology 2000, 99, 473–480. [Google Scholar] [CrossRef]
- Van Crevel, R.; Ottenhoff, T.H.M.; Van der Meer, J.W.M. Innate immunity to Mycobacterium tuberculosis. Clin. Microbiol. Rev. 2002, 15, 294–309. [Google Scholar] [CrossRef] [Green Version]
- Kishore, U.; Reid, K.B.M. Modular organization of proteins containing C1q-like globular domain. Immunopharmacology 1999, 42, 15–21. [Google Scholar] [CrossRef]
- Carroll, M.V.; Lack, N.; Sim, E.; Krarup, A.; Sim, R.B. Multiple routes of complement activation by Mycobacterium bovis BCG. Mol. Immunol. 2009, 45, 4168. [Google Scholar] [CrossRef]
- Hetland, G.; Wiker, H.G.; Høgåsen, K.; Hamasur, B.; Svenson, S.B.; Harboe, M. Involvement of antilipoarabinomannan antibodies in classical complement activation in tuberculosis. Clin. Diagn. Lab. Immunol. 1998, 5, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, J.S.; Weis, J.J.; Martin, J.L.; Schlesinger, L.S. Complement Protein C3 Binding to Mycobacterium tuberculosis Is Initiated by the Classical Pathway in Human Bronchoalveolar Lavage Fluid. Infect Immun. 2004, 72, 2564–2573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlesinger, L.S.; Bellinger-Kawahara, C.G.; Payne, N.R.; Horwitz, M.A. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J. Immunol. 1990, 144, 2771–2780. Available online: http://www.ncbi.nlm.nih.gov/pubmed/2108212 (accessed on 20 January 2021). [PubMed]
- Peyron, P.; Bordier, C.; N’Diaye, E.-N.; Maridonneau-Parini, I. Nonopsonic Phagocytosis of Mycobacterium kansasii by Human Neutrophils Depends on Cholesterol and Is Mediated by CR3 Associated with Glycosylphosphatidylinositol-Anchored Proteins. J. Immunol. 2000, 165, 5186–5191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.; Mayadas-Norton, T.; Tanaka, K.; Chan, J.; Salgame, P. Mycobacterium tuberculosis Infection in Complement Receptor 3-Deficient Mice. J. Immunol. 2000, 165, 2596–2602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohlson, S.S.; Strasser, J.A.; Bower, J.J.; Schorey, J.S. Role of complement in Mycobacterium avium pathogenesis: In vivo and in vitro analyses of the host response to infection in the absence of complement component C3. Infect Immun. 2001, 69, 7729–7735. [Google Scholar] [CrossRef] [Green Version]
- Bjorvatn, B.; Barnetson, R.S.; Kronvall, G.; Zubler, R.H.; Lambert, P.H. Immune complexes and complement hypercatabolism in patients with leprosy. Clin. Exp. Immunol. 1976, 26, 388–396. [Google Scholar]
- Dupnik, K.M.; Bair, T.B.; Maia, A.O.; Amorim, F.M.; Costa, M.R.; Keesen, T.S.L. Transcriptional changes that characterize the immune reactions of leprosy. J. Infect Dis. 2015, 211, 1658–1676. [Google Scholar] [CrossRef] [Green Version]
- Amorim, F.M.; Nobre, M.L.; Nascimento, L.S.; Miranda, A.M.; Monteiro, G.R.G.; Freire-Neto, F.P. Differential immunoglobulin and complement levels in leprosy prior to development of reversal reaction and erythema nodosum leprosum. PLoS Negl. Trop. Dis. 2019, 13, e0007089. [Google Scholar] [CrossRef]
- Kretzschmar, G.C.; Oliveira, L.C.; Nisihara, R.M.; Velavan, T.P.; Stinghen, S.T.; Stahlke, E.R.S. Complement receptor 1 (CR1, CD35) association with susceptibility to leprosy. PLoS Negl. Trop. Dis. 2018, 12, e0006705. [Google Scholar] [CrossRef]
- Kouser, L.; Abdul-Aziz, M.; Nayak, A.; Stover, C.M.; Sim, R.B.; Kishore, U. Properdin and factor H: Opposing players on the alternative complement pathway “see-saw”. Front. Immunol. 2013, 4, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pangburn, M.K.; Müller-Eberhard, H.J. The C3 convertase of the alternative pathway of human complement. Biochem. J. 1986, 235, 723–730. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1146747/pdf/biochemj00280-0100.pdf (accessed on 20 January 2021). [CrossRef] [PubMed]
- Higgins, J.M.; Wiedemann, H.; Timpl, R.; Reid, K.B. Characterization of mutant forms of recombinant human properdin lacking single thrombospondin type I repeats. Identification of modules important for function. J. Immunol. 1995, 155, 5777–5785. [Google Scholar]
- Al-Mozaini, M.A.; Tsolaki, A.G.; Abdul-Aziz, M.; Abozaid, S.M.; Al-Ahdal, M.N.; Pathan, A.A. Human properdin modulates macrophage: Mycobacterium bovis BCG interaction via thrombospondin repeats 4 and 5. Front. Immunol. 2018, 9, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdul-Aziz, M.; Tsolaki, A.G.; Kouser, L.; Carroll, M.V.; Al-Ahdal, M.N.; Sim, R.B. Complement factor H interferes with Mycobacterium bovis BCG entry into macrophages and modulates the pro-inflammatory cytokine response. Immunobiology 2016, 221, 944–952. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, S.; O’Sullivan, M.P.; Keane, J. IL-10 blocks phagosome maturation in Mycobacterium tuberculosis-infected human macrophages. Am. J. Respir. Cell Mol. Biol. 2011, 45, 172–180. [Google Scholar] [CrossRef]
- Lu, J.; Teh, C.; Kishore, U.; Reid, K.B.M. Collectins and ficolins: Sugar pattern recognition molecules of the mammalian innate immune system. Biochim. Biophys. Acta Gen. Subj. 2002, 1572, 387–400. [Google Scholar] [CrossRef]
- Murugaiah, V.; Tsolaki, A.G.; Kishore, U. Collectins: Innate Immune Pattern Recognition Molecules. In Lectin in Host Defense against Microbial Infections; Hsieh, S.L., Ed.; Springer Singapore: Gateway East, Singapore, 2020; pp. 75–127. [Google Scholar]
- Ip, E.W.K.; Takahashi, K.; Alan Ezekowitz, R.; Stuart, L.M. Mannose-binding lectin and innate immunity. Immunol. Rev. 2009, 230, 9–21. [Google Scholar]
- Jack, D.L.; Lee, M.E.; Turner, M.W.; Klein, N.J.; Read, R.C. Mannose-binding lectin enhances phagocytosis and killing of Neisseria meningitidis by human macrophages. J. Leukoc. Biol. 2005, 77, 328–336. [Google Scholar] [CrossRef]
- Kuhlman, M.; Joiner, K.; Ezekowitz, R.A.B. The human mannose-binding protein functions as an opsonin. J. Exp. Med. 1989, 169, 1733–1745. [Google Scholar] [CrossRef] [Green Version]
- Polotsky, V.Y.; Belisle, J.T.; Mikusova, K.; Ezekowitz, R.A.B.; Joiner, K.A. Interaction of human mannose-binding protein with Mycobacterium avium. J. Infect. Dis. 1997, 175, 1159–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levitz, S.M.; Tabuni, A.; Treseler, C. Effect of mannose-binding protein on binding of Cryptococcus neoformans to human phagocytes. Infect. Immun. 1993, 61, 4891–4893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Emmerik, L.C.; Kuijper, E.J.; Fijen, C.A.P.; Dankert, J.; Thiel, S. Binding of mannan-binding protein to various bacterial pathogens of meningitis. Clin. Exp. Immunol. 1994, 97, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Neth, O.; Jack, D.L.; Dodds, A.W.; Holzel, H.; Klein, N.J.; Turner, M.W. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect. Immun. 2000, 68, 688–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartlomiejczyk, M.A.; Swierzko, A.S.; Brzostek, A.; Dziadek, J.; Cedzynski, M. Interaction of lectin pathway of complement-activating pattern recognition molecules with Mycobacteria. Clin. Exp. Immunol. 2014, 178, 310–319. [Google Scholar] [CrossRef]
- Bahia, E.I.; Idrissi, N.; Hakobyan, S.; Ramaglia, V.; Geluk, A.; Morgan, B.P.; Das, P.K. Complement activation in leprosy: A retrospective study shows elevated circulating terminal complement complex in reactional leprosy. Clin. Exp. Immunol. 2016, 184, 338–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahia, E.I.; Idrissi, N.; Das, P.K.; Fluiter, K.; Rosa, P.S.; Vreijling, J.; Troost, D. M. leprae components induce nerve damage by complement activation: Identification of lipoarabinomannan as the dominant complement activator. Acta Neuropathol. 2015, 129, 653–667. [Google Scholar] [CrossRef] [Green Version]
- Świerzko, A.S.; Bartłomiejczyk, M.A.; Brzostek, A.; Łukasiewicz, J.; Michalski, M.; Dziadek,, J. Mycobacterial antigen 85 complex (Ag85) as a target for ficolins and mannose-binding lectin. Int. J. Med. Microbiol. 2016, 306, 212–221. [Google Scholar] [CrossRef]
- Luo, F.; Sun, X.; Wang, Y.; Wang, Q.; Wu, Y.; Pan, Q. Ficolin-2 Defends against Virulent Mycobacteria Tuberculosis Infection In Vivo, and Its Insufficiency Is Associated with Infection in Humans. PLoS ONE 2013, 8, e73859. [Google Scholar] [CrossRef] [Green Version]
- Eisen, D.P.; Minchinton, R.M. Impact of Mannose-Binding Lectin on Susceptibility to Infectious Diseases. Clin. Infect. Dis. 2003, 37, 1496–1505. [Google Scholar] [CrossRef] [Green Version]
- Thye, T.; Niemann, S.; Walter, K.; Homolka, S.; Intemann, C.D.; Chinbuah, M.A. Variant G57E of Mannose Binding Lectin associated with protection against tuberculosis caused by mycobacterium africanum but not by M. tuberculosis. PLoS ONE 2011, 6, e20908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.L.; Liu, Y.; Ban, W.J.; Sun, Q.; Shi, G.L. Association of mannose-binding lectin gene polymorphisms with the development of pulmonary tuberculosis in China. BMC Infect. Dis. 2017, 17, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffensen, R.; Thiel, S.; Varming, K.; Jersild, C.; Jensenius, J.C. Detection of structural gene mutations and promoter polymorphisms in the mannan-binding lectin (MBL) gene by polymerase chain reaction with sequence-specific primers. J. Immunol. Methods 2000, 241, 33–42. [Google Scholar] [CrossRef]
- Valdimarsson, H.; Vikingsdottir, T.; Bang, P.; Saevarsdottir, S.; Gudjonsson, J.E.; Oskarsson, O. Human Plasma-Derived Mannose-Binding Lectin: A Phase I Safety and Pharmacokinetic Study. Scand. J. Immunol. 2004, 59, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Takahashi, K.; Dundee, J.; Shahroor-Karni, S.; Thiel, S.; Jensenius, C. Mannose-binding lectin-deficient mice are susceptible to infection with Staphylococcus aureus. J. Exp. Med. 2004, 199, 1379–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Møller-Kristensen, M.; Ip, W.K.E.; Shi, L.; Gowda, L.D.; Hamblin, M.R.; Thiel, S. Deficiency of Mannose-Binding Lectin Greatly Increases Susceptibility to Postburn Infection with Pseudomonas aeruginosa. J. Immunol. 2006, 176, 1769–1775. [Google Scholar] [CrossRef] [Green Version]
- Gaynor, C.D.; McCormack, F.X.; Voelker, D.R.; McGowan, S.E.; Schlesinger, L.S. Pulmonary surfactant protein A mediates enhanced phagocytosis of Mycobacterium tuberculosis by a direct interaction with human macrophages. J. Immunol. 1995, 155, 5343–5351. [Google Scholar]
- Kronborg, G.; Weis, N.; Madsen, H.O.; Pedersen, S.S.; Wejse, C.; Nielsen, H. Variant mannose-binding lectin alleles are not associated with susceptibility to or outcome of invasive pneumococcal infection in randomly included patients. J. Infect. Dis. 2002, 185, 1517–1520. [Google Scholar] [CrossRef]
- Roy, S.; Knox, K.; Segal, S.; Griffiths, D.; Moore, C.E.; Welsh, K.I. MBL genotype and risk of invasive pneumococcal disease: A case-control study. Lancet 2002, 359, 1569–1573. [Google Scholar] [CrossRef]
- Garred, P.; Harboe, M.; Oettinger, T.; Koch, C.; Svejgaard, A. Dual role of mannan-binding protein in infections: Another case of heterosis? Int. J. Immunogenet. 1994, 21, 125–131. [Google Scholar] [CrossRef]
- Garred, P.; Richter, C.; Andersen, Å.B.; Madsen, H.O.; Mtoni, I.; Svejgaard, A. Mannan-binding lectin in the sub-saharan HIV and tuberculosis epidemics. Scand. J. Immunol. 1997, 46, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Ezekowitz, R.A.B. The role of the mannose-binding lectin in innate immunity. Clin. Infect. Dis. 2005, 41 (Suppl. S7), S440–S444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiel, S.; Frederiksen, P.D.; Jensenius, J.C. Clinical manifestations of mannan-binding lectin deficiency. Mol. Immunol. 2006, 43, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Klassert, T.E.; Slevogt, H. C-type lectin receptors in tuberculosis: What we know. Med. Microbiol. Immunol. 2016, 205, 513–535. [Google Scholar] [CrossRef] [PubMed]
- Hoal-Van Helden, E.G.; Epstein, J.; Victor, T.C.; Hon, D.; Lewis, L.A.; Beyers, N. Mannose-binding protein B allele confers protection against tuberculous meningitis. Pediatr. Res. 1999, 45, 459–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Liang, Y.; Li, W.; Wang, M.; Hu, L.; Abuaku, B.K. Impact of MBL and MASP-2 gene polymorphism and its interaction on susceptibility to tuberculosis. BMC Infect. Dis. 2015, 15, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalmers, J.D.; Matsushita, M.; Kilpatrick, D.C.; Hill, A.T. No Strong Relationship Between Components of the Lectin Pathway of Complement and Susceptibility to Pulmonary Tuberculosis. Inflammation 2015, 38, 1731–1737. [Google Scholar] [CrossRef]
- Henriksen, M.L.; Brandt, J.; Andrieu, J.-P.; Nielsen, C.; Jensen, P.H.; Holmskov, U. Heteromeric Complexes of Native Collectin Kidney 1 and Collectin Liver 1 Are Found in the Circulation with MASPs and Activate the Complement System. J. Immunol. 2013, 191, 6117–6127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troegeler, A.; Lugo-Villarino, G.; Hansen, S.; Rasolofo, V.; Henriksen, M.L.; Mori, K. Collectin CL-LK is a novel soluble pattern recognition receptor for Mycobacterium tuberculosis. PLoS ONE 2015, 10, e0132692. [Google Scholar] [CrossRef]
- Mehmood, A.; Kouser, L.; Kaur, A.; Holmskov, U.; Al-Ahdal, M.N.; Sim, R.B. Complement dependent and independent interaction between bovince congultinin and mycobacterium bovis BCG: Implications in bovince tuberculosis. Front. Immunol. 2019, 9, 3159. [Google Scholar] [CrossRef]
- Kishore, U.; Greenhough, T.J.; Waters, P.; Shrive, A.K.; Ghai, R.; Kamran, M.F. Surfactant proteins SP-A and SP-D: Structure, function and receptors. Mol. Immunol. 2006, 43, 1293–1315. [Google Scholar] [CrossRef] [PubMed]
- Watford, W.T.; Wright, J.R.; Hester, C.G.; Jiang, H.; Frank, M.M. Surfactant Protein A Regulates Complement Activation. J. Immunol. 2001, 167, 6593–6600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nepomuceno, R.R.; Henschen-Edman, A.H.; Burgess, W.H.; Tenner, A.J. cDNA cloning and primary structure analysis of C1qR(p), the human C1q/MBL/SPA receptor that mediates enhanced phagocytosis in vitro. Immunity 1997, 6, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Oosting, R.S.; Wright, J.R. Characterization of the surfactant protein A receptor: Cell and ligand specificity. Am. J. Physiol. Lung Cell Mol. Physiol. 1994, 267, L165–L172. [Google Scholar] [CrossRef]
- Ragas, A.; Roussel, L.; Puzo, G.; Rivière, M. The Mycobacterium tuberculosis cell-surface glycoprotein apa as a potential adhesin to colonize target cells via the innate immune system pulmonary C-type lectin surfactant protein A. J. Biol. Chem. 2007, 282, 5133–5142. [Google Scholar] [CrossRef] [Green Version]
- Beharka, A.A.; Gaynor, C.D.; Kang, B.K.; Voelker, D.R.; McCormack, F.X.; Schlesinger, L.S. Pulmonary Surfactant Protein A Up-Regulates Activity of the Mannose Receptor, a Pattern Recognition Receptor Expressed on Human Macrophages. J. Immunol. 2002, 169, 3565–3573. [Google Scholar] [CrossRef] [Green Version]
- Kudo, K.; Sano, H.; Takahashi, H.; Kuronuma, K.; Yokota, S.; Fujii, N. Pulmonary Collectins Enhance Phagocytosis of Mycobacterium avium through Increased Activity of Mannose Receptor. J. Immunol. 2004, 172, 7592–7602. [Google Scholar] [CrossRef] [Green Version]
- Chroneos, Z.C.; Abdolrasulnia, R.; Whitsett, J.A.; Rice, W.R.; Shepherd, V.L. Purification of a cell-surface receptor for surfactant protein A. J. Biol. Chem. 1996, 271, 16375–16383. [Google Scholar] [CrossRef] [Green Version]
- Weikert, L.F.; Edwards, K.; Chroneos, Z.C.; Hager, C.; Hoffman, L.; Shepherd, V.L. SP-A enhances uptake of bacillus Calmette-Guerin by macrophages through a specific SP-A receptor. Am. J. Physiol. Lung Cell Mol. Physiol. 1997, 272, L989–L995. [Google Scholar] [CrossRef]
- Weikert, L.F.; Lopez, J.P.; Abdolrasulnia, R.; Chroneos, Z.C.; Shepherd, V.L. Surfactant protein A enhances mycobacterial killing by rat macrophages through a nitric oxide-dependent pathway. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279. [Google Scholar] [CrossRef] [Green Version]
- Floros, J.; Lin, H.M.; Garcia, A.; Salazar, M.A.; Guo, X.; DiAngelo, S. Surfactant protein genetic marker alleles identify a subgroup of tuberculosis in a Mexican population. J. Infect. Dis. 2000, 182, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Madan, T.; Saxena, S.; Murthy, K.J.R.; Muralidhar, K.; Usha Sarma, P. Association of polymorphisms in the collagen region of human SP-A1 and SP-A2 genes with pulmonary tuberculosis in Indian population. Clin. Chem. Lab. Med. 2002, 40, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Greenwood, C.M.T.; Eguale, T.; Kifle, A.; Beyene, J.; Habte, A. Variants of the SFTPA1 and SFTPA2 genes and susceptibility to tuberculosis in Ethiopia. Hum. Genet. 2006, 118, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Vaid, M.; Kaur, S.; Taruna, M.; Singh, H.; Gupta, V.K.; Murthy, K.J.R. Association of SP-D, MBL and I-NOS genetic variants with pulmonary tuberculosis. Indian J. Hum. Genet. 2006, 12, 105–110. [Google Scholar]
- Yang, H.Y.; Li, H.; Wang, Y.; Xu, C.; Zhao, Y.; Ma, X. Correlation analysis between single nucleotide polymorphisms of pulmonary surfactant protein A gene and pulmonary tuberculosis in the Han population in China. Int. J. Infect. Dis. 2014, 26, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, L. Revisiting the role of the granuloma in tuberculosis. Nat. Rev. Immunol. 2012, 12, 352–366. [Google Scholar] [CrossRef]
- Shim, D.; Kim, H.; Shin, S.J. Mycobacterium tuberculosis Infection-Driven Foamy Macrophages and Their Implications in Tuberculosis Control as Targets for Host-Directed Therapy. Front. Immunol. 2020, 11, 910. [Google Scholar] [CrossRef]
- Ordway, D.; Henao-Tamayo, M.; Orme, I.M.; Gonzalez-Juarrero, M. Foamy Macrophages within Lung Granulomas of Mice Infected with Mycobacterium tuberculosis Express Molecules Characteristic of Dendritic Cells and Antiapoptotic Markers of the TNF Receptor-Associated Factor Family. J. Immunol. 2005, 175, 3873–3881. [Google Scholar] [CrossRef] [Green Version]
- Gleeson, L.E.; Sheedy, F.J.; Palsson-McDermott, E.M.; Triglia, D.; O’Leary, S.M.; O’Sullivan, M.P. Cutting Edge: Mycobacterium tuberculosis Induces Aerobic Glycolysis in Human Alveolar Macrophages That Is Required for Control of Intracellular Bacillary Replication. J. Immunol. 2016, 196, 2444–2449. [Google Scholar] [CrossRef] [Green Version]
- Garton, N.J.; Waddell, S.J.; Sherratt, A.L.; Lee, S.M.; Smith, R.J.; Senner, C. Cytological and transcript analyses reveal fat and lazy persister-like bacilli in tuberculous sputum. PLoS Med. 2008, 5, e75. [Google Scholar] [CrossRef]
- Greenwood, D.J.; Dos Santos, M.S.; Huang, S.; Russell, M.R.G.; Collinson, L.M.; MacRae, J.I. Subcellular antibiotic visualization reveals a dynamic drug reservoir in infected macrophages. Science 2019, 364, 1279–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, K.K.; Pritt, B.S.; Alexander, M.P. Histopathologic review of granulomatous inflammation. J. Clin. Tuberc. Other Mycobact. Dis. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, P.; Kolloli, A.; Shi, L.; Bushkin, Y.; Tyagi, S. Immunometabolism of Phagocytes During Mycobacterium tuberculosis Infection. Front. Mol. Biosci. 2019, 6, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, E.D.; Morris, K.R.; Belisle, J.T.; Hill, P.; Remigio, L.K.; Brennan, P.J. Induction of inducible nitric oxide synthase-NO• by lipoarabinomannan of Mycobacterium tuberculosis is mediated by MEK1-ERK, MKK7-JNK, and NF-κB signaling pathways. Infect. Immun. 2001, 69, 2001–2010. [Google Scholar] [CrossRef] [Green Version]
- Marino, S.; Cilfone, N.A.; Mattila, J.T.; Linderman, J.J.; Flynn, J.L.; Kirschner, D.E. Macrophage polarization drives granuloma outcome during Mycobacterium tuberculosis infection. Infect. Immun. 2015, 83, 324–338. [Google Scholar] [CrossRef] [Green Version]
- Lim, Y.J.; Yi, M.H.; Choi, J.A.; Lee, J.; Han, J.Y.; Jo, S.H. Roles of endoplasmic reticulum stress-mediated apoptosis in M1-polarized macrophages during mycobacterial infections. Sci. Rep. 2016, 6, 37211. [Google Scholar] [CrossRef] [Green Version]
- Flynn, J.A.L.; Goldstein, M.M.; Chan, J.; Triebold, K.J.; Pfeffer, K.; Lowenstein, C.J. Tumor necrosis factor-α is required in the protective immune response against mycobacterium tuberculosis in mice. Immunity 1995, 2, 561–572. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Prados, J.-C.; Través, P.G.; Cuenca, J.; Rico, D.; Aragonés, J.; Martín-Sanz, P. Substrate Fate in Activated Macrophages: A Comparison between Innate, Classic, and Alternative Activation. J. Immunol. 2010, 185, 605–614. [Google Scholar] [CrossRef] [Green Version]
- Galván-Peña, S.; O’Neill, L.A.J. Metabolic reprogramming in macrophage polarization. Front. Immunol. 2014, 5, 420. [Google Scholar]
- Hajishengallis, G.; Lambris, J.D. Microbial manipulation of receptor crosstalk in innate immunity. Nat. Rev. Immunol. 2011, 11, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Bohlson, S.S.; O’Conner, S.D.; Hulsebus, H.J.; Ho, M.M.; Fraser, D.A. Complement, C1Q, and C1q-related molecules regulate macrophage polarization. Front. Immunol. 2014, 5, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, C.D. M1 and M2 macrophages: Oracles of health and disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sica, A.; Bronte, V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Investig. 2007, 117, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Kaplan, M.H. Transcriptional regulation by STAT6. Immunol. Res. 2011, 50, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, H.L.; Schaible, U.E.; Kaufmann, S.H.E. Early IL-4 induction in bone marrow lymphoid precursor cells by mycobacterial lipoarabinomannan. J. Immunol. 1998, 161, 5546–5554. [Google Scholar] [PubMed]
- Wang, S.; Zhang, J.; Sui, L.; Xu, H.; Piao, Q.; Liu, Y. Antibiotics induce polarization of pleural macrophages to M2-like phenotype in patients with tuberculous pleuritis. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yang, H.; Li, Y.; Li, X.D.; Zeng, T.T.; Lin, S.X. Hypoxia restrains the expression of complement component 9 in tumor-associated macrophages promoting non-small cell lung cancer progression. Cell Death Discov. 2018, 4, 1–12. [Google Scholar] [CrossRef]
- Huang, L.; Nazarova, E.V.; Russell, D.G. Mycobacterium tuberculosis: Bacterial Fitness within the Host Macrophage. Microbiol. Spectr. 2019, 7, 127–138. [Google Scholar]
- Gupta, A.; Kaul, A.; Tsolaki, A.G.; Kishore, U.; Bhakta, S. Mycobacterium tuberculosis: Immune evasion, latency and reactivation. Immunobiology 2012, 217, 363–374. [Google Scholar] [CrossRef]
- Smith, D.; Hänsch, H.; Bancroft, G.; Ehlers, S. T-cell-independent granuloma formation in response to Mycobacterium avium: Role of tumour necrosis factor-α and interferon-γ. Immunology 1997, 92, 413–421. [Google Scholar] [CrossRef]
- Schwaeble, W.; Zwirner, J.; Schulz, T.F.; Linke, R.P.; Dierich, M.P.; Weiss, E.H. Human complement factor H: Expression of an additional truncated gene product of 43 kDa in human liver. Eur. J. Immunol. 1987, 17, 1485–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dannenberg, A.M. Delayed-type hypersensitivity and cell-mediated immunity in the pathogenesis of tuberculosis. Immunol. Today 1991, 12, 228–233. [Google Scholar] [CrossRef]
- Ehlers, S.; Benini, J.; Held, H.D.; Roeck, C.; Alber, G.; Uhlig, S. αβ T cell receptor-positive cells and interferon-γ, but not inducible nitric oxide synthase, are critical for granuloma necrosis in a mouse model of mycobacteria-induced pulmonary immunopathology. J. Exp. Med. 2001, 194, 1847–1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, T.C.; Thomas, L.H.; Friedland, J.S. Regulation of IL-10 secretion after phagocytosis of Mycobacterium tuberculosis by human monocytic cells. Cytokine 2000, 12, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Banerjee, S.; Majumder, S.; Chowdhury, B.P.; Goswami, A.; Halder, K. Immune subversion by Mycobacterium tuberculosis through CCR5 mediated signaling: Involvement of IL-10. PLoS ONE 2014, 9, e92477. [Google Scholar] [CrossRef] [Green Version]
- Demangel, C.; Bertolino, P.; Britton, W.J. Autocrine IL-10 impairs dendritic cell (DC)-derived immune responses to mycobacterial infection by suppressing DC trafficking to draining lymph nodes and local IL-12 production. Eur. J. Immunol. 2002, 32, 994–1002. [Google Scholar] [CrossRef]
- Tian, T.; Woodworth, J.; Sköld, M.; Behar, S.M. In Vivo Depletion of CD11c + Cells Delays the CD4 + T Cell Response to Mycobacterium tuberculosis and Exacerbates the Outcome of Infection. J. Immunol. 2005, 175, 3268–3272. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.H.; Zhang, M.; Modlin, R.L.; Linsley, P.S.; Iyer, D.; Lin, Y. Interleukin-10 downregulates Mycobacterium tuberculosis-induced Th1 responses and CTLA-4 expression. Infect. Immun. 1996, 64, 913–918. [Google Scholar] [CrossRef] [Green Version]
- Volkman, H.E.; Clay, H.; Beery, D.; Chang, J.C.W.; Sherman, D.R.; Ramakrishnan, L. Tuberculous granuloma formation is enhanced by a Mycobacterium virulence determinant. PLoS Biol. 2004, 2, e367. [Google Scholar] [CrossRef] [Green Version]
- Cronan, M.R.; Matty, M.A.; Rosenberg, A.F.; Blanc, L.; Pyle, C.J.; Espenschied, S.T. An explant technique for high-resolution imaging and manipulation of mycobacterial granulomas. Nat. Methods 2018, 15, 1098–1107. [Google Scholar] [CrossRef]
- Welsh, K.J.; Abbott, A.N.; Hwang, S.A.; Indrigo, J.; Armitige, L.Y.; Blackburn, M.R. A role for tumour necrosis factor-α, complement C5 and interleukin-6 in the initiation and development of the mycobacterial cord factor trehalose 6,6′-dimycolate induced granulomatous response. Microbiology 2008, 154, 1813–1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borders, C.W.; Courtney, A.; Ronen, K.; Laborde-Lahoz, M.P.; Guidry, T.V.; Hwang, S.A. Requisite role for complement C5 and the C5a receptor in granulomatous response to mycobacterial glycolipid trehalose 6,6′-dimycolate. Scand. J. Immunol. 2005, 62, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Welsh, K.J.; Hwang, S.A.; Hunter, R.L.; Kruzel, M.L.; Actor, J.K. Lactoferrin modulation of mycobacterial cord factor trehalose 6-6′-dimycolate induced granulomatous response. Transl. Res. 2010, 156, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagannath, C.; Hoffmann, H.; Sepulveda, E.; Actor, J.K.; Wetsel, R.A.; Hunter, R.L. Hypersusceptibility of A/J mice to tuberculosis is in part due to a deficiency of the fifth complement component (C5). Scand. J. Immunol. 2000, 52, 369–379. [Google Scholar] [CrossRef]
- Welsh, K.J.; Lewis, C.T.; Boyd, S.; Braun, M.C.; Actor, J.K. Complement factor C7 contributes to lung immunopathology caused by mycobacterium tuberculosis. Clin. Dev. Immunol. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Beckwith, K.S.; Beckwith, M.S.; Ullmann, S.; Sætra, R.S.; Kim, H.; Marstad, A. Plasma membrane damage causes NLRP3 activation and pyroptosis during Mycobacterium tuberculosis infection. Nat. Commun. 2020, 11, 2270. [Google Scholar] [CrossRef]
- Lerner, T.R.; Queval, C.J.; Lai, R.P.; Russell, M.R.G.; Fearns, A.; Greenwood, D.J. Mycobacterium tuberculosis cords in the cytosol of live lymphatic endothelial cells to evade host immune surveillance. JCI Insight 2020, 5, e136937. [Google Scholar] [CrossRef]
- Iacobino, A.; Piccaro, G.; Giannoni, F.; Mustazzolu, A.; Fattorini, L. Mycobacterium tuberculosis is selectively killed by rifampin and rifapentine in hypoxia at neutral pH. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [Green Version]
- Ryan, G.J.; Hoff, D.R.; Driver, E.R.; Voskuil, M.I.; Gonzalez-Juarrero, M.; Basaraba, R.J. Multiple M. tuberculosis phenotypes in mouse and guinea pig lung tissue revealed by a dual-staining approach. PLoS ONE 2010, 5, e11108. [Google Scholar] [CrossRef] [Green Version]
- Mukamolova, G.V.; Turapov, O.; Malkin, J.; Woltmann, G.; Barer, M.R. Resuscitation-promoting factors reveal an occult population of tubercle bacilli in sputum. Am. J. Respir. Crit. Care Med. 2010, 181, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Mukamolova, G.V.; Turapov, O.A.; Kazarian, K.; Telkov, M.; Kaprelyants, A.S.; Kell, D.B. The rpf gene of Micrococcus luteus encodes an essential secreted growth factor. Mol. Microbiol. 2002, 46, 611–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukamolova, G.V.; Murzin, A.G.; Salina, E.G.; Demina, G.R.; Kell, D.B.; Kaprelyants, A.S. Muralytic activity of Micrococcus luteus Rpf and its relationship to physiological activity in promoting bacterial growth and resuscitation. Mol. Microbiol. 2006, 59, 84–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurdle, J.G.; Deshpande, A. Bacterial persister cells tackled. Nature 2018, 556, 40–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tentori, L.; Graziani, G.; Porcelli, S.A.; Sugita, M.; Brenner, M.B.; Madaio, R. Rifampin increases cytokine-induced expression of the CD1b molecule in human peripheral blood monocytes. Antimicrob. Agents Chemother. 1998, 42, 550–554. [Google Scholar] [CrossRef] [Green Version]
- Yuhas, Y.; Berent, E.; Ovadiah, H.; Azoulay, I.; Ashkenazi, S. Rifampin augments cytokine-induced nitric oxide production in human alveolar epithelial cells. Antimicrob. Agents Chemother. 2006, 50, 396–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuhas, Y.; Azoulay-Alfaguter, I.; Berent, E.; Ashkenazi, S. Rifampin inhibits prostaglandin E2 production and arachidonic acid release in human alveolar epithelial cells. Antimicrob. Agents Chemother. 2007, 51, 4225–4230. [Google Scholar] [CrossRef] [Green Version]
- Mlambo, G.; Sigola, L.B. Rifampicin and dexamethasone have similar effects on macrophage phagocytosis of zymosan, but differ in their effects on nitrite and TNF-α production. Int. Immunopharmacol. 2003, 3, 513–522. [Google Scholar] [CrossRef]
- Bardou, F.; Raynaud, C.; Ramos, C.; Lanéelle, M.A.; Lanéelle, G. Mechanism of isoniazid uptake in Mycobacterium tuberculosis. Microbiology 1998, 144, 2539–2544. [Google Scholar] [CrossRef] [Green Version]
- Winder, F.G.; Collins, P.B. Inhibition by Isoniazid of Synthesis of Mycolic Acids in Mycobacterium tuberculosis. J. Gen. Microbiol. 1970, 63, 41–48. Available online: http://mic.microbiologyresearch.org/content/journal/micro/10.1099/00221287-63-1-41 (accessed on 20 January 2021). [CrossRef] [Green Version]
- Khan, S.R.; Aljuhani, N.; Morgan, A.G.M.; Baghdasarian, A.; Fahlman, R.P.; Siraki, A.G. Cytoprotective effect of isoniazid against H2O2 derived injury in HL-60 cells. Chem. Biol. Interact. 2016, 244, 37–48. [Google Scholar] [CrossRef]
- Tousif, S.; Singh, D.K.; Ahmad, S.; Moodley, P.; Bhattacharyya, M.; Van Kaer, L. Isoniazid Induces Apoptosis of Activated CD4 + T Cells. J. Biol. Chem. 2014, 289, 30190–30195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.J.; Lee, H.M.; Shin, D.M.; Kim, W.; Yuk, J.M.; Jin, H.S. Host cell autophagy activated by antibiotics is required for their effective antimycobacterial drug action. Cell Host Microbe 2012, 11, 457–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scorpio, A.; Zhang, Y. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat. Med. 1996, 2, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Manca, C.; Koo, M.S.; Peixoto, B.; Fallows, D.; Kaplan, G.; Subbian, S. Host Targeted Activity of Pyrazinamide in Mycobacterium tuberculosis Infection. PLoS ONE 2013, 8, e74082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cholo, M.C.; Mothiba, M.T.; Fourie, B.; Anderson, R. Mechanisms of action and therapeutic efficacies of the lipophilic antimycobacterial agents clofazimine and bedaquiline. J. Antimicrob. Chemother. 2017, 72, 338–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giraud-Gatineau, A.; Coya, J.M.; Maure, A.; Biton, A.; Thomson, M.; Bernard, E.M. The antibiotic bedaquiline activates host macrophage innate immune resistance to bacterial infection. Elife 2020, 9, e55692. [Google Scholar] [CrossRef]
- Genestet, C.; Bernard-Barret, F.; Hodille, E.; Ginevra, C.; Ader, F.; Goutelle, S. Antituberculous drugs modulate bacterial phagolysosome avoidance and autophagy in Mycobacterium tuberculosis-infected macrophages. Tuberculosis 2018, 111, 67–70. [Google Scholar] [CrossRef]
- Yano, T.; Kassovska-Bratinova, S.; Teh, J.S.; Winkler, J.; Sullivan, K.; Isaacs, A. Reduction of Clofazimine by Mycobacterial Type 2 NADH: Quinone Oxidoreductase. J. Biol. Chem. 2011, 286, 10276–10287. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.R.; Pan, F.; Parvez, S.; Fleig, A.; Chong, C.R.; Xu, J. Clofazimine inhibits human Kv1.3 potassium channel by perturbing calcium oscillation in T lymphocytes. PLoS ONE 2008, 3, e4009. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagatia, H.; Tsolaki, A.G. The Role of Complement System and the Immune Response to Tuberculosis Infection. Medicina 2021, 57, 84. https://doi.org/10.3390/medicina57020084
Jagatia H, Tsolaki AG. The Role of Complement System and the Immune Response to Tuberculosis Infection. Medicina. 2021; 57(2):84. https://doi.org/10.3390/medicina57020084
Chicago/Turabian StyleJagatia, Heena, and Anthony G. Tsolaki. 2021. "The Role of Complement System and the Immune Response to Tuberculosis Infection" Medicina 57, no. 2: 84. https://doi.org/10.3390/medicina57020084
APA StyleJagatia, H., & Tsolaki, A. G. (2021). The Role of Complement System and the Immune Response to Tuberculosis Infection. Medicina, 57(2), 84. https://doi.org/10.3390/medicina57020084




